ABSTRACT
The natural history of lung hyperinflation in patients with airway obstruction is unknown. In particular, little information exists about the extent of air trapping and its reversibility to bronchodilator therapy in those with mild airway obstruction. We completed a retrospective analysis of data from individuals with airway obstruction who attended our pulmonary function laboratory and had plethysmographic lung volume measurements pre- and post-bronchodilator (salbutamol). COPD was likely the predominant diagnosis but patients with asthma may have been included. We studied 2,265 subjects (61% male), age 65 ± 9 years (mean ± SD) with a post-bronchodilator FEV1/FVC <0.70. We examined relationships between indices of airway obstruction and lung hyperinflation, and measured responses to bronchodilation across subgroups stratified by GOLD criteria. In GOLD stage I, vital capacity (VC) and inspiratory capacity (IC) were in the normal range; pre-bronchodilator residual volume (RV), functional residual capacity (FRC) and specific airway resistance were increased to 135%, 119% and 250% of predicted, respectively. For the group as a whole, RV and FRC increased exponentially as FEV1 decreased, while VC and IC decreased linearly. Regardless of baseline FEV1, the most consistent improvement following bronchodilation was RV reduction, in terms of magnitude and responder rate. In conclusion, increases (above normal) in airway resistance and plethysmographic lung volumes were found in those with only minor airway obstruction. Indices of lung hyperinflation increased exponentially as airway obstruction worsened. Those with the greatest resting lung hyperinflation showed the largest bronchodilator-induced volume deflation effects. Reduced air trapping was the predominant response to acute bronchodilation across severity subgroups.
INTRODUCTION
Traditionally, the progression of respiratory impairment in susceptible smokers is charted by the accelerating rate of decline in FEV1 over time (Citation1). This approach, while useful and convenient, gives only limited information about the relentless deterioration of respiratory mechanics that characterizes chronic obstructive pulmonary disease (COPD). Progressive lung hyperinflation is another salient aspect of physiological deterioration that until recently has been neglected and is the main focus of this study. Given the clear association between lung hyperinflation, dyspnea, exercise intolerance (Citation2), and mortality (Citation3, 4), as well as its partial reversibility to treatment, there is considerable interest in evaluating the clinical utility of this physiological “biomarker” in COPD.
One reasonable hypothesis, recently reiterated by Peter Macklem (Citation5), is that the progressive decrements in FEV1 largely reflect the erosion of vital capacity (VC) as a consequence of increasing residual volume (RV). In other words, FEV1 decline mirrors the evolution of lung hyperinflation. Earlier small physiological studies have attested to remarkable heterogeneity in the pathophysiology of mild COPD. Thus, there is evidence that significant peripheral airway closure, maldistribution of ventilation, disruption of ventilation-perfusion relations and air trapping can exist (in highly variable combinations) in smokers with minimal or no reduction in FEV1 (Citation6–8). We have recently reported that resting lung volumes [RV, functional residual capacity (FRC) and total lung capacity (TLC)] were consistently increased in small groups of patients with significant exertional dyspnea who met criteria for Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage I COPD (Citation9, 10). The existence of air trapping in patients with ostensibly mild airway obstruction has potentially important clinical implications that remain to be studied (Citation11).
There is evidence that lung hyperinflation at rest is partially reversible to bronchodilator therapy and that lung deflation may in turn form the basis for improved respiratory symptoms (Citation12–15). Indeed, it can be argued that improvement in FEV1 following bronchodilator treatment mainly reflects recruitment of VC as a result of reduced air trapping (i.e., reduced RV). Thus, it is known that FEV1/FVC ratio remains unaltered (or even decreases) after bronchodilator treatment at least in more advanced COPD (Citation12–14). The pattern of bronchodilator reversibility in patients with milder airway obstruction (i.e., expired flow versus lung volume effects) is less well studied and will be examined in this study.
The main objectives of this study were to: 1) examine relations between increasing airway obstruction and changes in static lung volume components; 2) determine the nature and extent of physiological impairment in patients with milder airflow obstruction; and 3) determine if the pattern of change in airway function (i.e., flow versus volume response) following acute bronchodilator inhalation would differ in mild and severe airway obstruction.
