ABSTRACT
This study assessed the cardiovascular safety of QVA149, an inhaled, once daily, bronchodilator combination containing two 24-hour bronchodilators, the long-acting β2-agonist indacaterol and the long-acting muscarinic antagonist glycopyrronium (NVA237). In this randomised, double-blind, placebo-controlled, parallel-group study, 257 patients with moderate-to-severe chronic obstructive pulmonary disease (COPD) were randomised to receive QVA149 (indacaterol/NVA237) 600/100 μg, 300/100 μg or 150/100 μg, indacaterol 300 μg or placebo, once daily for 14 days. The primary endpoint was change from baseline in 24-h mean heart rate versus placebo on Day 14. 255 patients were included in the safety analysis (mean age 63.8 years, 76.5% male, post-bronchodilator forced expiratory volume in one second [FEV1] 53.2% predicted, FEV1/FVC [forced vital capacity] 50.0%, mean 24-h heart rate 79.6 bpm). There were no clinically significant differences in the 24-h mean heart rate on Day 14 between the three doses of QVA149 and placebo or indacaterol. The confidence intervals of these treatment differences (contrasts) were within the pre-specified equivalence limit (-5 to 5 bpm). No clinically relevant differences in QTc interval (Fridericia's) were observed between groups on Days 1, 7 and 14. Once-daily QVA149 was well tolerated in COPD patients with a cardiovascular safety profile and overall adverse event rates similar to placebo.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is characterised by progressive airflow limitation and is a leading cause of morbidity and mortality worldwide that causes a significant burden on society. It is the fourth leading cause of death and is projected to be the world's third leading cause of mortality by 2020 (Citation1).
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend the use of one or more bronchodilators as maintenance therapy for the treatment and management of COPD (Citation2). A number of studies have shown that combining bronchodilators of different pharmacologic classes may improve lung function compared with bronchodilators when used as monotherapies (Citation3–13).
Such combinations of bronchodilators with different mechanisms and durations of action may increase the degree of bronchodilation leading to better symptom control for COPD patients (Citation2, Citation10). The pharmacological basis for this may be that β2-agonists decrease the release of acetylcholine leading to subsequent increase in the bronchial smooth muscle relaxation induced by the anticholinergic agent (Citation14, 15). The addition of an anticholinergic agent can also reduce peripheral bronchoconstrictor effects of acetylcholine, consequently causing amplification of bronchodilation elicited by the β2-agonist through direct stimulation of smooth muscle β2-adrenoceptors (Citation14, 15).
LABAs may impact cardiac function due to the presence of β2-adrenoreceptors in the heart (Citation16, 17). Muscarinic-receptor antagonists act primarily by reducing the vagal cholinergic tone and thus reversibly antagonising the M1, M2, and M3 receptors, a property which may cause tachycardia (Citation18). Larger doses of anti-muscarinic agents are known to cause progressively increasing tachycardia by blocking vagal effects of M2 receptors on the sinoatrial nodal pacemaker (Citation19).
Thus, due to their pharmacologic action, LABAs and LAMAs may have the potential to increase heart rate and alter other cardiac events (Citation2). Indeed, cardiovascular safety concerns with LABAs such as salmeterol and formoterol and LAMAs such as tiotropium have been reported (Citation20–22). In addition, it has been suggested that clinicians and patients need to be made aware of the potential benefits and risks when using specific medications for treatment of COPD (Citation23).
Several studies have shown no increase in the frequency or intensity of adverse events with the free combination of once-daily tiotropium plus formoterol (given once or twice daily) compared with tiotropium or formoterol alone (Citation6, 7, Citation10). Salmeterol has also been used in combination with tiotropium (Citation3, Citation13) but safety data are limited. Also, formoterol and salmeterol have been approved for twice-daily dosing. Therefore, there is a need for a once daily LABA/LAMA combination for the management of COPD, which could simplify the treatment regimen and potentially improve patient compliance and outcomes.
QVA149 is an inhaled dual bronchodilator consisting of a fixed-dose combination of two 24-hour bronchodilators, the LABA indacaterol and the LAMA glycopyrronium (NVA237), and is in clinical development for the treatment of COPD. The efficacy of both mono-components of QVA149, indacaterol (Citation24–28); and NVA237 (Citation29, Citation30), has been demonstrated and they were found to have favorable cardiovascular safety and tolerability profiles.
