ABSTRACT
COPD patients are at increased risk for cardiovascular morbidity and mortality independent of smoking habits. Recent studies suggest CT emphysema is an independent predictor of cardiovascular risk as evidenced by its association with arterial stiffness and impaired endothelial function. We examined the relationship between demographics, lung function, CT emphysema and airway wall thickness and thoracic aortic calcification, another marker of cardiovascular risk, in the National Lung Screening Trial. We hypothesized that CT emphysema would be independently associated with thoracic aortic calcification. Two hundred forty current and former smokers were enrolled. After CT examination, we recorded subjects’ demographics and they performed spirometry. Subjects were classified into COPD and non-COPD subgroups. CT emphysema was quantified as a percentage of lung volume and measurements of the right upper lobe airway were performed using standard methods and expressed as wall area (%). Total calcification scores for the thoracic aorta were computed using TeraRecon image analysis. Univariate and multivariate analyses were performed to determine the associations between calcium score and subject characteristics. Subjects with COPD were older, more often male, heavier smokers and had more CT emphysema and greater aortic calcification than those without COPD. Calcium score was associated with age, pack-years, CT emphysema, wall area%, and lung function on univariate testing but only with age and CT emphysema on multivariate analysis. We conclude that CT emphysema is independently associated with thoracic calcification and thus may be used to assess cardiovascular risk in smokers with and without COPD.
BACKGROUND
Chronic obstructive pulmonary disease (COPD) is the fourth-leading cause of death in the United States and is the only major cause of death for which mortality continues to increase (Citation1, 2). Although many COPD patients die from respiratory failure, cardiovascular disease is a leading cause of death in individuals with COPD regardless of the severity of airflow obstruction (Citation3–5). Currently there is no COPD-specific marker of cardiovascular risk.
The mechanisms underlying the relationship between COPD and cardiovascular disease remain unclear, but appear to be independent of smoking history (Citation3, Citation6). It has been hypothesized that the endothelial dysfunction responsible for insults to the coronary vasculature similarly affects the pulmonary vasculature and can lead to emphysema (Citation7, 8). Others have suggested that the mechanistic link between COPD and cardiovascular disease is attributable to an inflammatory process whereby loss of elastin occurs in both the arterial wall and the alveolar architecture (Citation9–11). These hypotheses are supported by recent studies demonstrating associations between CT emphysema and impaired brachial artery flow-mediated vasodilation (FMD), a marker of endothelial dysfunction, and increased pulse wave velocity (PWV), a measure of arterial stiffness (Citation8, 9).
Alhough FMD and PWV are both highly predictive of cardiovascular events, there are limitations to the use of these markers in patients with COPD, as both are affected by cigarette smoking and other vasoactive drugs. Although the correlations between CT emphysema and these markers are intriguing, the findings are limited by the exclusion of active smokers in the former study (Citation8) and the use of carotid-brachial PWV rather than the “gold standard” carotid-femoral PWV in the latter (Citation9, Citation12).
Calcium deposits in the aorta signify the development of atherosclerotic disease, and populations with more extensive thoracic aortic calcification have an increased incidence of cardiovascular mortality independent of common risk factors such as age, smoking, hypertension, serum cholesterol, and diabetes (13–15).
To our knowledge, no study has examined the associations between the clinical and radiographic features of COPD and the presence of thoracic aortic calcification. The marker is attractive as it is unaffected by vasoactive medications or active cigarette smoking. The aim of this project was to examine the relationship between demographics, lung function, the presence of COPD, CT emphysema, and airway wall thickness and thoracic aortic calcification in the National Lung Screening Trial (NLST) population. We hypothesized that CT emphysema would correlate with the presence of aortic calcification. Some of the results of this study have been previously published in the form of an abstract (Citation16).
MATERIALS AND METHODS
The study received approval from the University of Alabama at Birmingham and Partners Health Care Institutional Review Boards as well as the NLST as an associate study.