We therefore conducted a retrospective analysis of a cohort of individuals from our pulmonary function laboratory database in whom plethysmographic lung volume measurements before and after a bronchodilator were available. Although our study population was likely comprised predominantly of patients with COPD, we cannot exclude the possibility that some patients with other obstructive airways diseases (i.e., asthma) were included since complete information on smoking history and clinical diagnosis was not available.
METHODS
Subjects and Design
This observational study involved a retrospective analysis of pulmonary function test records collected between May 1992 and April 2008 at the Kingston General Hospital's Pulmonary Function Laboratory. Patients selected from this database were referred for comprehensive pulmonary function tests with reversibility testing. Selection criteria included: males and females 40–80 years of age; body mass index 14–55 kg/m2; post-bronchodilator FEV1/FVC < 0.7; availability of post-bronchodilator static lung volumes (by body plethysmography); and the absence of a referral diagnosis of any lung disease other than COPD.
In the case of repeated follow-up testing within a patient, only the first visit meeting eligibility criteria was used in the analysis. For analysis, subjects were stratified by GOLD stage (Citation16). Since risk of harm to patients was minimal and obtaining patient informed consent impracticable, strict safeguards to ensure confidentiality of personal health data were implemented. The Queen's University and Affiliated Teaching Hospitals Research Ethics Board approved the use of this data and waived the need for patient informed consent.
A group of our previously studied (Citation17, 18), age-matched (40–80 yrs), healthy non-smokers were used as control subjects to test the validity of the pulmonary function predictive equations used in this analysis.
Procedures
Pulmonary function testing (spirometry, body plethysmography, single-breath diffusing capacity) was conducted by experienced respiratory therapists/technicians using automated pulmonary function testing equipment (2130 spirometer with 6200 Autobox DL or V6200 Autobox; SensorMedics, Yorba Linda, CA) in keeping with current recommended standards (Citation19–21). Patients were required to withdraw from all short-acting and long-acting bronchodilators for at least 4 and 12 hours, respectively. Reversibility testing (spirometry, body plethysmography) was performed using salbutamol 200 mcg by metered dose inhaler; post-dose measurements were performed 20-min after inhalation. Predicted normal values were those used in the laboratory at the time of testing (Citation22–25); predicted IC was calculated as predicted TLC minus predicted FRC, predicted ERV was calculated as predicted FRC minus predicted RV.
Statistical analysis
Values are reported as means ±SD unless otherwise specified. A p-value of < 0.05 was considered significant in all analyses. A chi-square test for homogeneity was used to assess the differences between the proportions of gender within the entire cohort and each GOLD stage. Differences between means across genders were examined using the unpaired t-test. Frequency distributions were analyzed for normality using the one-sample Kolmogorov-Smirnov test. Non-normal distributions were further examined for skewness (symmetry) or kurtosis (peakedness) which were considered significant if the skewness or kurtosis coefficients were >2. Comparisons across GOLD stages were analyzed using ANOVA; post-hoc testing of significant variables was performed using t-tests with Bonferroni adjustment for multiple comparisons.
Bronchodilator responsiveness
To avoid bias from differences in baseline measurements, changes in FEV1 and various lung volumes were assessed and compared as percentages of predicted normal values (%pr) (Citation26, 27). The occurrence of a positive bronchodilator response was evaluated as frequency statistics and compared using a chi-square test. A positive response cut-off for FEV1 of at least 10%predicted was selected after considering consensus statements and outcomes of previous studies (Citation12, Citation26, Citation27).
Table 1. Subject characteristics
Figure 1. Pre- and post-bronchodilator static lung volumes are shown for each GOLD stage group compared with an age-matched healthy control group. Residual volume (RV) and functional residual capacity (FRC) increased progressively as GOLD stage worsened. Total lung capacity increased in GOLD stage I in conjunction with a preserved inspiratory capacity (IC), vital capacity (VC) and expiratory reserve volume (ERV). IC and VC then decreased progressively in GOLD stages II and III/IV.