LABAs have been shown to cause changes in heart rate, QTc intervals, and glucose and potassium levels (Citation20, Citation31). LAMAs have been inconsistently associated with increased risk of cardiovascular events such as myocardial infarctions in COPD patients (Citation11, Citation21). Several meta-analyses have raised concerns over the cardiovascular safety profile of both LABAs (salmeterol, formoterol) and LAMAs (tiotropium) (Citation20–22, Citation32). The potential for a bronchodilator combination to have additive adverse effects on the cardiovascular system in COPD patients has not been tested. Therefore, the current study specifically investigated the cardiovascular safety of QVA149 (indacaterol/NVA237 compared with placebo and indacaterol 300 μg alone administered once daily in patients with moderate-to-severe stable COPD.
MATERIAL AND METHODS
Patients
Male and female patients (≥40 years) with moderate-to-severe COPD (GOLD 2006) and smoking history ≥10 pack-years (20 cigarettes/day for 10 years or 10 cigarettes/day for 20 years) were enrolled. To be included in the study, patients must have had: a post-bronchodilator forced expiratory volume in 1 second (FEV1) ≥30% and <80% of predicted normal and post-bronchodilator FEV1/forced vital capacity (FVC) <0.70, both measurements taken 30 minutes following inhalation of 4×100 μg puffs per inhalation of salbutamol.
Key exclusion criteria included the following: requiring long-term daily oxygen therapy for chronic hypoxemia; hospitalisation for a COPD exacerbation within 6 weeks prior to start of the study; history of asthma; history of cardiac failure, life-threatening arrhythmias, or acute ischemic changes; history of long QT syndrome or prolonged QTc at screening; uncontrolled diabetes; respiratory tract infection within 6 weeks prior to study start; history of malignancy in the previous 5 years; and patients with concomitant pulmonary disease, pulmonary tuberculosis, or clinically significant bronchiectasis. In addition, pregnant or nursing (lactating) women and women of child-bearing potential not using acceptable methods of contraception were excluded.
Patients with a history of untoward reactions to sympathomimetic amines, inhaled medication or any component thereof, or any of the study drugs or to drugs with similar chemical structures, and patients unable to use the Breezhaler®* device, a Single-Dose Dry Powder Inhaler (SDDPI) or a pressurised metered dose inhaler (rescue medication) or perform spirometry measurements were excluded from the study.
During the screening period, any ongoing LABA therapy was changed to regular short-acting β2-agonist (SABA) therapy. Only salbutamol was permitted as a rescue medication. The use of a fixed-dose combination of a SABA and SAMA was permitted during the screening period if, in the opinion of the treating physician, the patient's condition warranted such treatment. Inhaled corticosteroids (ICS) and leukotriene antagonists in recommended and constant dose regimens were allowed only if treatment had been stabilised for at least 1 month prior to start of the study. The steroid component of any fixed combination (ICS and β2-agonist) therapy was replaced with an equivalent monotherapy ICS. Unacceptable medications included treatment with sympathomimetic agents, methylxanthines, other anticholinergics, non-selective β-blocking agents, cardiac anti-arrhythmics, any drugs with a potential to significantly prolong the QT interval, tricyclic antidepressants, and monoamine oxidase inhibitors.
Study design
This was a 5-arm, randomised, double-blind, placebo-controlled, parallel-group, multicentre study. Following a pre-randomisation screening phase that lasted up to 15 days, patients were randomised at Day 1 to one of the following once-daily regimens: QVA149 (indacaterol/NVA237) 600/100 μg, 300/100 μg, or 150/100 μg; indacaterol 300 μg; or placebo. All treatments were administered via the Breezhaler®. Study drug was administered in the clinic in the morning on Days 1, 2, 7, and 14.
The study protocol and any amendments were reviewed and approved by the Independent Ethics Committee or Institutional Review Board of each participating centre. The study was conducted according to the Declaration of Helsinki. Written informed consent was obtained from each patient before enrolment.
Measurements
The primary endpoint was change from baseline in mean 24-h heart rate (ventricular rate) on Day 14 measured by Holter monitoring, a surrogate measure for cardiovascular safety. Other endpoints included change from baseline in mean 24-h heart rate on Day 1, the number of sustained ventricular ectopic events over 24 h on Days 1 and 14 and analyses of corrected QT (QTc) intervals (Fridericia's method). Continuous 24-h Holter monitoring (Holter Mortara H12+24H; eResearch Technology Limited, Peterborough, UK) was performed at screening to assess eligibility and provide a baseline recording and on Days 1 and 14.
All Holter monitoring recordings were initiated in the morning at approximately the same time; Day 1 Holter monitoring was initiated at least 45 min prior to the administration of the morning dose of the trial medication. Holter monitor recordings were to contain a minimum of 18 h of acceptable quality recording out of each 24-h recording. A single electrocardiogram (ECG) (Mortara Eli 250, eResearchTechnology Limited, Peterborough, UK) was performed at screening to assess eligibility. ECGs included all 12 standard leads and a Lead II rhythm strip of at least 10 s duration. All ECGs were centrally reviewed by an independent cardiologist.
