Abstract
Triple therapy for COPD consists of a long-acting anti-cholinergic bronchodilator, a long-acting beta-agonist bronchodilator, and an inhaled corticosteroid. Guidelines from the Canadian Thoracic Society advocate triple therapy for some patients with moderate-to-severe COPD. The objective of this review was to evaluate the evidence based clinical efficacy of triple therapy compared to dual bronchodilator therapy (long-acting anti-cholinergic bronchodilator + beta-agonist bronchodilator) or long-acting anti-cholinergic bronchodilator monotherapy for managing COPD. A systematic literature search was conducted to identify relevant clinical evaluations of triple therapy in the management of moderate to severe COPD. Databases searched included: Medline; EMBASE; CINAHL and PubMed (non-Medline records only). Of 2,314 publications, 4 articles evaluated triple therapy for the management of COPD. Hospitalization rates for COPD exacerbations, reported in 2 trials, were significantly reduced with triple therapy compared to long-acting anti-cholinergic bronchodilator monotherapy, with reported relative risks of 0.53 (95% CI: 0.33, 0.86, p = 0.01) and 0.35 (95% CI: 0.16–0.78, p = 0.011). Exacerbation data is inconsistent between the two trials reporting this outcome. Lung function, dyspnea and quality of life data show statistical significant changes with triple therapy compared to long-acting anti-cholinergic bronchodilator monotherapy but the changes do not reach clinical importance. Triple therapy does decrease the number of hospitalizations for severe/acute COPD exacerbations compared with long-acting anti-cholinergic bronchodilator monotherapy. There is insufficient evidence to determine if triple therapy is superior to dual bronchodilator therapy.
BACKGROUND
Chronic obstructive pulmonary disease (COPD) is a respiratory disorder largely caused by smoking characterized by progressively partially reversible airway obstruction and hyperinflation, systemic manifestations and increasing frequency and severity of exacerbations (1). Airway inflammation in COPD is predominantly neutrophilic rather than eosinophilic as seen in asthma which is responsive to corticosteroids (Citation2). COPD is predicted to be the third leading cause of death worldwide by 2020 (Citation3).
The reported COPD prevalence varies worldwide, with estimates from Canada varying from 7.3% (Citation4) to 17.4%; 8.9% from Columbia, (Citation5) Turkey 6.9%, (Citation6) Southeastern Kentucky 19.6%, (Citation7) and Rotterdam 11.6%. (Citation8) COPD prevalence is reported to be higher in men, smokers, and older persons. (Citation4–9) The prevalence reported in a recent population-based study targeting smokers who underwent diagnostic spirometry was 20.7%. (Citation10) The large BOLD international cohort study (Citation11) (general population ≥ 40 years old) was designed to determine the worldwide prevalence reported estimates from 12 countries by gender. The estimates for COPD in men varied from 8.5% to 15.4% and for women the estimates varied from 3.7% to 16.7%.
COPD exacerbations contribute considerably to the morbidity and mortality of the disease. Patients with frequent exacerbations have worse health-related quality of life (QoL) and larger healthcare expenditures than those without frequent exacerbations (Citation12). A recent economic review (Citation13) of COPD reported the cost, in U.S. dollars, of treating a moderate exacerbation, which varied from $248 to $1,011, and the cost for treating a severe exacerbation varied from $4,172 to $9,060. Belgium/Netherlands had the highest moderate exacerbation costs (Citation14) and Canada had the highest severe exacerbation costs (Citation15).
Because COPD is not curable, the goal of treatment is to slow disease progression and to control symptoms. Deterioration of lung function over time usually requires reassessment and more aggressive recourse of both non-pharmacological (e.g., diet, smoking cessation, exercise, pulmonary rehabilitation) and pharmacological therapy (e.g., vaccinations) including introduction of more inhaled treatments in an attempt to better manage symptoms.
Three classes of inhaled drugs could be prescribed for the treatment of moderate to severe COPD: anticholinergic bronchodilators, both short-acting (SAAC) and long-acting (LAAC); beta-agonist bronchodilators, both short-acting (SAAB) and long-acting (LABA), and inhaled corticosteroids (ICS). Both classes of bronchodilators produce smooth muscle relaxation with bronchodilation, but have different mechanisms of action, therefore enabling them to be used in combination (Citation16). A triple therapy drug regimen for the management of COPD consists of a LAAC and a combination inhaler containing both LABA and ICS, and a dual therapy regimen consists of a LAAC + LABA. Monotherapy with a LAAC has been the foundation therapy for the management of COPD (Citation17). All maintenance therapy regimens for moderate to severe COPD also require short-acting reliever medication.