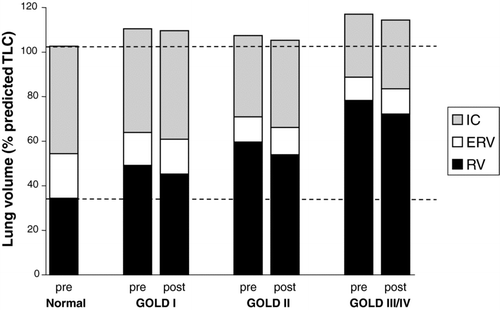
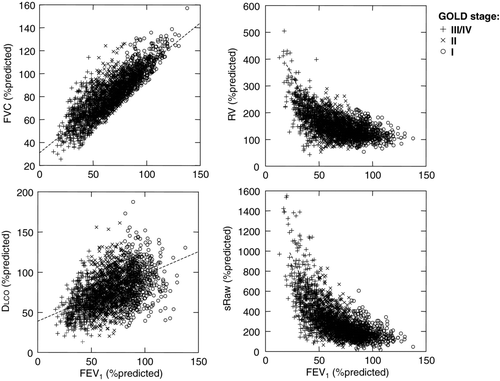
Figure 2. Relationships between forced vital capacity (FVC), residual volume (RV), diffusing capacity of the lung (DLCO) and specific airway resistance (sRaw) are shown against FEV1 (all measurements expressed as% of predicted normal values). FVC changed linearly with FEV1, RV and sRaw both increased exponentially as FEV1 decreased, and DLCO decreased as FEV1 decreased.
Because no recognized criteria are available, we arbitrarily selected a similar cut-off of at least 10% predicted for IC and other measured lung volumes. A change of this magnitude falls outside the 95% confidence interval for these measurements, as well as outside the coefficient of variation for repeated measurements, in COPD (Citation28, 29). In addition, we have previously estimated that an increase in IC of ∼10%predicted (or ∼0.3 L) results in clinically important improvements in exertional dyspnea intensity (i.e., reductions of at least 0.5 Borg Scale units) and in exercise endurance (i.e., increases of 20% or more) in moderate to severe COPD (Citation14, Citation28).
Regression models
Post-bronchodilator measurements expressed as %pr were used for regression analyses evaluating the effect of worsening airflow obstruction (FEV1) on lung volumes, specific airway resistance (sRaw) and diffusing capacity (DLCO). We first evaluated whether the relationship was linear or non-linear using Box-Cox transformation (Citation30). Regardless of linearity/nonlinearity, we used GOLD stage grouping as a categorical variable for FEV1 and performed a two-way ANOVA (with interaction) using GOLD stage grouping and sex as categorical variables. A significant interaction term (sex*GOLD) would indicate a difference between men and women in the volume-FEV1 relationship. All model fitting was examined using residual analysis and regression diagnostics were performed to identify possible outliers and influential observations.
RESULTS
Of the 2,265 subjects included in this analysis, 1,378 (61%) were male and 887 (39%) were female. Pre-bronchodilator DLCO measurements were available in 2,167 of the included subjects. As there were only 74 patients with a post-bronchodilator FEV1< 30%predicted and the presence of chronic respiratory failure could not be confirmed, GOLD stages III and IV were combined into one larger (n = 516) severe-to-very severe group for evaluation in this analysis. There was a similar mean age of 65 yrs within each gender group. GOLD stage distribution was relatively similar in men and women, with the majority in each gender meeting stage II criteria (48 and 53%, respectively). Measurements across age- and height-matched GOLD stage subgroups are reported in . Lung volume components are summarized in .
Table 2. Bronchodilator reversibility: pre- to post-bronchodilator changes
Measurements from our healthy control group () indicated good validity of predictive equations for the majority of pulmonary function measurements but underestimated our laboratory's normative values by at least 5% for FEV1, VC, IC, ERV, sRaw, DLCO and DLCO/VA. Comparisons between the GOLD I group and this age-matched control group showed that all pre-bronchodilator pulmonary function measurements expressed as%predicted were significantly different (p < 0.0005) between groups except for VA and the SVC-FVC difference, which were similar.