Other safety assessments included collecting all adverse events (AEs), serious adverse events (SAEs), with their severity and relationship to study drug. Vital signs (pulse rate, blood pressure), physical condition, and laboratory parameters (including glucose and potassium) were also assessed regularly.
Efficacy was assessed in terms of FEV1 and FVC. Both FEV1 and FVC were measured at -45 min and -15 min prior to dosing, and 30 min and 2 h after dosing on Days 1 and 14. Measurements were also taken at 23 h 15 min and 23 h 45 min post-dosing when patients returned to the clinic at Days 2 and 15, respectively, or at the time of discontinuation for patients who prematurely withdrew from the study.
Statistical analyses
The intention-to-treat (ITT) population included all randomised patients and the safety population included all patients who received at least one dose of study drug. Patients were analysed according to the treatment received. Patients were randomly allocated to treatment groups in a 1:1:1:1:1 ratio using an automated system. Blinding was maintained from randomisation until database lock.
The primary variable, mean change from baseline in 24-h heart rate after 14 days, was analysed using an analysis of covariance (ANCOVA) model with treatment as a fixed effect and baseline 24-h heart rate and baseline FEV1 reversibility as covariates. All data were included regardless of rescue medication usage. The assessment tested the null hypothesis of a relevant change from baseline in heart rate (equal to or more than ±5 bpm versus placebo) against the alternative hypothesis (change of less than ±5 bpm versus placebo) for each dose of QVA149, using repeated multiple testing with a Bonferroni two one-sided test (Citation33). Results are presented as least squares (LS) means with standard errors and associated 98.3% confidence intervals (CI) for the differences between QVA149 and placebo (95% CI for other comparisons).
No imputation was made for missing data. The analysis of the primary variable was repeated using the same ANCOVA but with age, sex and smoking history as covariates. A similar ANCOVA with appropriate baseline values was used to assess change from baseline in mean 24-h heart rate on Day 1 before testing the secondary variable. Comparisons between doses were not adjusted for multiplicity.
QTc interval, blood pressure and pulse rate, and the efficacy variables, were analysed using a similar ANCOVA incorporating the appropriate baseline value, without adjustment for multiplicity. Other safety data were summarised for each treatment group for the safety population. QTc interval was calculated using Fridericia's formula. Although no commonly recognised safety standard has been established for heart rate data, a difference of 5 bpm was used as the threshold based on previous cardiovascular safety study (Citation34). Based on this, and a standard deviation of 5.26 bpm for the change from baseline in heart rate on Day 14 based on previous findings, a sample size of 47 evaluable patients per treatment arm was needed to reject the null hypothesis with 96% power at a significance level of 0.0167%.
RESULTS
Patient disposition
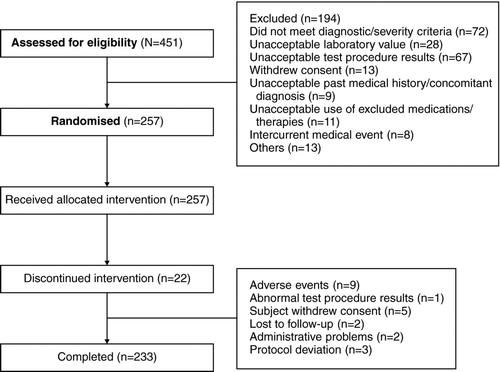
A total of 257 patients were enrolled (ITT population) and 233 (90.7%) completed the study (). The percentage of patients discontinuing the study early was highest in the QVA149 600/100 μg group (12.0%) and lowest in the QVA149 300/100 μg group (5.9%). The safety population included 255 patients.
Table 1. Patient baseline demographics and characteristics (Safety population)
Table 2. Patient cardiovascular baseline characteristics (Safety population)
Patient demographics and baseline clinical characteristics
Treatment groups were well matched for baseline demographics and COPD disease history (). The mean age of all patients was 63.8 years and the majority of patients were male (76.5%) and Caucasian (98.8%). The mean duration of COPD was 6.8 years. Mean FEV1 30 min after the inhalation of a SABA was slightly lower in the QVA149 600/100 μg and placebo groups than in the other groups. However, FEV1 reversibility and FEV1 percent predicted after SABA use were similar across groups (mean FEV1 reversibility 13.9, mean FEV1 percent predicted 53.2). FEV1 reversibility was lowest for the QVA149 150/100 μg group. Baseline demographics and lung function () and cardiovascular characteristics () were similar between the groups.