Patient population
The NLST is sponsored by the National Cancer Institute and was designed to compare annual chest radiographs with low-dose CT for the early detection of lung cancer. The primary outcome of the NLST is lung cancer mortality and approximately 50,000 subjects were recruited from 2002–2004. Participants are men and women aged 55 to 74 years with a minimum 30-pack-year history of cigarette smoking. Exclusion criteria included prior lung cancer, pulmonary resection, or recent respiratory infection. We approached consecutive NLST participants at the University of Alabama at Birmingham site for participation in the study at the time of one of three annual CT screenings.
Study procedures
After CT screening, subjects signed informed consent and their demographic and medical information was recorded. A smoking history was obtained and subjects performed pre-bronchodilator spirometry according to American Thoracic Society standards (Citation17). FEV1 was recorded as percent predicted using race-adjusted reference values (Citation18). Subjects with FEV1/FVC ratio ≥ 0.70 and FEV1% predicted < 80% were classified as restricted and excluded from analysis. COPD was defined by the presence of an FEV1/FVC ratio < 0.70.
CT analysis
NLST CT scans were obtained with a multidetector helical scanner at 120 kilovolt peak, 40 to 60 mA, and 1-s scan time using 2.5-mm collimation and contiguous reconstructions. Quantitative densitometric analysis was performed, and areas of CT emphysema were defined as low attenuation areas (LAA) (≤ −950 Hounsfield units). The percentage of LAA (LAA%) was then determined for the entire lung using open-source software (3D Slicer; Brigham and Women's Hospital; Boston, MA) as described previously (Citation19, 20). Airway wall measurements of the right upper lobe bronchus were performed using standard methods and expressed as wall area% (WA%) (Citation21, 22).
Measurements of aortic calcification were made using a post-imaging processing application (Aquarius Work Station, TeraRecon, San Mateo, CA) using the Calcium Analysis Tool (). We determined the total aortic calcification score, or Agatston score (Citation23), by obtaining the sum of each CT slice's area of calcification multiplied by a coefficient based on the peak CT number. Areas of calcification were defined by the standard scoring threshold (> 130 HU). To minimize the effect of CT image noise on the calcification measures, these values were divided by the standard deviation of the CT attenuation (HU) measured in the middle of the ascending aorta at the level of the origin of the left main coronary artery (Citation24). Investigators determining the extent of aortic calcification were unaware of subjects’ lung function, smoking history and quantitative emphysema.
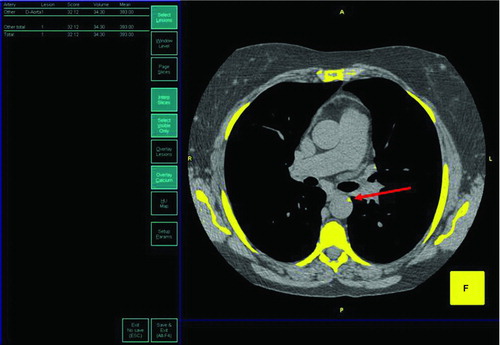
Figure 1. Aortic calcification measurement. Shown is a representative screen image of the Aquarius Workstation highlighting areas of calcification within the thorax (>130 Hounsfield Units). The red arrow highlights an area of thoracic aortic calcification. The total aortic calcification score, or Agatston calcium score, was determined by obtaining the sum of each CT slice's area of calcification multiplied by a coefficient based on the peak CT number. Calcifications in the aortic valve and coronary arteries were excluded from analysis.
Statistical analysis
Differences in characteristics between subjects with and without COPD were examined using t-tests for continuous variables and homogeneity chi-squared analysis for categorical variables. Simple and multiple regression analysis was used to determine the associations of calcium score with age, sex, race, pack-years, LAA%, WA%, lung function and the presence of COPD. P values < 0.05 were considered significant. Statistical analyses were performed using SPSS for Windows version 15.0.
Table 1. Subject characteristics
RESULTS
A total of 240 subjects were included in the study. The demographics, smoking history, lung function, LAA%, WA%, and thoracic aortic calcification scores for the COPD and non-COPD groups are shown in . Subjects were predominately male (64%) and Caucasian (91%) and had an extensive smoking history (55% were current smokers).