Frequency distributions of pulmonary function measurements
Pre- and post-bronchodilator measurements expressed as%predicted showed normal distributions for: FEV1, FVC, IC, SVC and TLC; as well as the IC/TLC, FRC/TLC and RV/TLC ratios. These measurements were also normally distributed within each GOLD stage subgroup. Positive skewness (significantly longer right tail) was shown in FEF50, FRC, RV, ERV, sRaw and DLCO. However, the skewed distributions for the group as a whole were largely combinations of normally distributed GOLD subgroups, each with different means.
Relationships with worsening airway obstruction
There was a linear relation between FEV1%pr and FVC%pr (r = 0.84, p < 0.0005) (), SVC%pr (r = 0.79, p < 0.0005) and IC%pr (r = 0.67, p < 0.0005). Relations between FEV1 and each of sRaw (), RV (), FRC and TLCwere curvilinear (exponential), each measurement expressed as%predicted. These relationships were unaffected by gender (i.e., non-significant GOLD*sex interaction), except sRaw%pr in which females had higher sRaw (interaction p = 0.0003). RV%pr increased in association with worsening sRaw%pr (r = 0.66, p < 0.0005), FEV1/FVC (r = −0.62, p < 0.0005),FEV1%pr (r = −0.60, p < 0.0005) and FEF25–75%pr (r = −0.46, p < 0.0005). RV and FRCincreased together (r = 0.90, p < 0.0005).
The DLCO-FEV1 relation was nonlinear (squared) with wide scatter ()while the relation between DLCO corrected for alveolar volume (VA) and FEV1 was poor. As FEV1 worsened, single-breath VA decreased linearly (r = 0.65, p < 0.0005) and the TLC-VA difference increased exponentially.
Bronchodilator reversibility
The magnitude of pre- to post-dose change in lung function measurements across GOLD stages is shown in and . There was a small but significant (p < 0.0005) increase in post-bronchodilator FEV1 in all GOLD subgroups; however, this increase was significantly smaller in stage III/IV than either I or II. FEV1/FVC changes were not consistent across subgroups with a small increase of 0.4% (p = 0.002), no change (p = 0.97), and a decrease of 1.4% (p < 0.0005) in stages I, II and III/IV, respectively.
Figure 3. Bronchodilator-induced changes in lung volumes are shown as (A) measured changes expressed as % of predicted normal and (B) frequencies of a positive response defined as at least a 10 % of predicted improvement in that measurement. As GOLD stage worsened, the FEV1 response became less important while other lung volume responses increased progressively.
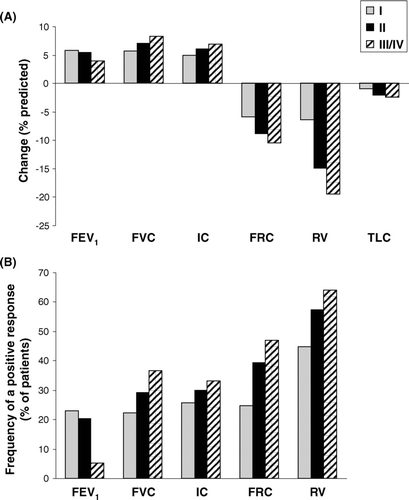
The magnitude of the FEV1 response correlated significantly with the FVC response (r = 0.67, p < 0.0005). In all GOLD groups, the RV response was greatest in magnitude; however, the magnitude of reduction in RV became greater as GOLD stage worsened (III/IV>II>I; p < 0.0005). Bronchodilator-induced reductions in RV%pr correlated with increases in FEV1%pr (r = −0.39, p < 0.0005) and sRaw%pr (r = 0.48, p < 0.0005); reductions in RV and FRC were strongly related (r = 0.81, p < 0.0005).
