Abstract
There is an increasing awareness of the role of distal airways in the pathophysiology of obstructive lung diseases including asthma and chronic obstructive pulmonary disease. We hypothesize that during induced bronchoconstriction: 1) disparity between distal and proximal airway reactivity may occur; and 2) changes in distal airway function may explain symptom onset in subjects with minimal FEV1 change. 185 subjects underwent methacholine challenge testing (MCT). In addition to spirometry, oscillometry was performed at baseline and after maximum dose of methacholine; 33/185 also underwent oscillometry after each dose. Oscillometric parameters included resistance at 5 and 20 Hz (R5, R20) and heterogeneity of distal airway mechanics assessed by frequency dependence of resistance 5–20 Hz (R5–20) and reactance area (AX). R5 varied widely during MCT (range -0.8 – 11.3 cmH2O/L/s) and correlated poorly with change in FEV1 (r = 0.17). Changes in R5 reflected changes in both R20 and R5–20 (r = 0.59, p<0.05; r = 0.87, p<0.0001). However, R20 increased only 0.3 cmH2O/L/s, while R5–20 increased 0.7 cmH2O/L/s for every 1cmH2O/L/s change in R5, indicating predominant effect of distal airway mechanics. 9/33 subjects developed symptoms despite minimal FEV1 change (<5%), while R5 increased 42% due to increased distal airway heterogeneity. These data indicate disparate behavior of proximal airway resistance (FEV1 and R20) and distal airway heterogeneity (R5–20 and AX). Distal airway reactivity may be associated with methacholine-induced symptoms despite absence of change in FEV1. This study highlights the importance of disparity between proximal and distal airway behavior, which has implications in understanding pathophysiology of obstructive pulmonary diseases and their response to treatment.
INTRODUCTION
There is an increasing awareness of the role of distal airways in the pathophysiology of obstructive lung diseases including asthma and chronic obstructive pulmonary disease. Distal airways abnormalities are evident on histologic examination in early stages of obstructive pulmonary diseases (Citation1–3). Disparity between proximal and distal airway biology and physiology has been documented in a variety of disease states (Citation4–9). This disparity has important clinical implications in the assessment of bronchial reactivity, which is traditionally assessed by the spirometric change in FEV1 in response to methacholine (Citation10). However, the distal airways represent a potential “silent zone,” which may or may not be reflected in the FEV1 response to induced bronchoconstriction (Citation11, 12).
An effect of methacholine on distal airway function has been demonstrated directly by measurements of small airway resistance utilizing a wedged bronchoscope (Citation7, Citation13) and indirectly based on development of ventilation defects and/or air trapping on computed tomography (Citation14, 15). Since reduction in FEV1 could reflect reduced airflow or reduced lung volume, Gibbons et al. and Chapman et al. proposed that the evaluation of the simultaneous change in the vital capacity during methacholine challenge testing (MCT) might be useful to determine the contribution of airway closure to the observed change in the FEV1 (Citation16, 17). However, airway closure could be a proximal or distal phenomenon (Citation18); thus, the relative contribution of proximal versus distal airway reactivity cannot be determined from spirometry alone (Citation15).
Impedance oscillometry is utilized to assess airway resistance and has been proposed as a surrogate for FEV1 during MCT (Citation10). However, elevated airway resistance may be detectable by oscillometry despite normal FEV1 and oscillometry may detect frequency dependence of resistance, which is influenced by distal airway mechanics (Citation9, Citation19). Frequency dependence of resistance has been shown to correlate with frequency dependence of compliance, an accepted parameter reflecting distal airway dysfunction (Citation19). Furthermore, imaging studies have confirmed that methacholine-induced changes in frequency dependence of resistance are indicative of distal airway reactivity (airway diameter <Citation2 mm) (Citation15, Citation20).
Based on the above considerations, we hypothesize that disparity between distal and proximal airway reactivity could be demonstrated during induced bronchoconstriction by including oscillometry. In addition, we hypothesize that changes in frequency dependence of resistance could be associated with onset of methacholine-induced symptoms in subjects without significant spirometric response providing clinical relevance to the evaluation of distal airway reactivity.
METHODS AND MATERIALS
This study retrospectively analyzed data from 185 consecutive subjects who were evaluated for persistent respiratory symptoms (cough, dyspnea and/or wheeze) and normal chest radiograph. Symptoms were unexplained since FEV1/FVC was greater than 0.70 in all subjects and was above the age-adjusted lower limit of normal in all but 4 (Citation21). These subjects underwent methacholine challenge testing after simultaneous measurements of airway resistance with IOS were added to our clinical testing protocol. Spirometry was performed in accord with ATS/ERS recommendations (Citation22, 23).