Table 3. Overall incidence of notable QTc values, change from baseline and most significant ECG results (Safety population), n (%)
Mean 24-h heart rate
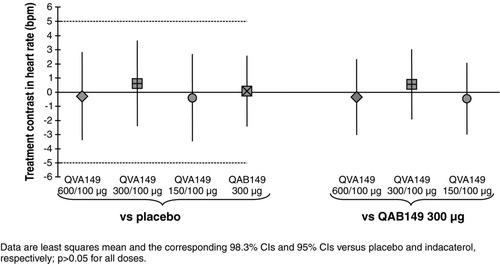
None of the QVA149 doses showed a statistically significant differences from placebo in mean 24-h heart rate on Day 14, and the 98.3% CIs of these differences were contained within the pre-specified equivalence limit of (−5 to +5 bpm)(). There was no apparent dose-response relationship between the different QVA149 doses. The results of a confirmatory ANCOVA model including additional demographic and smoking history covariates produced the same outcome. When the QVA149 treatment groups were compared with indacaterol 300 μg, there was again no statistical difference in changes in mean 24-h heart rate; similarly there was no difference between indacaterol 300 μg and placebo.
A similar analysis was performed for Day 1 mean 24-h heart rate. All treatments tended to decrease heart rate, with the greatest change from baseline in the QVA149 600/100 μg treatment group (least squares mean −2.877 bpm compared with −2.770, −0.547, −1.849, and −0.329 bpm for QVA149 300/100 μg, QVA149 150/100 μg, indacaterol 300 μg, and placebo, respectively). The two highest QVA149 doses had differences from placebo approaching, but not reaching, statistical significance (p = 0.05).
Ventricular and supraventricular ectopic events
The distribution of counts for ventricular ectopic events was highly skewed and comparisons were therefore made based on median data. At baseline, the median total number of ventricular events and single premature contractions was highest in the QVA149 600/100 μg group. The number of events did not appear to be dose- or time-related. No patient experienced periods of sustained ventricular tachycardia, Torsades de pointes, ventricular fibrillation or ventricular flutter. The presence of non-sustained tachycardia was greatest in the QVA149 150/100 μg group at baseline (14.3%). Presence of such events increased on Day 1 for the QVA149 600/100 μg group (14.9%), but fell on Day 14 (9.8%).
Supraventricular ectopic events were most common at baseline in the QVA149 150/100 μg treatment group with no apparent trend towards an increase or decrease over time within or between any treatment groups. There were very few incidences of atrial flutter, and the median values for all treatment groups on all days were zero. The frequency of short episodes of non-sustained supraventricular tachycardia was highest in the QVA149 150/100 μg treatment group, but percentages fell from baseline to Day 14. There was no change in the frequency of these episodes in the other groups and there was little difference among groups for other supraventricular events. There was also little difference in the median change from baseline for supraventricular ectopic events on Day 1 or Day 14.
Table 4. Overall incidence of abnormal vital signs (Safety population), n (%)
QTc intervals
The percentage of patients with notable QTc intervals (maximum post-baseline value of >470 ms in females and >450 ms in males) was similar at all time points. There were no clinically relevant differences in mean QTc interval (Fridericia's) among the groups (). The highest incidence of notable QTc interval values was in the QVA 600/100 μg group (12.2%) compared with the lower doses of 300/100 μg (7.8%) and 150/100 μg (2.0%); however, none of the values with any of the active treatments involved a notable increase (>60 ms) from baseline (). No females reported a QTc interval >470 ms and the maximum increase from baseline in QTc was predominantly <30 ms when using Fridericia's formula.
The differences among groups with regards to LS mean of QTc interval were minimal and there was no dose-response relationship. The QVA149 groups showed significant differences from placebo only at 3 time points in the 600/100 μg treatment group: LS mean (SE) on Day 1 at 4 h and 23 h 45 minutes’ post-dose was 408.3 (1.67) and 408.2 (1.77) respectively, while on Day 7 at 30 minutes’ post-dose, it was 409.7(1.91). Clinically insignificant abnormalities were more frequent with increasing QVA149 dose (150/100 μg: 39.2%; 300/100 μg: 54.9%; 600/100 μg: 57.1%) ().
Two patients reported a clinically significant ECG result post-baseline, both of whom had reported clinically significant abnormalities at baseline. The general trend for all treatment groups, except QVA149 150/100 μg, was a mean decrease from baseline in QT interval (<10 ms). For the QVA149 150/100 μg group there was an increase from baseline on Days 7 and 14. However, pre-dose values on Day 7 and Day 14 were lower than baseline, and the actual post-dose values showed little change.