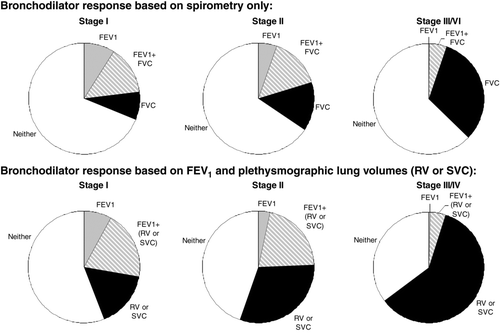
Using the ATS criteria of at least a 12% and 200 mL improvement in FEV1 (Citation31), the frequency of a positive response was 22.6%, 28.6% and 16.7% in the GOLD I, II and III/IV subgroup, respectively. The frequency of a positive bronchodilator response assessed by an improvement of at least 10%pr in each lung volume measurement across GOLD stages is shown in ; again, there were fewer FEV1 responders in the GOLD III/IV subgroup. The frequency of a positive RV response was greater than for any other lung volume within each GOLD stage subgroup, but increased progressively across GOLD stages (p < 0.0005). The frequency of a positive response determined by spirometry alone or in combination with plethysmographically-determined lung volumes is shown in . By considering changes in SVC and/or RV in addition to FEV1, a larger proportion of each GOLD subgroup showed a positive response to a bronchodilator, especially in GOLD stage III/IV. Reductions in sRaw by at least 10%predicted were seen in 84, 86 and 85% of subjects in GOLD I, II and III/IV, respectively.
Figure 4. The frequency of a significant bronchodilator response (change of at least 10% predicted) is shown when only spirometric measurements are taken into account (FEV1 alone, FVC alone or combination of FEV1 and FVC) (top). By considering plethysmographic lung volume (RV and SVC) improvements in addition to spirometric FEV1, greater rates of reversibility were uncovered (bottom). When volumes were taken into account, the frequency of a positive bronchodilator response increased with worsening GOLD stage.
DISCUSSION
The main findings of this study were as follows: 1) RV, FRC and TLC were significantly increased above predicted values in GOLD I; by contrast, VC and IC were largely preserved; 2) for the whole group, the relation between increasing airflow obstruction and decreasing VC and IC was linear, whereas indices of lung hyperinflation increased exponentially; 3) reduced air trapping (reduced RV) was the most consistent response to a bronchodilator across severity subgroups.
The unique features of this database are the inclusion of a large population who met the criteria for mild airway obstruction and the availability of reliable pre- and post- bronchodilator plethysmographic lung volumes. Given that these patients were referred to a specialized pulmonary unit for assessment, it is reasonable to assume that respiratory symptoms were present in the majority. The three subgroups stratified by GOLD criteria were well matched for age and height and had similar sex representation (i.e., 30–40% female). We validated our pulmonary function normative reference values in a control group of age- and height-matched non-smokers. Plethysmographic lung volumes in our control group were well within the normal range as predicted for the reference population, whereas spirometric volume measurements in the control sample tended to exceed the predicted normal values.
Mild airway obstruction
Individuals fitting GOLD I criteria (average post-bronchodilator FEV1 of 93% predicted) had evidence of significant respiratory impairment which included increased lung hyperinflation, increased specific airway resistance (to 250% predicted) and reduced mid-volume expiratory flow rates (to 39% predicted) (all pre-bronchodilator values). RV was 0.73 or 35% above the predicted value, on average. The presence of an increased RV signifies increased air trapping due to enhanced airway closure during full expiration.
There is no consensus with respect to a definition of air trapping but a RV exceeding the predicted value by more than 20% is generally thought to be significant. A greater than normal RV has previously been reported in our own studies in GOLD I patients (Citation9, 10), as well as in an earlier (1966) Canadian study in younger male smokers who had symptoms of chronic bronchitis (Citation32). Not surprisingly, the increased RV correlated well with increased airway resistance and inversely with other measures of reduced expiratory flow rates.
FRC was similarly increased by an average of 0.75 or 19% above predicted normal in this group. In numerical terms, the increase in FRC was mainly explained by the increase in RV with preservation of ERV. Increased FRC values correlated inversely with FEV1. Mechanistically, it could not be determined whether the increased FRC was due to the effects of increased lung compliance or to heterogeneous alterations in the mechanical time constants for gas emptying, or both. An earlier physiological study showed that smokers with COPD with preserved FEV1 and FVC had measurable increases in static lung compliance (Citation7). It is therefore conceivable (but unproven) that alteration of the elastic properties of the lung contributed to the consistent increases in TLC and FRC that were present in the GOLD I group.