Methacholine challenge testing
MCT was performed in accord with ATS recommendations.(Citation10) Increasing doses of methacholine were administered with a 2-min tidal breathing protocol up to a maximum dose of 16 mg/ml. IOS and spirometry were assessed within 2 min after administration of the methacholine. The calculated provocative concentration that resulted in a 20% fall in FEV1 (PC20) was used to determine the degree of bronchial hyperreactivity (BHR) as defined by the ATS guidelines for MCT: negative = PC20>16 mg/ml (n = 94), borderline = PC20 4–16 mg/ml (n = 19) and positive = PC20<4 mg/ml (n = 73) (Citation10). Subjects abstained from using short acting β2-agonists for 12 h, long acting β2-agonist for 48 h, anticholinergics for 24 h and inhaled corticosteroids for 14 days prior to MCT. There were no subjects treated with theophylline or leukotriene antagonists.
Impulse oscillometry
Impulse oscillometry (IOS) was measured with the Jaeger Impulse Oscillation System (Jaeger USA; Yorba Linda, CA) as previously described (Citation9, Citation19, Citation24). Respiratory resistance and reactance were calculated during 30 s of tidal breathing, while subjects supported their cheeks to minimize “shunt effect” (Citation24). IOS testing was performed pre- and post-bronchodilator in 117/185 subjects prior to the day of MCT. IOS was performed in all 185 subjects undergoing MCT at baseline and after the maximal dose of methacholine. A subset of 33 subjects underwent IOS after each methacholine dose followed by spirometry.
Fast Fourier transformation of the data obtained from the pressure impulse generated by IOS yielded data for resistance and reactance within a range of frequencies evaluated in this study (Citation25). Parameters selected included: 1) resistance at an oscillation frequency of 5Hz (R5); 2) resistance at an oscillation frequency of 20Hz (R20); 3) frequency dependence of resistance calculated as a difference between R5 and R20 (R5–20); and 4) frequency dependence of reactance as assessed by reactance area (AX).
AX was calculated as the area above the reactance curve from 5Hz to the resonant frequency. Only trials with constant tidal volume and coherence >0.85 at oscillation frequencies of 10 Hz and higher were analyzed. Interpretation of IOS parameters were based on the models of DuBois et al., Mead et al. and Otis et al. (Citation26–28). R20 was assumed to reflect resistance in proximal airways although a specific level of the tracheo-bronchial tree cannot be definitively established. Oscillation frequency dependent parameters (R5–20 and AX) were assumed to reflect heterogeneity of distal airway mechanics (Citation15, Citation29).
Data analysis
Data are presented as raw data and are compared to an upper limit of normal. Upper limits of normal for R5 (3.96 cmH2O/L/s), R20 (3.2 cmH2O/L/s), R5–20 (0.76 cmH2O/L/s) and AX (3.6 cmH2O/L) were chosen to approximate the upper limit of the 95% confidence interval (Citation9, Citation30–34). Data for change in IOS parameters in response to methacholine is presented as absolute numeric change to allow analysis of the relative contribution of changes in R20 and R5–20 to the observed change in R5. Data were summarized either as median (interquartile range [IQR]) or as mean ± standard error (SE). Differences between groups were assessed utilizing a Student's t-test, Mann-Whitney U-test or analysis of variance with post hoc pair wise testing using Tukey's HSD. Analyses were performed utilizing SPSS for Windows version 13.0. This study was approved by the Institutional Review Board of the NYU School of Medicine.
RESULTS
Baseline data
One hundred and eighty-five subjects with unexplained respiratory symptoms were referred for evaluation of bronchial hyper-reactivity. The majority of the subjects (69%) were referred from the WTC/Environmental Health Center following exposure to inhaled irritants, 13% were referred for evaluation of asthma and remaining subjects were referred for evaluation of COPD. Baseline data for the 185 subjects is illustrated in . Mean age was 45 years (range 13–76). 56% were female. Prior history of smoking was reported by 25% and only 2.7% were active smokers.
Table 1. Baseline data (n = 185)
also illustrates baseline spirometric and oscillometric parameters. FEV1/FVC was greater than 0.70 in all subjects and was above the age-adjusted lower limit of normal in all but 4. There was wide range of oscillometric parameters ranging from normal to significantly abnormal. IOS parameters were abnormal in more than 50% of subjects indicating varying degrees of distal airway dysfunction at baseline not detected by spirometry. These abnormalities in the baseline IOS did not differ based on smoking history, age or provisional diagnosis (p = ns).