Approximately two-thirds of participants met spirometric criteria for COPD based on pre-bronchodilator spirometry. Subjects in the COPD group were older (62.6 vs. 60.7 years, p = 0.009), more often male (70% vs. 52%, p = 0.006), and were heavier smokers (67 vs. 54 pack-years, p = 0.002). Subjects with COPD also had more extensive CT emphysema (7.91% vs. 3.34%, p< 0.001) and higher calcium scores (75.8 vs. 47.6, p = 0.046). Subjects with COPD also exhibited a trend toward greater wall thickness (WA%) (78.1% vs. 76.6%, p = 0.08)
displays the univariate correlations between subject demographics, lung function, CT parameters and calcium score. Age, pack-years, LAA%, and the presence of COPD were each positively associated with calcium score (p< 0.05 for all) while WA% and FEV1 (% predicted) were negatively correlated with calcium score (p<0.05). Gender, race and current smoking were not associated with calcium score.
Table 2. Univariate analyses between subject characteristics and calcium score
On multivariate analysis, age (8.63 (6.18–11.1); p<0.001) and LAA% (2.16 (0.10–4.22); p = 0.04) remained significant predictors for calcium score after adjustment for the other covariates (). The estimate for pack-years approached statistical significance (0.38 (−0.02–0.79); p = 0.06). The R2 for the final model was 0.26. LAA% remained associated with calcium scores when the absolute FEV1/FVC ratio was used rather than the presence of COPD (2.36 (0.01–4.70); p = 0.049). Similar results were also obtained when FEV1 (% predicted) was included in the model, rather than the presence of COPD though the estimate for CT emphysema did not reach statistical significance (p = 0.10). When the presence of COPD and FEV1 (% predicted) were both included in the multivariate model the estimate for CT emphysema was again non-significant (p = 0.10). When uncorrected calcium scores were used we did not observe an independent correlation with LAA% (p = 0.35).
DISCUSSION
We have found that in a population of current and former smokers with and without COPD, CT emphysema (LAA%) is associated with the presence of thoracic aortic calcification, a well-established marker of cardiovascular risk (Citation13–15). This association persists after adjustment for subject demographics, smoking history, a CT metric of airways disease and the presence of airflow obstruction. These data complement and extend prior reports demonstrating associations between LAA% and arterial stiffness and impaired flow-mediated vasodilation and argue that CT emphysema is a valid tool to stratify cardiovascular risk among smokers (Citation8, 9).
In the current study, COPD subjects had higher thoracic calcium scores than healthy smokers and both the presence of airflow obstruction and FEV1 (%) were highly correlated with that measure of cardiovascular risk in univariate analyses. This is in keeping with prospective studies that have repeatedly shown that cardiovascular disease is a major cause of morbidity and mortality in COPD patients, accounting for between 25% and 50% of deaths (Citation4, 5, Citation25).
In addition, it has been clearly demonstrated that reduced lung function as measured by FEV1 is highly associated with cardiovascular events (Citation3, Citation26, Citation27) and that this correlation is independent of well-established cardiovascular risk factors such as smoking, hypercholesterolemia, and hypertension. Though these studies demonstrate the strong association between reduced lung function and cardiovascular morbidity and mortality, the mechanistic link remains unclear. It has been postulated that pathological events common to both emphysema and atherosclerosis, such as endothelial dysfunction and degradation of elastin, could explain this link and recent reports that demonstrate associations between CT emphysema and markers of cardiovascular disease support this assertion (Citation8, 9).
McAllister has shown that CT emphysema is linearly associated with arterial stiffness as measured by PWV (Citation9) extending the results reported by Sabit who demonstrated increased arterial stiffness in COPD patients as compared to control subjects matched for age, sex, blood pressure, and other cardiovascular risks (Citation28). The results argue that elastin degradation may occur systemically due to exaggerated matrix-metalloproteinase driven inflammation and could lead to emphysema in the lung, stiffness of the arterial vasculature and accelerated atherosclerosis, and even loss of skin elasticity as suggested by animal models (Citation11, Citation28–30).