In keeping with previous physiological studies (Citation9, 10), the VC and IC in the GOLD I group were not reduced in the face of consistent increases in RV and FRC, respectively. This was explained by the accompanying increase in TLC. Derived ratios (RV/TLV, IC/TLC and FRC/TLC) were also all within the expected ranges. The clinical relevance of increased air trapping of this magnitude in GOLD I is unknown but it is reasonable to assume that this may be linked to development of respiratory symptoms such as exertional dyspnea in some individuals (Citation9, 10).
DLCO measurements were lower than in the healthy control group but varied widely. We could not control for the potential effects of active tobacco smoking (Citation33). However, the finding that approximately 20% of the GOLD I subgroup had a DLCO <70%predicted suggests that the surface area for gas exchange was abnormal in some. Recent small studies have shown that ventilation-perfusion disequilibrium and a widened alveolar to arterial O2 tension gradient occur in the majority of patients with mild COPD (Citation34, 35).
The continuum of lung hyperinflation
Relations between increasing airway obstruction (FEV1%predicted) and decreasing VC and IC (all post-bronchodilator) were linear in this population. By contrast, the relations between increasing airway obstruction and increasing RV, FRC and TLC were exponential. Thus, there was a disproportionate increase in indices of lung hyperinflation as FEV1 and VC worsened to a critically reduced value. In general, these data support the conclusion that progressive decline in FEV1 in part reflects the concomitant reduction in VC as a result of an increased RV.
However, it seems that at the extremes of airway obstruction, RV and related volume components rise more precipitously in tandem with sharper increases in sRaw and increases in the TLC-VA difference. The disparity between worsening spirometry and increasing hyperinflation is not surprising given the fact that FEV1 gives little information about the physiological determinants of air trapping such as peripheral airway function, the heterogeneity of mechanical time constants within the lungs and breathing pattern (Citation36, 37).
Bronchodilator reversibility of lung hyperinflation
The pattern of reversibility varied across severity subgroups. Thus, improvement in FEV1 was more likely in those fitting GOLD I criteria than those with more severe airway obstruction. Additional measurements of change in static lung volumes following bronchodilator therapy added little to evaluation of bronchodilator efficacy in those with mild airway obstruction. Among all lung volume components, the greatest effect (in terms of effect size and responder rate) was reduction of RV. This was true regardless of GOLD stage. RV changes correlated inversely with changes in FEV1 and FVC across all three groups. The fact that FEV1 mainly changed in proportion to changes in VC supports the idea that lung volume recruitment as a result of reduced RV is an important contributor to improved expiratory flow rates.
sRaw consistently decreased after bronchodilator in the majority of subjects in all GOLD groups and was correlated with change in RV. The magnitude of this change fell within one standard deviation of the mean across groups, therefore, the clinical significance of this improvement is unclear.
Incremental decrements in FRC and reciprocal increases in IC also occurred as GOLD severity increased. Consistent with results of previous studies (Citation12, Citation29), the largest lung volume deflation effect was seen in those with the most severe resting lung hyperinflation. In GOLD III/IV, the majority (64%) showed a significant volume reduction response while fewer in this group had an FEV1 response, i.e., 5% and 17% by ERS and ATS criteria, respectively. The true magnitude of bronchodilator reversibility may have been underestimated in our study due to the fact that only a single beta2-agonist bronchodilator was used and that there was a relatively short period of withdrawal of any long-acting bronchodilators (12 hours) prior to testing. However, the disparity in flow versus volume responses is striking and indicates that important improvements in the mechanical time constants for lung emptying may occur independently of change in FEV1. Pharmacological lung volume reduction of the magnitude seen in GOLD stages II-IV is likely important but the clinical relevance of smaller such changes in GOLD I is less certain (Citation10).
Limitations
We utilized a hospital database consisting exclusively of caucasians where information on smoking history, respiratory symptoms, comorbidities, medications and healthcare utilization was not available. Therefore, the generalizability of our results to a broader population of patients with COPD is unclear. However, our study sample is representative of the population of older individuals with airway obstruction that is not fully reversible who seek pulmonary subspecialist care. We used GOLD fixed ratio spirometric criteria for airway obstruction which may lead to over-diagnosis of COPD in the elderly (Citation38,39). However, corroborating evidence of increased air trapping, increased airway resistance and configurational changes in the expiratory flow-volume loop (all referenced to an age-matched control group) suggest the presence of respiratory impairment beyond the effects of healthy aging in the GOLD I subgroup.