Relationship of BHR to baseline IOS
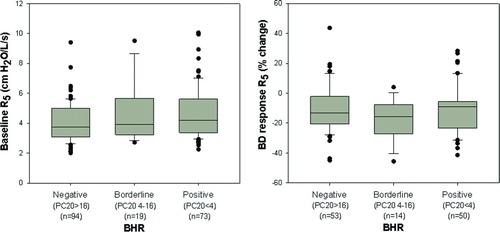
A wide range of responsiveness of FEV1 to methacholine was noted. The percent change in FEV1 varied from 1 to −63% at maximum methacholine doses ranging from 0.063 to 16 mg/ml. Patients were classified into positive, borderline and negative BHR groups based on the calculated PC20 (Citation10). examines whether degree of BHR relates to the presence of pre-existing airway abnormality as assessed by baseline IOS. The left panel illustrates baseline R5 in all subjects prior to methacholine administration; the right panel illustrates the bronchodilator response of R5 assessed on a prior day (117/185 subjects). There was no relationship between the degree of BHR to either baseline R5 or response to bronchodilator (p = ns).
Figure 1. Left panel illustrates data for baseline R5 (median and IQR) obtained in all subjects prior to methacholine administration. Right panel illustrates the bronchodilator response of R5 assessed on a prior day in 117/185 subjects. Baseline R5 and bronchodilator response did not relate to the degree of BHR (p = ns).
Relationship of BHR to change of IOS parameters during methacholine
shows the relationship between degree of BHR and changes of oscillometric parameters during MCT. Oscillometric parameters were evaluated at the maximum dose of methacholine (16 mg/ml or dose at which FEV1 decreased ≥20%) and expressed as absolute change from baseline. The top panel illustrates that the change in R5 varied widely (range −0.8 – 11.3cmH2O/L/s) with significant overlap in all 3 groups. Despite this overlap, the change in R5 was significantly larger in positive and borderline groups as compared with negative BHR group (ΔR5 = median [IQR] 2.84 [ 1 .75– 4 . 53 ], 3.22 [ 2 . 33 – 4 .67], 1.98 [ 1 .04– 3 . 36 ] cmH2O/L/s, respectively, p = 0.025). The bottom two panels illustrate similar data for R20 and R5–20. There was minimal change in proximal resistance (R20), which did not relate to degree of BHR as assessed by FEV1 (ΔR20 = 0.82 [0. 21 – 1 . 27 ], 0.60 [0. 19 – 1 . 40 ], 0.82 [0. 39 – 1 . 34 ] cmH2O/L/s, respectively p = 0.43).
Figure 2. Relationship between degree of BHR and changes in oscillometric parameters during MCT. The top panel illustrates that the change in total airway resistance as assessed by R5 was significantly larger in the positive and borderline BHR groups compared with the negative BHR group (p = 0.025). The bottom two panels illustrate minimal change in R20 during MCT while there was a larger change in distal airway mechanics as assessed by R5–20 (p<0.001 for comparison of R5–20 between positive and borderline vs. negative BHR groups).
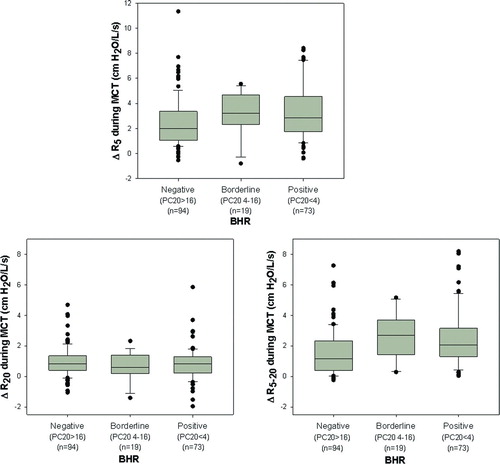
In contrast, there was a large change in the frequency dependent parameter (R5–20), which was significantly higher in positive and borderline BHR groups as compared with negative group (ΔR5–20 = 2.07 [ 1 . 29 – 3 . 15 ], 2.69 [ 1 . 43 – 3 .68], 1.17 [0. 38 – 2 . 33 ] cmH2O/L/s, respectively, p<0.001). Despite these trends, the overlap between the groups was such that a threshold value for oscillometry to assess the degree of BHR could not be identified; thus, IOS could not be used as a surrogate for FEV1 to predict the degree of BHR.