Vital signs
The overall incidence of abnormal vital signs was similar among the groups (). All treatment groups showed a tendency towards a non-clinically relevant decrease in pulse rate and blood pressure (both systolic and diastolic) post-dose. There were no notably low or high pulse rates in any of the groups, while there was single instance each of notably low or high diastolic blood pressure and systolic blood pressure in the QVA149 300/100 μg groups.
For all active treatment groups there was a mean decrease in heart rate following inhalation of study drug on Days 1, 7, and 14 of between -1.0 and -5.0 bpm. The mean change from baseline was greater in the QVA149 groups than in the indacaterol 300 μg group, but there was no evidence of a dose-response relationship. Heart rates returned to approximately baseline values at pre-treatment time points (indicating that the effect was transient), and there was no evidence that the change from baseline was greater at subsequent visits.
For all treatment groups, a heart rate ≥90 bpm was most frequently reported 4 hours post-dose, while abnormal blood pressure values were no more frequent post-dose than pre-dose. Mean change from baseline in mean sitting diastolic blood pressure (msDBP) decreased for all groups. The maximum/minimum post-dose change from baseline in msDBP was less than 20 mmHg for all groups. All treatment groups had a decrease in post-dose msDBP on Days 1, 7 and 14; however, no mean decrease was greater than 4 mmHg, and the median change was predominantly 0. On Day 1, all treatment groups showed a decrease from baseline in mean sitting systolic blood pressure (msSBP) at post-baseline time points that was not significant.
Table 5. Most common adverse events (occurring in >3% in any group) (Safety population), n (%)
Adverse events and serious adverse events
The most common AEs are summarised in . Overall, 25.9% of patients experienced an AE. The majority of AEs were mild or moderate in severity, and there was no apparent relationship between QVA149 dose and AE frequency. The percentage of patients reporting AEs was highest in the QVA149 150/100 μg group (33.3%), and was the lowest in the indacaterol 300 μg group (19.6%). The most frequently reported AEs were cough (4.3%) and COPD exacerbation (3.9%). Seven (2.7%) patients experienced severe AEs; 3 (5.9%) patients in the QVA149 150/100 μg group, 3 (5.9%) patients in the indacaterol 300 μg group and 1 (1.9%) patient in the placebo group. In the QVA149 150/100 μg group the severe AEs were COPD exacerbation, ventricular tachycardia, and anemia. In the indacaterol 300 μg group, the SAEs were COPD exacerbation, spondylitis, and cataract. In the placebo group one patient reported a severe toothache.
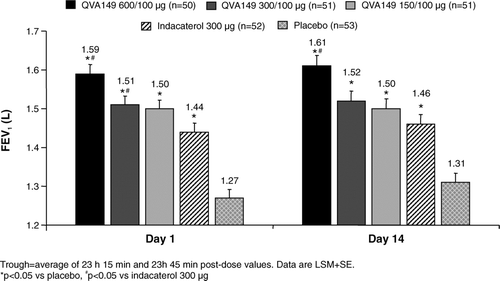
Figure 3. Trough FEV1 (ITT population). Trough = average of 23 h 15 min and 23 h 45 min post-dose values. Data are LSM+SE. *p < 0.05 vs placebo, #p < 0.05 vs indacaterol 300 μg.
SAEs were reported in 5 (2.0%) patients. Of these patients, 3 (1.2%) discontinued due to SAEs. SAEs reported as related to study medication included ventricular tachycardia (n = 1 [2.0%]; QVA149 150/100 μg group), atrial fibrillation (n = 1 [2.0%]; QVA149 600/100 μg), and hyperkalaemia (n = 1 [2.0%]; QVA149 600/100 μg). These cardiovascular events were based on the Holter monitoring readings and not on reports and patient's symptoms. SAEs not considered to be study-drug related included anaemia (n = 1 [2.0%]; QVA149 150/100 μg) and COPD exacerbation (n = 1 [1.9%]; placebo). No deaths were reported during the study.
Efficacy
In all treatment groups, QVA149 showed significant increases (p < 0.05) in trough FEV1 compared with placebo on Days 1 and 14 (). Trough FEV1 for the QVA149 600/100 and 300/100 μg groups was significantly higher (p < 0.05) than indacaterol 300 μg on Day 1 and QVA 600/100 μg was significantly higher (p < 0.05) than indacaterol 300 μg on Day 14. The change from baseline in FVC showed a pattern similar to FEV1. All doses of QVA149 were statistically greater than placebo (p < 0.05) at all post-dose time points on Days 1 and 14.