CONCLUSIONS AND CLINICAL IMPLICATIONS
The novel findings of this study were as follows. Individuals with mild airway obstruction had relatively preserved vital and inspiratory capacities but showed consistent evidence of increased airway resistance and air trapping. This is the first population study to demonstrate an exponential relation between worsening airflow obstruction and indices of lung hyperinflation. The most consistent physiological improvement following acute bronchodilator therapy across GOLD stages was reduced RV. Collectively, these results provide new insights into the possible course of physiological deterioration in COPD and prompt the question of whether pharmacological reduction of air trapping in patients with milder airway obstruction is clinically beneficial.
Declaration of interest
The authors report no conflicts of interest related to this manuscript. The authors alone are responsible for the content and writing of the paper.
ACKNOWLEDGMENTS
The authors wish to acknowledge the pulmonary function laboratory staff Cathy Muir (RRT, RCPT(P)), Denis Faubert (B.Sc., RRT) and Robin McHardy (RCPT(P)) for their careful attention to detail when conducting the tests reported as part of this study.
REFERENCES
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977; 1(6077):1645–1648.
- O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in COPD. Am J Respir Crit Care Med 2001; 64:770–777.
- Tantucci C, Donati P, Nicosia F, Bertella E, Redolfi S, de Vecchi M, Corda L, Grassi V, Zulli R. Inspiratory capacity predicts mortality in patients with chronic obstructive pulmonary disease. Respir Med 2008; 102:613–619.
- Casanova C, Cote C, de Torres JP, Aguirre-Jaime A, Marin JM, Pinto-Plata V, Celli BR. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171:591–597.
- Macklem PT. Therapeutic implications of the pathophysiology of COPD. Eur Respir J 2010; 35:676–680.
- Cosio M, Ghezzo H, Hogg JC, Corbin R, Loveland M, Dosman J, Macklem PT. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med 1977; 298:1277–1281.
- Corbin RP, Loveland M, Martin RR, Macklem PT. A four-year follow-up study of lung mechanics in smokers. Am Rev Respir Dis 1979; 120:293–304.
- Woolcock AJ, Vincent NJ, Macklem PT. Frequency dependence of compliance as a test for obstruction in small airways. J Clin Invest 1969; 48:1097–1106.
- Ofir D, Laveneziana P, Webb KA, Lam YM, O’Donnell DE. Mechanisms of dyspnea during cycle exercise in symptomatic patients with GOLD stage I chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 177:622–629.
- O’Donnell DE, Laveneziana P, Ora J, Webb KA, Lam Y-M, Ofir D. Evaluation of acute bronchodilator reversibility in patients with symptoms of GOLD stage I COPD. Thorax 2009; 64:216–223.
- Raghavan N, Ora J, Webb KA, O’Donnell DE. “Mild” COPD – is there a case for earlier treatment? Ann Respir Med 2010; 1(1):23–30.
- O’Donnell DE, Forkert L, Webb KA. Evaluation of bronchodilator responses in patients with “irreversible” emphysema. Eur Respir J 2001; 18: 914–920.
- O’Donnell DE, Hamilton AL, Webb KA. Sensory-mechanical relationships during high-intensity, constant-work-rate exercise in COPD. J Appl Physiol 2006; 101:1025–1035.
- O’Donnell D, Flüge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, Make B, Magnussen H. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004; 23:832–840.
- Maltais F, Hamilton A, Marciniuk D, Hernandez P, Sciurba FC, Richter K, Kesten S, O’Donnell D. Improvements in symptom-limited exercise performance over eight hours with once-daily tiotropium in patients with COPD. Chest 2005; 128:1168–1178.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. GOLD executive summary. Am J Respir Crit Care Med 2007; 176:532–555.
- Ofir D, Laveneziana P, Webb KA, Lam Y-M, O’Donnell DE. Sex differences in the perceived intensity of breathlessness during exercise with advancing age. J Appl Physiol 2008; 104:1583–1593.