Assessment of simultaneous spirometric and oscillometric response to methacholine in individual subjects
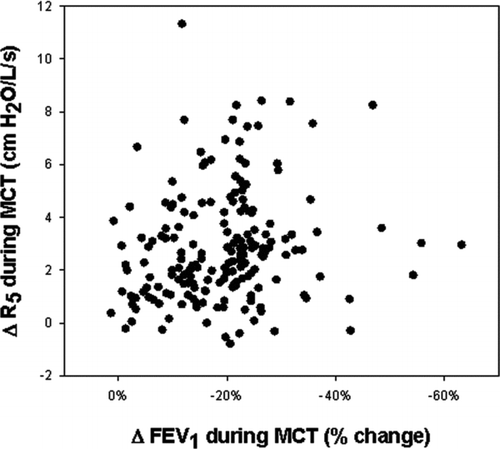
The FEV1 change in response to methacholine was examined in reference to the simultaneous change in oscillometric measurement of resistance for each subject () While many of the subjects demonstrated concordant changes in spirometric and oscillometric parameters during MCT, there was still disparity between change in FEV1 and change in R5 for the group as a whole (r = 0.17, p = 0.019). This disparity between the change in FEV1 and the change in R5 did not differ based on smoking history, age or provisional diagnosis. There were subjects with marked reduction in FEV1 during forced exhalation with minimal to no change in R5 during tidal breathing suggesting predominant dynamic airway compression. In contrast, other subjects demonstrated a wide change in R5 in presence of minimal to no change in FEV1, suggesting predominant distal airway reactivity.
Figure 3. Relationship between change in FEV1 obtained after the highest dose of methacholine with the simultaneous change in R5. Although many of the subjects demonstrated concordant changes in spirometric and oscillometric parameters during MCT, there was disparity between change in FEV1 and change in R5 (r = 0.17, p = 0.019).
Relative contribution of frequency dependence of resistance to change in R5
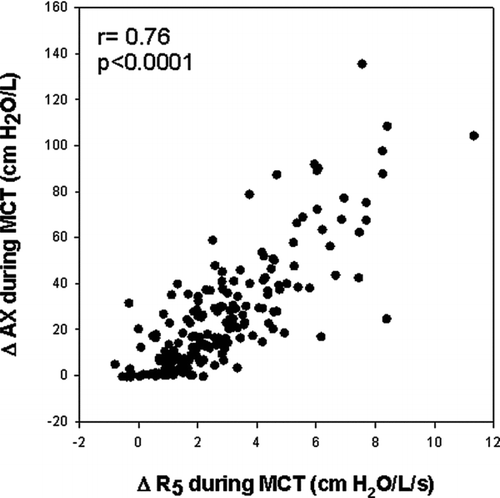
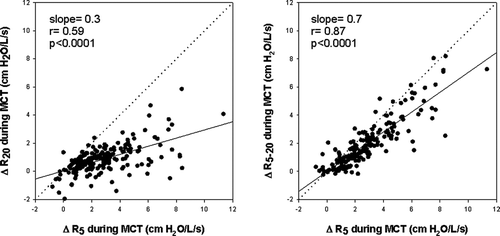
analyzes the changes in R5 during MCT with respect to changes in proximal airway resistance (R20) and development of frequency dependence of resistance (R5–20). Changes in R20 and R5–20 were significantly related to change in R5. However, analysis of the slopes indicate that the effect of methacholine on resistance at 5Hz was predominantly driven by changes in distal airway mechanics: R20 increased only 0.3 cmH2O/L/s for every 1cmH2O/L/s change in R5 while R5–20 increased 0.7cmH2O/L/s for every 1cmH2O/L/s change in R5. This conclusion is reinforced in , which shows that the change in R5 was also tightly correlated to frequency dependence of reactance as assessed by AX.
Figure 4. Relationship between the change in R5 during methacholine administration to changes in R20 and frequency dependence of resistance between 5–20 Hz. Analysis of the slopes of these relationships indicated that R20 increased only 0.3 cmH2O/L/s for every 1 cmH2O/L/s change in R5. In contrast, R5–20 increased 0.7 cmH2O/L/s for every 1 cmH2O/L/s change in R5 indicating that the effect of methacholine on resistance at 5Hz was predominantly driven by increased frequency dependence of resistance.