The American Thoracic Society (ATS)/European Respiratory Society (ERS) (Citation18) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) (Citation19) conceptaluse the severity of COPD related to FEV1. Although they currently do not specifically recommend triple therapy for the management of COPD in their current iterations they promote stepwise addition of therapy as patients progress towards moderate and severe COPD. Both documents recommend one or more long-acting bronchodilators for treatment of moderate to severe COPD and adding an ICS for severe to very severe COPD. However, the guidelines from the Canadian Thoracic Society (CTS) currently recommend use of triple therapy for management of COPD patients under two clinical scenarios. (Citation20) Scenario one includes patients with moderate to severe COPD (based upon GOLD classification (Citation19) and infrequent acute disease exacerbations (less than one per year, on average, for two consecutive years) and who have persistent disability despite the use of a LAAC plus a LABA. Scenario two includes patients with moderate to severe COPD who have persistent symptoms and a history of exacerbations (one or more per year, on average, for two consecutive years).
There is widespread use of triple therapy for COPD even in primary care where patients have predominantly mild disease and occasional bronchitis. For a publically funded health care system the inappropriate use of triple therapy has major financial consequences. A full health technology assessment was undertaken to determine the clinical- and cost-effectiveness of triple therapy to assist with reimbursement decisions. Reported here are the results of the systematic review of the current literature to determine the clinical efficacy of triple therapy compared with LAAC + LABA therapy or LAAC therapy alone for the management of moderate to severe COPD.
MATERIALS AND METHODS
Literature searches
The following bibliographic databases were systematically searched: Medline; EMBASE; CINAHL; PubMed (for non-Medline records only) and Thomson Reuters’ ISI Web of Science, and BIOSIS Previews. The Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Methodology Register, and HTA Database were also searched (via Wiley's Cochrane Library).
Both controlled vocabulary terms and keywords were used, including terms for “Chronic Obstructive Pulmonary Disease” or “chronic bronchitis” or “emphysema”. The terms for the drugs were separated into concepts of triple therapy, dual therapy, and monotherapy. See Appendix 1 for the detailed search strategy.
Selection criteria and methods
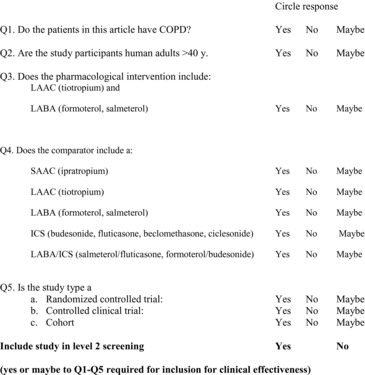
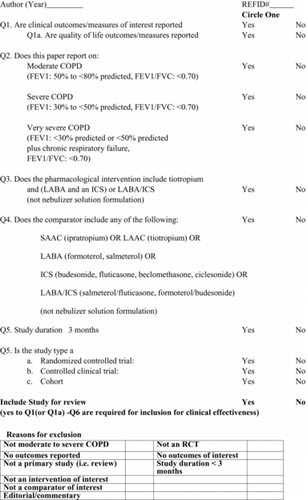
Studies utilizing a Randomized Controlled Trial (RCT), controlled clinical trial, or cohort design; enrolling patients with moderate to severe COPD; and evaluating triple therapy compared to LAAC plus LABA, LABA/ICS or LAAC alone were included. Two reviewers (KG, NA) independently screened the titles and abstracts and then the full text of relevant articles using predefined checklists (Appendices 2 and 3). Kappa statistics for inter-rater agreement were calculated and discrepancies between reviewers were discussed until consensus was reached.
Data from all included studies were extracted using a predefined data extraction form (Appendix 4). The outcomes available for extraction were: the number of exacerbations, number of hospitalizations, number of deaths, FEV1 measures (defined as the forced expiratory volume in the first second of expiration), adverse events, dyspnea, and quality of life (QoL). The St. George's Respiratory Questionnaire (SGRQ) (Citation21) was used to measure changes in the patients QoL. This is a respiratory disease specific QoL measurement tool consisting of 76 questions split into three domains: symptoms; activity; and impacts. Each domain is scored separately in the range of zero to 100.