DISCUSSION
The results of this study indicate that once-daily QVA149, a dual bronchodilator containing the LABA indacaterol and the LAMA NVA237, has an acceptable safety and tolerability profile in patients with COPD. QVA149 produced no significant effect on mean 24-h heart rate after 14 days of treatment. The effect of QVA149 on other cardiovascular assessments (ventricular and supraventricular ectopic events, QTc interval, vital signs) appeared to be minimal and with a profile similar to placebo. In addition, it was well tolerated, with overall AE rates similar to placebo.
The results of this study are important since previous studies have questioned the cardiovascular safety profile of LABAs and LAMAs. In a meta-analysis of randomised, placebo-controlled trials using β2-agonists (short- and long-acting), Salpeter et al. (Citation20) evaluated the short-term effects on heart rate, potassium concentrations and long-term effects on adverse cardiovascular events. These bronchodilators were shown to cause increases in heart rate and reduced potassium concentrations compared to placebo and by this mechanism also precipitate cardiovascular events like ischaemia, congestive heart failure, arrythmias and sudden death. Additional studies have shown LABAs to be associated with systemic adverse effects such as skeletal muscle tremor, headache, nervousness, palpitations with changes in heart rate, prolongation of the QTc interval, and increased glucose and potassium blood levels in COPD patients and healthy volunteers (Citation31).
A meta-analysis by Singh et al. (Citation21) indicated that inhaled muscarinic antagonists significantly increased the risk of cardiovascular death, myocardial infarction or stroke compared with placebo/active control. Conversely, the results of the Understanding Potential Long-Term Impacts on Function with Tiotropium (UPLIFT) trial, did not demonstrate an increase in cardiovascular events. Instead, patients receiving tiotropium had shown a decreased risk of mortality and reduced cardiac and respiratory morbidity compared with patients in the control group (Citation11). Celli et al. (Citation35) analysed the incidence rates of cardiovascular events (including composite cardiovascular end points encompassing cardiovascular deaths, nonfatal myocardial infarction, nonfatal stroke, and the terms sudden death, sudden cardiac death and cardiac death), and all-cause mortality from the pooled results of 30 randomised, parallel-group and placebo-controlled trials in stable COPD patients. Their results also indicated that tiotropium reduced the risk of cardiovascular events, cardiovascular mortality and all-cause mortality in COPD patients.
The results of our study indicated that once-daily QVA149 has an acceptable cardiac safety profile similar to placebo. Additional safety data will be determined in Phase III studies to investigate the long-term cardiovascular safety of QVA149 in COPD patients. These results are consistent with studies on LABA/LAMA combinations in COPD patients (Citation6, 7, Citation10, 11). Although formoterol has been approved for twice-daily administration, it has been shown that formoterol used once daily in combination with once-daily tiotropium did not show drug-related increase either in frequency or in intensity of adverse events compared to either agent administered alone.
Furthermore, measurements of post-study ECG recordings, blood pressure, pulse rates and biochemical tests did not reveal any difference between the combination and the single drug periods (Citation6). No clinically relevant changes have been observed with the combination of twice-daily formoterol and once-daily tiotropium in safety parameters like blood pressure, pulse rate, ECG, and standard laboratory tests (Citation7). Similar results for changes in mean heart rate and QTc intervals have been reported by Tashkin et al. (Citation11) and Vogelmeier et al. (Citation10) for the combination of formoterol and tiotropium. In addition, combined therapy did not appear to confer any increased risk compared with individual therapies (Citation10).
The potential limitations of this study were that it only included patients that did not have any clinically significant cardiovascular conditions. However, in ‘real life’ practice, patients will likely have multiple co-morbidities and thus be at considerably greater risk than the patients studied in this trial. Another limitation was that the 14-day duration of this study was too short to make any definitive conclusions regarding the long-term safety of QVA149. Indeed, future and ongoing long-term studies in larger patient populations will additionally establish the efficacy and safety profile of QVA149 in patients with COPD.
In conclusion, the present study demonstrated that once-daily QVA149 was well tolerated with an acceptable overall safety profile in patients with moderate-to-severe COPD. The effect of the combination of indacaterol and NVA237 on cardiovascular assessments, even at the highest dose (600/100 μg), appeared to be minimal. Furthermore, the results also show the 24-h bronchodilator efficacy of QVA149. This combination of the two 24-h bronchodilators with different mechanisms of action in a single inhaler may increase compliance and further simplify COPD management.