Relationship of symptom onset to changes in spirometry and IOS during MCT
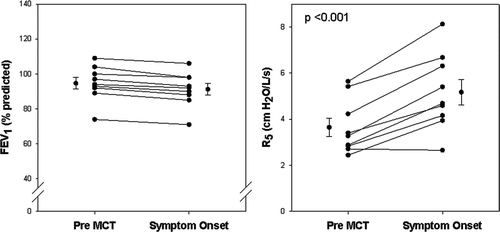
The relationship between onset of respiratory symptoms (dyspnea, wheeze, chest tightness and/or cough) to changes in expiratory flow rate and corresponding change in IOS was evaluated in the subgroup of 33 subjects in whom IOS was performed following each dose of methacholine. 9/33 subjects (27%) developed symptoms with minimal to no change in FEV1 (≤5%). This group had similar age range (35–76) and smoking prevalence (ever smoker 2/9) as compared to the entire study population. Spirometric and oscillometric data from these 9 subjects obtained at baseline and at the onset of symptoms are illustrated in . Symptoms developed at methacholine doses ranging from 0.063 to 1 mg/ml. FEV1 decreased minimally (mean change -3.4%) despite development of symptoms (left panel). In contrast, R5 increased significantly at the onset of symptoms in all but one subject (right panel). On average, R5 increased 42% from baseline. This increase in R5 was predominantly attributable to increases in R5–20 and AX (150% and 278%) rather than R20 (18%) suggesting that isolated distal airway abnormality was responsible for the onset of symptoms. Despite this distal airway reactivity, FVC decreased by only 2.7% in these subjects. Furthermore, none of these 9 subjects developed spirometric evidence of BHR despite completing MCT at a maximal dose of 16 mg/ml.
Figure 6. Relationship between onset of respiratory symptoms to changes in airflow and changes in R5. Data are illustrated for the 9/33 subjects who developed symptoms with minimal change in FEV1 (mean change = -3.4%, left panel). The mean increase in R5 was 42% from the baseline value in these subjects (right panel).
DISCUSSION
The disparity between spirometric and oscillometric responses to methacholine demonstrated in this study likely reflects disparity between proximal and distal airway behavior. Thus, oscillometry cannot be used as a surrogate for spirometry in evaluation of BHR, but may provide additional information about distal airway mechanics. This disparity between distal airway reactivity and spirometry was seen across multiple clinical phenotypes based on age, smoking status and provisional diagnosis. Moreover, distal airway reactivity was associated with methacholine-induced respiratory symptoms even in the absence of changes in airway function as assessed by spirometry, adding potential clinical relevance to the evaluation of distal airway mechanics.
Multiple studies have compared oscillometric parameters with either the “gold standard” FEV1 change or assessment of specific airway conductance by plethysmography during MCT (Citation10, Citation33, Citation35–39). ATS guidelines support use of oscillometry during MCT for subjects who cannot perform spirometry adequately (Citation10); however, a threshold to define BHR by this methodology has not been identified. Oscillometric abnormalities were manifested predominantly in frequency-dependent parameters (R5–20, AX), compatible with development of distal airway reactivity in response to methacholine. The poor correlation between spirometric and oscillometric responses observed in the present study provides support for the conclusion that oscillometry cannot be used as a surrogate for FEV1 to evaluate BHR.
There is non-uniformity of airway behavior in response to a variety of stimuli (Citation13, Citation20, Citation40–42). There is accumulating evidence that the biology and mechanics of distal airways differ from the behavior of more proximal airways. Gillis et al. used a morphometric model of the lung to demonstrate heterogeneous constriction of peripheral airways with diameter<2 mm, in response to bronchoconstriction (Citation43). Distal airway reactivity was demonstrated in human subjects following direct administration of methacholine to the distal airways through a wedged bronchoscope (Citation7). There is evidence that distal airway wall inflammation and remodeling are associated with smooth muscle shortening and peripheral constriction (Citation6, 7, Citation43–45). Moreover, heterogeneous airway closure in the lung periphery is an important component of reactivity in obstructive airway diseases (Citation20, Citation46–48).
Several of the preceding studies (Citation7, Citation41, Citation43) have demonstrated development of frequency dependence of resistance as a physiologic manifestation of the distal airway dysfunction. In accord with these observations, the present study demonstrated that the oscillometric response to methacholine was predominantly associated with changes in frequency dependence of resistance expressed as R5–20. Interpretation of R5–20 as a reflection of distal heterogeneity is further supported by its correlation to reactance as shown in the present study and to prior studies demonstrating correlation to independent measurement of frequency dependence of lung compliance (Citation19, Citation49, Citation50). These considerations provide a mechanism for disparity between spirometric and oscillometric assessments of airway resistance observed in response to methacholine in the present study.