Each domain score has been shown to correlate with measures of disease activity (e.g., FEV1, wheezing, dyspnea etc). (Citation21) A total SGRQ score is computed by applying an empirically derived weight (Citation22) to each question which provides an estimate of the distress associated with the symptom or state in the question. The question scores are then summed to provide the total SGRQ score which also ranges from zero to 100. Dyspnea was measured using three different instruments: the Morning Activities and Symptom Questionnaire's (MASQ) global chest symptom domain, the Transitional Dyspnea Index and a visual analogue scale. The methodological quality of all included studies was assessed using the Jadad scale (Citation23) and the Schultz treatment allocation concealment questionnaire (Citation24) (Appendix 5).
Data analysis
When two or more comparable studies (similar population characteristics, interventions, outcome measures) were identified, a meta-analysis was conducted to generate a pooled estimate of effects. Using Review Manager software (The Cochrane Collaboration 2008, Copenhagen), a random-effects model was used to derive the pooled estimates and the associated 95% confidence intervals (CI). Risk ratios (RR) were used to summarize estimates of effect for dichotomous outcomes, while mean differences were used for continuous data.
RESULTS
Literature selection
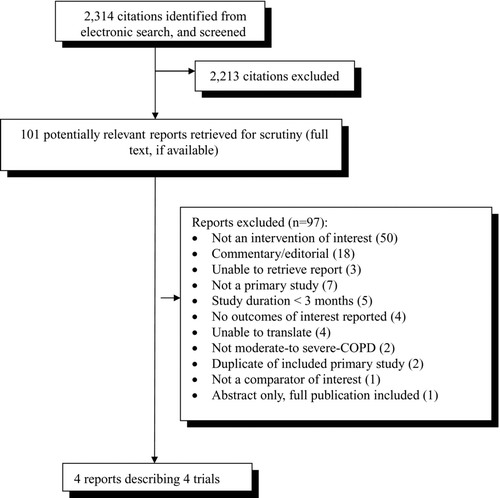
Four studies from the 2,314 potentially relevant citations identified were included in the review (see ). The agreement between the two reviewers for title and abstract screening was good, with a Kappa score of 0.84. The agreement for the full text screening was also good, with a Kappa score of 0.74. A list of the excluded studies with reasons for exclusion is displayed in Appendix 6.
Study characteristics
displays the study and population characteristics, as well as, the interventions evaluated in the trials and the primary outcome. All 4 trials excluded patients with a history of asthma or other respiratory diseases other than COPD. Three trials (Citation25–27) utilized a two-week run-in period, where patients were not allowed to use any inhaled medications except for their rescue medication, (Citation25, Citation26) or used only tiotropium plus a rescue medication (Citation27) prior to randomization. Two of these three trials excluded patients if they had an exacerbation requiring hospitalization, or oral/inhaled steroids, or antibiotics during the run-in period. (Citation26, Citation27) Cazzola et al. (Citation26) also excluded patients from the analysis if they experienced an exacerbation during the intervention period. The fourth trial (Citation28) randomized all eligible patients after informed consent was obtained. If a patient had experienced a COPD exacerbation requiring oral or intravenous antibiotics or steroids within the previous 28 days, they were required to wait 28 days after stopping the antibiotic/steroid before entering the study.
Table 1. Study characteristics
TRIPLE THERAPY COMPARED WITH LAAC MONOTHERAPY
Hospitalizations
Both Aaron et al. (Citation28) and Welte et al. (Citation27) report hospitalizations due to exacerbations. Aaron et al. (Citation28) reported the number of events but Welte et al. (Citation27) reported the outcome as a rate; therefore, no pooling of data was possible. Aaron et al. (Citation28) reports an incident RR of 0.53 (95% CI: 0.33, 0.86, p = 0.01) for the triple therapy group compared to LAAC alone and Welte et al. (Citation27) reports a RR of 0.35 (95% CI: 0.16–0.78, p = 0.011) for triple therapy versus LAAC alone. Aaron et al. (Citation28) also reports significantly lower all cause hospitalization rates for patients using triple therapy compared to LAAC alone with a RR of 0.67 (95% CI:0.45, 0.99).
Data were pooled for the comparison of triple therapy versus LAAC monotherapy for the following outcomes: exacerbations, FEV1, SGRQ, mortality, and adverse events and the results are reported next.