Declaration of interests
BVM has received speaker fees and reimbursement for attending conferences in Belgium and abroad from Medtronics, Glaxo Smith Kline, and Astra Zeneca; he and his staff received fees for conducting clinical trials for following companies: Boehringer Ingelheim, Glaxo Smith Kline, AstraZeneca, and Novartis. LMF has served as a consultant to AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Merck Sharp and Dohme, Novartis, Nycomed, Roche, Pfizer, and Sigma-Tau; been paid lecture fees by AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Merck Sharp and Dohme, Novartis, Nycomed, Roche, and Pfizer; and received grant support from AstraZeneca, Boehringer Ingelheim, Menarini, Schering-Plough, Chiesi Farmaceutici, GlaxoSmithKline, Merck Sharp and Dohme, Nycomed, Union Chimique Belge, Pfizer, Sigma-Tau, Italian Ministry of Health, and Italian Ministry for University and Research. CM, RH, MD and TO are employees of Novartis.
ACKNOWLEDGMENTS
The authors acknowledge Shaik Asma Sultana, professional medical writer (Novartis), Mark Fedele (Novartis) and Gary Cotter (ACUMED UK) for assistance in the preparation of this manuscript. This study was supported by Novartis Pharma AG, Basel, Switzerland.
Notes
*Breezhaler is a registered trademark of Novartis Pharm AG, Basel, Switzerland.
REFERENCES
- World Health Organisation (WHO). World Health Statistics. Available at: http://www.who.int/whosis/whostat/EN_WHS08_Full.pdf Accessed: 10 November 2008.
- GOLD. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease, Updated 2009. Available at http://www.goldcopd.com Accessed: 23 December 2009.
- Cazzola M., Santus P, Verga M, Mondoni M, Di Marco F, Matera MG. The functional impact of adding salmeterol to tiotropium in patients with stable COPD. Respir Med 2004; 98:1214–1221.
- Brashier B, Jantikar A, Maganji M, Raghupathy A, Valsa S, Gokhale P, Mahadik P, Gogtay J, Salvi SS. Addition of formoterol to tiotropium produces better FEV1 and FVC responses when measured over 24 hours following single-dose administration in subjects with moderate-to-severe COPD (Abstract). Chest 2005; 128:258s.
- Cazzola M, Noschese P, Salzillo A, De Giglio C, D’Amato G, Matera MG. Bronchodilator response to formoterol after regular tiotropium or to tiotropium after regular formoterol in COPD patients. Respir Med 2005; 99:524–528.
- van Noord JA, Aumann JL, Janssens E, Smeets JJ, Verhaert J, Disse B, Mueller A, Cornelissen PJG. Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. Eur Respir J 2005; 26:214–222.
- van Noord JA, Aumann JL, Janssens E, Verhaert J, Smeets JJ, Mueller A, Cornelissen PJG. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest 2006; 129:509–517.
- Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, Balter M, O’Donnell D, McIvor A, Sharma S, Bishop G, Anthony J, Cowie R, Field S, Hirsch A, Hernandez P, Rivington R, Road J, Hoffstein V, Hodder R, Marciniuk D, McCormack D, Fox G, Cox G, Prins HB, Ford G, Bleskie D, Doucette S, Mayers I, Chapman K, Zamel N, FitzGerald M. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2007; 146:545–555.
- Rabe KF, Timmer W, Sagkriotis A, Viel K. Comparison of a combination of tiotropium plus formoterol to salmeterol plus fluticasone in moderate COPD. Chest 2008; 134:255–262.
- Vogelmeier C, Kardos P, Harari S, Gans SJ, Stenglein S, Thirlwell J. Formoterol mono- and combination therapy with tiotropium in patients with COPD: a 6-month study. Respir Med 2008a; 102:1511–1520.
- Tashkin DP, Littner M, Andrews CP, Tomlinson L, Rinehart M, Denis-Mize K. Concomitant treatment with nebulized formoterol and tiotropium in subjects with COPD: a placebo-controlled trial. Respir Med 2008; 102:479–487.
- Tashkin D, Pearle J, Iezzoni D, Varghese ST. Treatment with formoterol plus tiotropium is more effective than treatment with tiotropium alone in patients with stable chronic obstructive pulmonary disease: Findings from a randomized, placebo-controlled trial. J COPD 2009; 6:17–25.
- van Noord JA, Aumann JL, Janssens E, Smeets JJ, Zaagsma J, Mueller A, Combining tiotropium and salmeterol in COPD: Effects on airflow obstruction and symptoms. Respir Med 2010; 10(7):995–1004.
- Cazzola M, Matera MG. The effective treatment of COPD: Anticholinergics and what else? Drug Disc Today: Therapeut Strateg 2006; 3(3):277–286.