Spirometry assesses expiratory airflow as a surrogate for resistance during forced exhalation, whereas IOS assesses airway resistance during tidal breathing. Thus, dynamic airway compression would contribute to disparity between these two tests and is relevant to those subjects with marked reduction of flow during forced expiration (FEV1) and minimal to no change of resistance during tidal breathing (R5) in response to methacholine (). Nevertheless, dynamic airway compression cannot account for disparity in subjects with change in oscillometric parameters (tidal breathing) but minimal change in FEV1 (forced expiration), thus reflecting isolated distal airway reactivity; up to 4-fold increase in R5 was seen in subjects where the FEV1 decrease was less than 20%. Moreover, distal airway reactivity can be discerned even with concomitant change in spirometry and oscillometry.
Tgavalekos et al. have shown that changes in distal heterogeneity cannot be due to large airway constriction alone (Citation15). Oppenheimer et al. (Citation19) and Galetke et al. (Citation51) have demonstrated correlation between R5–20 and frequency dependence of compliance, a marker of distal heterogeneity (Citation52). Campana et al. demonstrated that oscillometric frequency-dependent parameters reflect distal airway behavior during MCT, even in the presence of changes in spirometric parameters.(Citation53) In the present study, the change in frequency dependent parameters (R5–20 and AX) during MCT with minimal change of proximal airway resistance as assessed by R20 is in accord with these considerations. Thus, development of distal airway heterogeneity during MCT is indicative of distal airway reactivity, which may occur with or without spirometric changes supporting disparate reactivity of proximal and distal airways.
The development of symptoms during methacholine administration in association with changes in frequency dependent IOS parameters and in absence of changes in spirometric parameters reinforces the importance of the evaluation of distal airway behavior during MCT. Mansur et al. demonstrated that methacholine-induced symptoms correlated better with changes in R5 than with change in FEV1; however, all subjects had changes in FEV1 (Citation54). The present study extends this observation by relating the onset of symptoms to development of isolated changes in oscillometric parameters when FEV1 did not change. This finding indicates a role for evaluation of distal airway behavior when spirometry changes minimally in response to methacholine and extends prior observations from this laboratory demonstrating a role for IOS in evaluation of symptomatic subjects when spirometry is normal (Citation9).
In summary, the present study demonstrates that there is disparate behavior of proximal airway resistance (assessed by expiratory flow rate and R20) and distal airway heterogeneity (assessed by R5–20 and AX) during MCT. This dissociation indicates that oscillometry cannot be used as a surrogate for spirometry but can provide additional information about behavior of distal airways that is not reflected in spirometry alone. The presence of methacholine-induced symptoms in a subgroup with isolated distal airway heterogeneity (absence of spirometric and R20 change) gives further potential clinical relevance to evaluation of distal airway function during MCT. However, identification of a threshold using oscillometry to define hyperreactivity in the distal airways would require further study. This study highlights the importance of recognition of disparity between proximal and distal airway behavior, which has implications in a variety of clinical, and research settings devoted to understanding pathophysiology of obstructive pulmonary diseases and their response to treatment.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med 1968; 278(25):1355–1360.
- Cosio M, Ghezzo H, Hogg JC, Corbin R, Loveland M, Dosman J, The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med 1978; 298(23):1277–1281.
- Kraft M, Djukanovic R, Wilson S, Holgate ST, Martin RJ. Alveolar tissue inflammation in asthma. Am J Respir Crit Care Med 1996; 154(5):1505–1510.
- Kraft M, Djukanovic R, Wilson S, Holgate ST, Martin RJ. Alveolar tissue inflammation in asthma. Am J Respir Crit Care Med 1996; 154(5):1505–10.
- Haley KJ, Sunday ME, Wiggs BR, Kozakewich HP, Reilly JJ, Mentzer SJ, Inflammatory cell distribution within and along asthmatic airways. Am J Respir Crit Care Med 1998; 158(2):565–572.
- Minshall EM, Hogg JC, Hamid QA. Cytokine mRNA expression in asthma is not restricted to the large airways. J Allergy Clin Immunol 1998; 101(3):386–390.
- Kaminsky DA, Irvin CG, Lundblad L, Moriya HT, Lang S, Allen J, Oscillation mechanics of the human lung periphery in asthma. J Appl Physiol 2004; 97(5):1849–1858.
- Macklem PT, Mead J. Resistance of central and peripheral airways measured by a retrograde catheter. J Appl Physiol 1967; 22(3):395–401.