Exacerbations
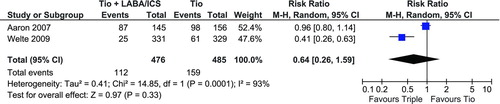
Welte et al. (Citation27) reported 25 (7.6%) patients experienced at least one severe exacerbation while on triple therapy and Aaron et al. (Citation28) reported 87 (60.0%) patients experienced an acute exacerbation. The number of exacerbations reported for tiotropium therapy alone in these trials was higher, 61 (18.5%) and 98 (62.8%), respectively. As seen in pooling the exacerbation events resulted in a non-significant RR of 0.64 (95% CI: 0.26, 1.59). This result does have to be interpreted with caution because of the significant heterogeneity between these two studies (I2 = 93%).
Lung function
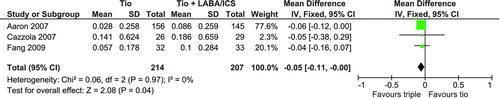
Lung function was measured with spirometry and reported as a FEV1 measure. Three of the four trials reporting a FEV1 outcome were used for a pooled analysis. (Citation25;Citation26;Citation28) displays the pooled analysis which resulted in a statistically significant mean difference of 0.05L (95% CI: 0.00, 0.11) favouring triple therapy.
Quality of life
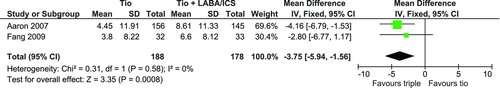
Three trials (Citation25,Citation27,Citation28) used the St. George's Respiratory Questionnaire (SGRQ) (Citation21) to measure changes in the patients QoL. Data reported in Aaron et al. (Citation28) and Fang et al. (Citation25) could be used for a pooled analysis but Welte et al. (Citation27) did not report the standard deviation of the changes in their report and therefore was not included. As seen in , there was a significant mean difference of 3.75 (95% CI: 1.56, 5.94) in the SGRQ score favoring triple therapy.
Dyspnea
Welte et al. (Citation27) reported a statistically significantly large improvement in the patients’ breathlessness as measured with the MASQ global chest symptom domain for the triple therapy group compared to the tiotropium alone group (p<0.001). Aaron et al. (Citation28) used the Transitional Dyspnea Index to measure dyspnea while Cazzola et al. (Citation26) used a visual analogue scale. Neither of these two studies reported a statistically significant difference in dyspnea measures between triple therapy and monotherapy.
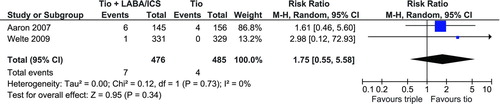
Mortality
Both Aaron et al. (Citation28) and Welte et al. (Citation27) reported the number of deaths that occurred during the study. displays the pooled estimate for mortality with a non-significant RR of 1.75 (95% CI: 0.55, 5.58).
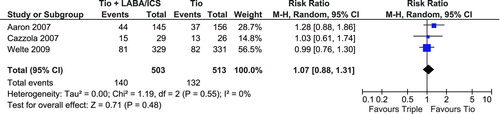
Adverse Events
All trials were of relatively short duration or had small sample sizes and therefore there is limited adverse event data available. Reported adverse events associated with triple therapy include: respiratory failure leading to mechanical ventilation or death, pneumonia leading to mechanical ventilation or death, cancer, myocardial infarction, acute arrhythmia, dry or sore mouth, oral candidiasis, tremor, nasopharyngitis, bronchitis, hypertension, upper respiratory tract infection, dysphonia/aphonia, palpitations, cough, dyspnea, pleuritic pain. Only 3 of the 4 trials reported data that could be used in a pooled analysis (Citation26–28) and displays the pooled estimate for any adverse event. The resulting non-significant RR is 1.07 (95% CI: 0.88, 1.31). Of note the Aaron et al. (Citation28) trial used a high dose ICS (500 μg) and reported a large but non-significant increase in the risk of an adverse event, compared to the Cazzola et al. (Citation26) trial which used a low dose ICS (250 μg).
Triple therapy compared with dual bronchodilator therapy (LAAC plus LABA)
There were no comparisons of triple therapy with LAAC + LABA in any of the four trials reviewed. Therefore, we were unable to compare the incremental effect of triple therapy compared with dual bronchodilator therapy.