- Jensen D, Ora J, Webb KA, O’Donnell DE. Effects of dead space loading on the intensity, quality and unpleasantness of perceived respiratory discomfort during incremental cycle exercise in the healthy elderly. Am J Respir Crit Care Med 2010; 181(abstracts):A6487.
- Miller MR, Hankinson J, Brusasco V, et al; on behalf of the American Thoracic Society/European Respiratory Society (ATS/ERS) Task Force. Standardisation of spirometry. Eur Respir J 2005; 26:319–338.
- Wanger J, Clausen JL, Coates A, et al; on behalf of the ATS/ERS Task Force. Standardisation of the measurement of lung volumes. Eur Respir J 2005; 26:511–522.
- MacIntyre N, Crapo RO, Viegi G, et al; on behalf of the ATS/ERS Task Force. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005; 26:720–735.
- Morris JF, Koski A, Temple WP, Claremont A, Thomas DR. Fifteen-year interval spirometric evaluation of the Oregon predictive equations. Chest 1988; 92:123–127.
- Crapo RO, Morris AH, Clayton PD, Nixon CR. Lung volumes in healthy nonsmoking adults. Bull Europ Physiopath Resp 1982; 18:419–425.
- Briscoe WA, Dubois AG. The relationship between airway resistance, airway conductance, and lung volumes in subjects of different age and body size. J Clin Invest 1959; 37:1279–1285.
- Burrows B, Kasik JE, Niden AH, Barclay WR. Clinical usefulness of the single-breath pulmonary diffusing capacity test. Am Rev Respir Dis 1961; 84:789–806.
- Siafakas NM, Vermeire P, Pride NB, Paoletti P, Gibson J, Howard P, Yernault JC, Decramer M, Higenbottam T, Postma DS, Rees J; on behalf of the European Respiratory Society Task Force. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). Eur Respir J 1995; 8:1398–1420.
- Brand LP, Quanzer PH, Postma DS, Kerstjens HAM, Koëter GH, Dekhuijzen PNR, Sluiter HJ, and the Dutch chronic non-specific lung disease (CNSLD) study group. Interpretation of bronchodilator response in patients with obstructive airways disease. Thorax 1992; 47:429–436.
- O’Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 168:1557–1565.
- Box GEP, Cox DR. An analysis of transformations. Journal of the Royal Statistics Society 1964;26(B):211–234.
- American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am J Respir Crit Care Med 1991; 144:1202–1218.
- Bates DV, Gordon CA, Paul GI, Place REG, Snidal DP, Woolf CR. Chronic bronchitis: report on the third and forth stages of the co-ordinated study of chronic bronchitis in the Department of Veterans Affairs, Canada. Med Serv J Can 1966; 22:1–59.
- Watson A, Joyce H, Hopper L, Pride NB. Influence of smoking habits on change in carbon monoxide transfer factor over 10 years in middle aged men. Thorax 1993; 48:119–124.
- Rodriguez-Roisin R, Drakulovic M, Rodriguez DA, Ventilation-perfusion imbalance and chronic obstructive pulmonary staging severity. J Appl Physiol 2009; 106:1902–1908.
- Barbera JA, Ramirez J, Roca J, Wagner PD, Sanchez-Lloret J, Rodriguez-Roisin R. Lung structure and gas exchange in mild chronic obstructive pulmonary disease. Am Rev Respir Dis 1990; 141:895–901.
- Pride NB, Macklem PT. Lung mechanics in disease. In: Fishman AP, ed. Handbook of Physiology, section 3, volume III, part 2: The Respiratory System. Bethesda: American Physiological Society 1986:659–692.
- Vinegar A, Sinnett EE, Leith DE. Dynamic mechanisms determine functional residual capacity in mice, mus musculus. J Appl Physiol 1979; 46:867–871.
- Newton MF, O’Donnell DE, Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest 2002; 121:1042–1050.
- Schermer TRJ, Smeele IJM, Thoonen BPA, Lucas AEM, Grootens JG, van Boxem TJ, Heijdra YF, van Weel C. Current clinical guideline definitions for airflow obstruction leads to substantial overdiagnosis of COPD in primary care. Eur Respir J 2008; 32:945–992.
- Hansen JE, Sun XG, Wasserman K. Spirometric criteria for airway obstruction. Chest 2007; 131:349–355.