- Cazzola M, Molimard M. The scientific rationale for combining long-acting β2-agonists and muscarinic antagonists in COPD. Pulm Pharmacol Ther 2010; 23(4):257–267.
- Roig J, Hernando R, Mora R. Indacaterol, A Novel Once Daily Inhaled β2-Adrenoreceptor Agonist. The Open Respiratory Medicine Journal 2009, 3:27–30.
- Restrepo RD. Use of inhaled anticholinergic agents in obstructive airway disease. Respir Care 2007;52:833–851.
- Ray NC, Alcaraz L. Muscarinic antagonist-β2–adrenergic agonist dual pharmacology molecules as bronchodilators: a patent review Expert Opin. Ther Patents 2009;19(1):1–12.
- Sarria B, Naline E, Zhang Y, Cortijo J, Molimard M, Moreau J, Therond P, Advenier C, Morcillo EJ. Muscarinic M2 receptors in acetylcholine-isoproterenol functional antagonism in human isolated bronchus. Am J Physiol Lung Cell Mol Physiol 2002; 283(5):L1125–L1132.
- Salpeter SR, Omiston TM, Salpeter EE. Cardiovascular effects of β2-agonists in patients with asthma and COPD: a meta-analysis. Chest 2004; 125:2309–2321.
- Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA 2008; 300:1439–1450.
- Rodrigo GJ, Nannini LJ. Tiotropium for the treatment of stable chronic obstructive pulmonary disease: a systematic review with meta-analysis. Pulmon Pharmacol Ther 2007; 20:495–502.
- Rabe KF. Anticholinergic Drugs for the Treatment of COPD Are Safe… Are They? Chest 2010; 137:1–3.
- Rennard S, Bantje T, Centanni S, Chanez P, Chuchalin A, D’Urzo A, Kornmann O, Perry S, Jack D, Owen R, Higgins M. A dose-ranging study of indacaterol in obstructive airways disease, with a tiotropium comparison. Respir Med 2008; 102:1033–1044.
- Beier J, Chanez P, Martinot JB, Schreurs AJ, Tkácová R, Bao W, Jack D, Higgins M. Safety, tolerability and efficacy of indacaterol, a novel once-daily β2-agonist, in patients with COPD: A 28-day randomised, placebo controlled clinical trial. Pulm Pharmacol Ther 2007; 20:740–749.
- Dahl RP, Chung KF, Buhl R, Magnussen H, Nonikov V, Jack D, Efficacy of a new once-daily long-acting inhaled β2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax 2010; 65:473–479.
- Donohue JF, Fogarty C, Lötvall J, Mahler DA, Worth H, Yorgancioglu A, Once-daily Bronchodilators for Chronic Obstructive Pulmonary Disease: Indacaterol versus Tiotropium. Am J Respir Crit Care Med 2010; 182:155–162.
- Khindri S, Sabo R, Harris S, Jennings S, Drollman AF. Cardiac safety of indacaterol—no clinical effect on QT in healthy subjects. Eur Respir J 2009; 34(Suppl. 53):2031.
- Vogelmeier C, Verkindre C, Cheung D, Galdiz JB, Güçlü SZ, Spangenthal S, Overend T, Henley M, Mizutani G, Zeldin RK. Safety and tolerability of NVA237, a once-daily long-acting muscarinic antagonist, in COPD patients. Pulm Pharmacol Ther. 2010; 23:438–444.
- Verkindre C, Fukuchi Y, Flémale A, Takeda A, Overend T, Prasad N, Dolker M. Sustained 24-h efficacy of NVA237, a once-daily long-acting muscarinic antagonist, in COPD patients. Respir Med. 2010; 104:1482–1489.
- Bremmer P, Woodmarn K, Burgess C, Crane J, Purdie G, Pearce N, Beasley RA. Comparison of the cardiovascular and metabolic effects of formoterol, salbutamol and fenoterol. Eur Respir J 1993; 6:204–210.
- Rodrigo G, Nannini LJ, Rodríguez-Roisin R. Safety of long-acting β-agonists in stable COPD. Chest 2008; 133:1079–1087.
- Welleck S. Testing Statistical Hypotheses of Equivalence, 1st Edition, London: Chapman and Hall, 2002.
- Ferguson G, Funck-Bretano C, Fischer T, Darken P, Reisner C. Cardiovascular safety of salmeterol in COPD. Chest 2003; 123:1817–1824.
- Celli B, Decramer M, Leimer I, Vogel U, Kesten S, Tashkin DP. Cardiovascular safety of tiotropium in patients with COPD. Chest 2010; 137:20–30.