Table 3. Final multivariate model for predictors of calcium score
Importantly, McAllister found no association between CRP and arterial stiffness, suggesting that systemic inflammation may not be the driver of accelerated vascular disease in COPD though many inflammatory markers were not examined (Citation31, 32) and other authors have suggested such a relationship (Citation33). Although these results are intriguing, the adoption of CT emphysema as a specific marker of cardiovascular risk is limited, as the correlation was observed between LAA% and carotid-brachial PWV rather than the preferred carotid-femoral PWV. Carotid-femoral measurements are thought superior as they are largely unaffected by changes in smooth muscle tone, better correlate with central artery stiffness, and predict cardiovascular risk in longitudinal studies (Citation12).
In another recent study, Barr found that LAA% is linearly associated with endothelial dysfunction as measured by FMD of the brachial artery (Citation8). The authors propose that the link between COPD and cardiovascular disease could be explained by vascular insults that affect both the coronary and pulmonary vasculature. It has been shown that apoptotic endothelial cells are present in the lungs of patients with COPD and that nitric oxide-mediated endothelial relaxation is reduced in their excised pulmonary vessels (Citation8, Citation34, Citation35).
In their population of former smokers with COPD, Barr demonstrated a similar reduction in in vivo endothelial function as evidenced by reduced brachial FMD, which correlates well with intracoronary endothelial vasoactivity (Citation36). Again, it is important to recognize that the use of CT emphysema as a marker of cardiovascular risk as supported by these data is limited by the exclusion of current smokers and by other factors such as medication use that may have confounded the observed associations. In addition, though no measures of CT emphysema were obtained, Maclay has recently reported that men with COPD, in fact, have normal endothelial function as compared to healthy controls raising questions about the utility of FMD as a marker of cardiovascular risk in this population (Citation37).
Like PWV and FMD, aortic calcification is well-established marker of cardiovascular risk. Although the extent of aortic calcification has been shown to be associated with age and smoking (as we also observed); diabetes, and hypertension, it remains independently predictive of cardiovascular morbidity and mortality after adjustment for these traditional cardiovascular risk factors (Citation13–15). In a population-based cohort study, Iribarren demonstrated that men and women with aortic arch calcifications were 25% and 22% more likely to develop coronary heart disease compared to those without aortic arch calcifications after adjustment for other risks (Citation13). Other studies have shown similar associations between aortic calcification and an increased incidence of coronary artery disease and all-cause mortality (Citation14, 15).
No prior study has examined the relationship between a metric of airways disease and a marker of cardiovascular risk in smokers. Though WA% was not an independent predictor of calcium score in the multivariate models it was negatively correlated when examined alone. As increasing WA% is associated with lower lung function, and therefore more advanced COPD, this may appear unexpected (Citation21, Citation38); however, we observed an inverse correlation between LAA% and WA% (B = −0.15 (−0.28 to −0.02); p = 0.025) which may explain this finding. An inverse relationship between quantitative measures of airways disease and LAA% has also been described previously (Citation39).
The independent correlation between LAA% and thoracic aortic calcification in our study strengthens prior reports and supports the concept that CT emphysema conveys additional information about cardiovascular risk in smokers that may also be associated with the underlying mechanism of disease. The findings are also important as measures of aortic calcification are not subject to instability caused by smoking or other vasoactive drugs or technical issues that limit the utility of FMD and PWV.
Our study has several limitations. The low-dose NLST CT protocol is not ideal for the measurement of airway wall thickness, and thus the determination of WA% could be impacted. We did observe a trend towards higher WA% among subjects with COPD, which would be expected. The low-dose CT protocol may also overestimate LAA% compared to higher dose scans though the technique has been used before and the effect is likely uniform across subjects (Citation19, 20).
Measurements of aortic calcification are also subject to image noise due to the low-resolution scans, respiratory motion artifact, and cardiac pulsation artifact (Citation23). We attempted to minimize the effect of image noise by standardization as described in the methods and indeed, an independent correlation between LAA% and aortic calcification was only observed when corrected calcium measures were used. In our preliminary analysis of these data, which was published as an abstract (Citation16), a univariate association was observed between uncorrected calcium scores and LAA% but an independent association was not found on multivariate testing. Corrected calcium scores could not be reliably obtained on 26 of the 266 patients included in the abstract and thus these patients were excluded from the final analysis.