Limitations of the included studies
The Aaron et al. (Citation28) trial was a well designed study of 12 months duration with a moderate sample size. There were high withdrawal from therapy rates with 26% of triple therapy users and 47% of LAAC alone users withdrawing from therapy. Fang et al. (Citation25) also utilized a 12 month study duration but with a very small sample size. Patients in the fourth arm of this trial did not utilize any inhaled medications but the authors do not report what medications were used by these patients.
Therefore, the significant differences reported throughout the Feng et al. study may actually be from a comparison of suboptimal treatment (no inhaled medications) with optimal treatment (three inhaled medications). Welte et al. (Citation27) study was well designed with a large sample size but the study duration was too short, making it difficult to compare the trial results (exacerbations, QoL) with those from the other studies that are of longer duration. Also, this trial excluded any patient experiencing an exacerbation after they discontinued all their inhaled medications (except for tiotropium) prior to randomization. Patients that have suffered from frequent exacerbations in the past have a high probability of suffering from frequent exacerbations in the future (Citation29). Excluding this group may have artificially lowered the exacerbation rate of the trial. The fourth trial (Cazzola et al. (Citation26)) was a pilot study with a small sample size and short duration.
DISCUSSION
The evidence for the clinical efficacy of triple therapy from published RCT's in patients with moderate-to-severe COPD is limited compared to its application in clinical practice. Although triple therapy has been recommended by the CTS for certain patient populations with COPD, as defined earlier, more evidence is needed to determine its clinical efficacy when compared with current management strategies.
To determine the incremental benefit of triple therapy the appropriate comparison should have been LAAC + LABA therapy or even LABA/ICS therapy not LAAC monotherapy which was the comparator in 3 of the 4 identified RCTs. The guidelines for the management of COPD recommend a step-up treatment regimen as COPD worsens (Citation18–20) except for COPD patients who experience frequent severe exacerbations. Therefore, the majority of patients would be using two inhaled medications before becoming triple therapy users and currently we do not have RCT data that supports the widespread adoption of triple therapy.
The current evidence does show that triple therapy reduces the hospitalization rate for severe/acute exacerbations compared to LAAC monotherapy. Hospitalization is a major component of the cost of COPD (Citation13) and depending upon country varied from 58% to 90% of the overall costs. One study reported that the average length of stay for patients with a severe COPD exacerbation was 10 days, which accounted for 73% of the total cost (Citation15). A Swedish study (Citation30) reported that exacerbations accounted for 30–45% of the total health care costs of COPD and that 67% of the cost is due to hospitalizations. The total cost of exacerbations in patients with severe COPD was 45 times greater than the costs for patients with mild COPD.
Lung function and dyspnea data show statistically significant changes with triple therapy compared to LAAC monotherapy and the changes came close to the minimally important difference for the SGRQ (reduction of score by ≥ 4 points) (Citation31). These results are similar to the large 4-year UPLIFT study (Citation32). This study randomized participants to either LAAC or placebo and allowed all other forms of COPD medications (excluding another anti-cholinergic) to be taken concurrently. Forty-six percent of the participants were using a LABA/ICS combination inhaler. The change in FEV1 and SGRQ scores were statistically significant favouring LAAC but the changes did not reach the minimally important difference.
Exacerbation data is inconsistent between the two trials (Citation27, 28) reporting this outcome because this outcome was not consistently defined and measured. This could be one reason for the significant heterogeneity across these studies (I2 of 93%). Other possible sources of this heterogeneity could be: length of follow-up (52 weeks versus 12 weeks); differences in the baseline FEV1 measure required for inclusion (< 65% predicted versus < 50% predicted); use of a run-in period prior to randomization where patients were excluded from randomization if they experienced an exacerbation during this time; and differing components of triple therapy (fluticasone/salmeterol versus formoterol/budesonide).
The trials also had different therapy discontinuation rates. In Aaron et al. (Citation28) we see a differential withdrawal rate with 26% of triple therapy users and 47% of LAAC monotherapy users withdrawing from therapy; whereas, in Welte et al. (Citation27) only 8% and 9% of the sample withdrew from therapy in the triple therapy and the LAAC monotherapy treatment arms, respectively. The lower withdrawal rates seen in the Welte et al. (Citation27) study may be due to the short duration of the trial.