- Oppenheimer BW, Goldring RM, Herberg ME, Hofer IS, Reyfman PA, Liautaud S, Distal airway function in symptomatic subjects with normal spirometry following World Trade Center dust exposure. Chest 2007; 132(4):1275–1282.
- Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, Guidelines for methacholine and exercise challenge testing-1999. Am J Respir Crit Care Med 2000; 161(1):309–329.
- Pedley TJ, Schroter RC, Sudlow MF. The prediction of pressure drop and variation of resistance within the human bronchial airways. Respir Physiol 1970; 9(3):387–405.
- Mead J. The lung's “quiet zone”. N Engl J Med 1970; 282(23):1318–1319.
- Wagner EM, Bleecker ER, Permutt S, Liu MC. Direct assessment of small airways reactivity in human subjects. Am J Respir Crit Care Med 1998; 157(2):447–452.
- Cohen J, Douma WR, van Ooijen PM, Willems TP, Dicken V, Kuhnigk JM, Localization and quantification of regional and segmental air trapping in asthma. J Comput Assist Tomogr 2008; 32(4):562–569.
- Tgavalekos NT, Tawhai M, Harris RS, Musch G, Vidal-Melo M, Venegas JG, Identifying airways responsible for heterogeneous ventilation and mechanical dysfunction in asthma: an image functional modeling approach. J Appl Physiol 2005; 99(6):2388–2397.
- Gibbons WJ, Sharma A, Lougheed D, Macklem PT. Detection of excessive bronchoconstriction in asthma. Am J Respir Crit Care Med 1996; 153(2):582–589.
- Chapman DG, Berend N, King GG, Salome CM. Increased airway closure is a determinant of airway hyperresponsiveness. Eur Respir J 2008; 32(6):1563–1569.
- Brown RH, Mitzner W. The myth of maximal airway responsiveness in vivo. J Appl Physiol 1998; 85(6):2012–2017.
- Oppenheimer BW, Goldring RM, Berger KI. Distal airway function assessed by oscillometry at varying respiratory rate: comparison with dynamic compliance. COPD 2009; 6(3):162–170.
- Bayat S, Strengell S, Porra L, Janosi TZ, Petak F, Suhonen H, Methacholine and ovalbumin challenges assessed by forced oscillations and synchrotron lung imaging. Am J Respir Crit Care Med 2009; 180(4):296–303.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159(1):179–187.
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Interpretative strategies for lung function tests. Eur Respir J 2005; 26(5):948–968.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Standardisation of spirometry. Eur Respir J 2005; 26(2):319–338.
- Oostveen E, MacLeod D, Lorino H, Farre R, Hantos Z, Desager K, The forced oscillation technique in clinical practice: Methodology, recommendations and future developments. Eur Respir J 2003; 22(6):1026–1041.
- Smith HJ, Reinhold P, Goldman MD. Forced oscillation technique and impulse oscillometry. Eur Respir Mon 2005 (31):72–105.
- Mead J. Contribution of compliance of airways to frequency-dependent behavior of lungs. J Appl Physiol 1969; 26(5):670–673.
- Dubois AB, Brody AW, Lewis DH, Burgess BF, Jr. Oscillation mechanics of lungs and chest in man. J Appl Physiol 1956; 8(6):587–594.
- Otis AB, McKerrow CB, Bartlett RA, Mead J, McIlroy MB, Selver-Stone NJ, Mechanical factors in distribution of pulmonary ventilation. J Appl Physiol 1956;8(4):427–443.
- Bates JH, Lutchen KR. The interface between measurement and modeling of peripheral lung mechanics. Respir Physiol Neurobiol 2005; 148(1–2):153–164.
- Goldman MD, Saadeh C, Ross D. Clinical applications of forced oscillation to assess peripheral airway function. Respir Physiol Neurobiol 2005; 148(1–2):179–194.
- Niven AS, Backenson TJ, Goldman MD, Hnatiuk OW, Weisman IM. Resistance measured by forced oscillation (IOS) is mouthpiece dependent and reduced in normal subjects using a free flow mouthpiece. Am J Respir Crit Care Med 2003; 167:A419.
- Skloot G, Goldman M, Fischler D, Goldman C, Schechter C, Levin S, Respiratory symptoms and physiologic assessment of ironworkers at the World Trade Center disaster site. Chest 2004; 125(4):1248–1255.
- Kohlhaufl M, Brand P, Scheuch G, Schulz H, Haussinger K, Heyder J. Impulse oscillometry in healthy nonsmokers and asymptomatic smokers: effects of bronchial challenge with methacholine. J Aerosol Med 2001; 14(1):1–12.