We did not examine the association between subject characteristics and coronary artery calcification, another powerful marker for cardiovascular risk, but a strong relationship between aortic calcification and coronary artery calcification as detected by CT has been previously shown (Citation15, Citation23, Citation40). We were also unable to adjust our analysis for other cardiovascular risk factors not available in the database, such as blood pressure, lipids, diabetes, hypertension, and inflammatory markers. These factors may certainly be associated with the extent of aortic calcification though a larger study would be required to adequately correct for their effects. Lastly, though only pre-bronchodilator spirometry measures were available, which almost certainly led to an overestimation of the number of subjects with COPD (Citation41), classification of COPD based on pre-bronchodilator spirometry measures has been previously shown to predict COPD-related hospitalization and death (Citation42).
In conclusion, these results support the use of CT emphysema as a tool to stratify cardiovascular risk in smokers with and without COPD. Additional large-scale studies are needed to confirm these findings and to further examine the association between CT emphysema and other markers of cardiovascular disease as well as the occurrence of cardiovascular events.
Declaration of interest
The authors have no conflict of interests to declare. The authors are solely responsible for the content, writing and approval of the manuscript.
REFERENCES
- Viegi G, Pistelli F, Sherrill DL, Maio S, Baldacci S, Carrozzi L. Definition, epidemiology and natural history of COPD. Eur Respir J 2007; 30:993–1013.
- Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 1997; 349:1436–1442.
- Sin DD, Man SF. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc 2005; 2:8–11.
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356:775–789.
- Anthonisen NR, Connett JE, Enright PL, Manfreda J. Lung Health Study Research Group. Hospitalizations and mortality in the Lung Health Study. Am J Respir Crit Care Med 2002; 166:333–339.
- Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004; 59:574–580.
- Kanazawa H, Asai K, Hirata K, Yoshikawa J. Possible effects of vascular endothelial growth factor in the pathogenesis of chronic obstructive pulmonary disease. Am J Med 2003; 114:354–358.
- Barr RG, Mesia-Vela S, Austin JH, Basner RC, Keller BM, Reeves AP, Shimbo D, Stevenson L. Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: the Emphysema and Cancer Action Project (EMCAP) Study. Am J Respir Crit Care Med 2007; 176:1200–1207.
- McAllister DA, Maclay JD, Mills NL, Mair G, Miller J, Anderson D, Newby DE, Murchison JT, Macnee W. Arterial stiffness is independently associated with emphysema severity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 176:1208–1214.
- Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 2005; 25:932–943.
- Shifren A, Mecham RP. The stumbling block in lung repair of emphysema: elastic fiber assembly. Proc Am Thorac Soc 2006; 3:428–433.
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27:2588–2605.
- Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA 2000; 283:2810–2815.
- Rodondi N, Taylor BC, Bauer DC, Lui LY, Vogt MT, Fink HA, Browner WS, Cummings SR, Ensrud KE. Association between aortic calcification and total and cardiovascular mortality in older women. J Intern Med 2007; 261:238–244.
- Itani Y, Watanabe S, Masuda Y. Aortic calcification detected in a mass chest screening program using a mobile helical computed tomography unit: relationship to risk factors and coronary artery disease. Circ J 2004; 68:538–541.
- Dransfield MT, Huang F, Washko GR, Nath H. CT measures of emphysema and airway disease do not predict aortic calcification. Am J Respir Crit Care Med 2009:A4024.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, Van Der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J; ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 2005; 26:319–338.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159:179–187.
- Hersh CP, Washko GR, Jacobson FL, Gill R, Estepar RS, Reilly JJ, Silverman EK. Interobserver variability in the determination of upper lobe-predominant emphysema. Chest 2007; 131:424–431.
- Dransfield MT, Washko GR, Foreman MG, Estepar RS, Reilly J, Bailey WC. Gender differences in the severity of CT emphysema in COPD. Chest 2007; 132:464–470.
- Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, Nishimura K, Itoh H, Paré PD, Hogg JC, Mishima M. Computed tomographic measurements of airway dimensions and emphysema in smokers: correlation with lung function. Am J Respir Crit Care Med 2000; 162:1102–1108.
- Hasegawa M, Nasuhara Y, Onodera Y, Makita H, Nagai K, Fuke S, Ito Y, Betsuyaku T, Nishimura M. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 173:1309–1315.
- Takasu J, Budoff MJ, O’Brien KD, Shavelle DM, Probstfield JL, Carr JJ, Katz R. Relationship between coronary artery and descending thoracic aortic calcification as detected by computed tomography: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2009; 204:440–446.
- Wu MT, Yang P, Huang YL, Chen JS, Chuo CC, Yeh C, Chang RS. Coronary arterial calcification on low-dose ungated MDCT for lung cancer screening: concordance study with dedicated cardiac CT. AJR Am J Roentgenol 2008; 190:923–928.
- Camilli AE, Robbins DR, Lebowitz MD. Death certificate reporting of confirmed airways obstructive disease. Am J Epidemiol 1991; 133:795–800.
- Beaty TH, Newill CA, Cohen BH, Tockman MS, Bryant SH, Spurgeon HA. Effects of pulmonary function on mortality. J Chronic Dis 1985; 38:703–710.
- Engström G, Wollmer P, Hedblad B, Juul-Möller S, Valind S, Janzon L. Occurrence and prognostic significance of ventricular arrhythmia is related to pulmonary function: a study from “men born in 1914,” Malmö, Sweden. Circulation 2001; 103:3086–3091.
- Sabit R, Bolton CE, Edwards PH, Pettit RJ, Evans WD, McEniery CM, Wilkinson IB, Cockcroft JR, Shale DJ. Arterial stiffness and osteoporosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 175:1259–1265.
- Laurent S, Boutouyrie P, Lacolley P. Structural and genetic bases of arterial stiffness. Hypertension 2005; 45: 1050–1055.
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997; 390:45–51.
- Schols AM, Buurman WA, Staal Van Den Brekel AJ, Dentener MA, Wouters EF. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax 1996; 51:819–824.
- Wouters EF. Local and systemic inflammation in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005; 2:26–33.
- Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003; 107:1514–1519.
- Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res 2006; 7:53.
- Peinado VI, Barbera JA, Ramirez J, Gomez FP, Roca J, Jover L, Gimferrer JM, Rodriguez-Roisin R. Endothelial dysfunction in pulmonary arteries of patients with mild COPD. Am J Physiol 1998; 274:L908–913.
- Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, Satomura K, Ohsuzu F, Kurita A. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol 1998; 82:1535–1539.
- Maclay JD, McAllister DA, Mills NL, Paterson FP, Ludlam CA, Drost EM, Newby DE, Macnee W. Vascular dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009; 180:513–520.
- Hasegawa M, Nasuhara Y, Onodera Y, Makita H, Nagai K, Fuke S, Ito Y, Betsuyaku T, Nishimura M. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 173:1309–1315.
- Kim WJ, Silverman EK, Hoffman E, Criner GJ, Mosenifar Z, Sciurba FC, Make BJ, Carey V, Estépar RS, Diaz A, Reilly JJ, Martinez FJ, Washko GR; NETT Research Group. CT metrics of airway disease and emphysema in severe COPD. Chest 2009; 136:396–404.
- Eisen A, Tenenbaum A, Koren-Morag N, Tanne D, Shemesh J, Imazio M, Fisman EZ, Motro M, Schwammenthal E, Adler Y. Calcification of the thoracic aorta as detected by spiral computed tomography among stable angina pectoris patients: association with cardiovascular events and death. Circulation 2008; 118:1328–1334.
- Johannessen A, Omenaas ER, Bakke PS, Gulsvik A. Implications of reversibility testing on prevalence and risk factors for chronic obstructive pulmonary disease: a community study. Thorax 2005; 60:842–847.
- Mannino DM, Sonia Buist A, Vollmer WM. Chronic obstructive pulmonary disease in the older adult: what defines abnormal lung function? Thorax 2007; 62:237–241.