- Shiota S, Katoh M, Fujii M, Aoki S, Matsuoka R, Fukuchi Y, Predictive equations and the reliability of the impulse oscillatory system in Japanese adult subjects. Respirology 2005; 10(3):310–315.
- Chinet T, Pelle G, Macquin-Mavier I, Lorino H, Harf A. Comparison of the dose-response curves obtained by forced oscillation and plethysmography during carbachol inhalation. Euro Respir J J Euro Soc Clin Respir Physiol 1988; 1(7):600–605.
- Vink GR, Arets HG, Van Der Laag J, Van Der Ent CK. Impulse oscillometry: A measure for airway obstruction. Pediatr Pulmonol 2003; 35(3):214–219.
- Kopriva F, Szotkowska J, Plocova A, Zavodska J, Zapalka M, Smatanova D, The use of oscillometry as a measure of airway responsiveness in asthmatic children after histamine and methacholine bronchoprovocation with dosimeter-MedicAid and DeVilbiss nebulizers. J Asthma 2007; 44(4):267–271.
- van Noord JA, Clement J, van de Woestijne KP, Demedts M. Total respiratory resistance and reactance as a measurement of response to bronchial challenge with histamine. Am Rev Respir Dis 1989; 139(4):921–926.
- Descatha A, Fromageot C, Ameille J, Lejaille M, Falaize L, Louis A, Is forced oscillation technique useful in the diagnosis of occupational asthma? J Occup Environ Med 2005; 47(8):847–853.
- Ingram RH, Jr., McFadden ER, Jr. Localization and mechanisms of airway responses. N Engl J Med 1977; 297(11):596–600.
- Pimmel RL, Fullton JM, Ginsberg JF, Hazucha MJ, Haak ED, McDonnell WF, Correlation of airway resistance with forced random noise resistance parameters. J Appl Physiol 1981; 51(1):33–39.
- Gelb AF, Flynn Taylor C, Krishnan A, Fraser C, Shinar CM, Schein MJ, Central and peripheral airway sites of nitric oxide gas exchange in COPD. Chest 2010; 137(3):575–584.
- Gillis HL, Lutchen KR. Airway remodeling in asthma amplifies heterogeneities in smooth muscle shortening causing hyperresponsiveness. J Appl Physiol 1999; 86(6):2001–2012.
- Macklem PT. The physiology of small airways. Am J Respir Crit Care Med 1998; 157(5 Pt 2):S181–183.
- Tulic MK, Hamid Q. The role of the distal lung in asthma. Semin Respir Crit Care Med 2002; 23(4):347–359.
- Gillis HL, Lutchen KR. Airway remodeling in asthma amplifies heterogeneities in smooth muscle shortening causing hyperresponsiveness. J Appl Physiol (Bethesda, Md : 1985) 1999; 86(6):2001–2012.
- Thorpe CW, Bates JH. Effect of stochastic heterogeneity on lung impedance during acute bronchoconstriction:Aa model analysis. J Appl Physiol 1997; 82(5):1616–1625.
- Lutchen KR, Gillis H. Relationship between heterogeneous changes in airway morphometry and lung resistance and elastance. J Appl Physiol 1997; 83(4):1192–1201.
- Kjeldgaard JM, Hyde RW, Speers DM, Reichert WW. Frequency dependence of total respiratory resistance in early airway disease. Am Rev Respir Dis 1976; 114(3):501–508.
- Van Den Elshout FJ, van Herwaarden CL, Folgering HT. Oscillatory respiratory impedance and lung tissue compliance. Respir Med 1994; 88(5):343–347.
- Galetke W, Randerath WJ, Feier C, Muth T, Borsch-Galetke E. Esophageal pressure method and impulse oscillometry to assess mechanical properties of the respiratory system in healthy men. Med Sci Monit 2009; 15(8):CR429–435.
- Woolcock AJ, Vincent NJ, Macklem PT. Frequency dependence of compliance as a test for obstruction in the small airways. J Clin Invest 1969; 48(6):1097–1106.
- Campana L, Kenyon J, Zhalehdoust-Sani S, Tzeng YS, Sun Y, Albert M, Probing airway conditions governing ventilation defects in asthma via hyperpolarized MRI image functional modeling. J Appl Physiol 2009; 106(4):1293–1300. PMCID: 2698646.
- Mansur AH, Manney S, Ayres JG. Methacholine-induced asthma symptoms correlate with impulse oscillometry but not spirometry. Respir Med 2008; 102(1):42–49