The significantly lower RR for exacerbations reported by Welte et al. (Citation27) should be interpreted with caution because of the short duration of the study. The ATS/ERS jointly created a task force to examine outcomes for COPD pharmacological trials (Citation33). Based upon the rate of disease progression and frequency of exacerbations the ATS/ERS recommend that trials to support treatment claims should be at least 6 months in duration. ATS/ERS further recommend that due to seasonal variation, exacerbation frequency should be evaluated over a period of at least 1 year.
The UPLIFT (Citation32) study also reported a 14% reduction in the mean number of exacerbations with LAAC therapy (p<0.001). This outcome also has to be interpreted with caution because the reduction in the mean number of exacerbations is not reported by treatment regiments (triple, LAAC + LABA, LAAC + ICS). Also the exacerbations in the Welte et al. (Citation27) were not verified, as they were in the Aaron et al. (Citation28) trial. Since COPD is a risk factor for pneumonia (Citation34–36) and the presenting symptoms of pneumonia and a COPD exacerbation may overlap, this may lead to an inflated exacerbation rate.
Therefore, it is crucial to remember that the findings reported here cannot be generalized to all patients with moderate-to-severe COPD. Three of the 4 trials reviewed included patients with moderate to severe COPD but the results are not reported by severity. Also no trials included patients with FEV1 measures between 70% and 80% of predicted. Both moderate and severe groups are at risk of exacerbations and should be considered for escalation of therapy. As the disease progresses and symptoms become more frequent and debilitating, pharmacotherapy will need to be increased. Triple therapy is effective in managing COPD, and therefore should be considered when patients continue to have recurrent exacerbations while compliant with foundation therapy.
CONCLUSIONS
Triple therapy does decrease the number of hospitalizations for severe/acute COPD exacerbations compared with LAAC monotherapy. There is insufficient evidence to determine if triple therapy is superior to the LAAC + LABA treatment regimen.
ACKNOWLEDGMENTS
This work was funded by the Canadian Agency for Drugs and Technologies in Health, Ottawa, Ontario.
Declaration of interest
Kathryn Gaebel, Gord Blackhouse, Diana Robertson, Nazila Assasi, Feng Xie and Ron Goeree have no conflicts of interests. Andrew McIvor has received honoraria for providing education and attending pharmaceutical advisory boards for AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKlein, Pfizer, Merck and Novartis. Paul Hernandez has participated on medical advisory boards, received travel funds to attend educational events, conducted continuing health education activities and/or industry-sponsored clinical research trials for the following companies: Abbott, Actelion, Altana, AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Merck Frosst, Novartis, Nycomed, Paringenix, Pfizer, Shering, and CSL Behring.
REFERENCES
- Todd DC, McIvor RA, Pugsley SO, Cox G. Approach to chronic obstructive pulmonary disease in primary care. Can Fam Physician 2008 May; 54(5):706–711.
- McIvor RA. Future options for disease intervention: Important advances in phosphodiesterase 4 inhibitors. Eur Respir Rev 2007; 16(105):105–112.
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997 May 24; 349(9064):1498–1504.
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, Menezes AM, Sullivan SD, Lee TA, Weiss KB, Jensen RL, Marks GB, Gulsvik A, Nizankowska-Mogilnicka E, BOLD Collaborative Research Group. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007; 370(9589):741–750.
- Caballero A, Torres-Duque CA, Jaramillo C, Bolivar F, Sanabria F, Osorio P, Orduz C, Guevara DP, Maldonado D. Prevalence of COPD in five Colombian cities situated at low, medium, and high altitude (PREPOCOL study). Chest 2008;133(2):343–9.
- Gunen H, Hacievliyagil SS, Yetkin O, Gulbas G, Mutlu LC, Pehlivan E. Prevalence of COPD: first epidemiological study of a large region in Turkey. Eur J Intern Med 2008; 19(7):499–504.
- Methvin JN, Mannino DM, Casey BR. COPD prevalence in southeastern Kentucky: the burden of lung disease study. Chest 2009; 135(1):102–107.
- van Durme YM, Verhamme KM, Stijnen T, van Rooij FJ, Van Pottelberge GR, Hofman A, Joos GF, Stricker BH, Brusselle GG. Prevalence, incidence, and lifetime risk for the development of COPD in the elderly: the Rotterdam study. Chest 2009; 135(2):368–377.
- Dales RE, Aaron SD, Vandemheen KL, Mehdizadeh A, Clinch J. The prevalence of airflow obstruction in rural primary care. Respir Med 2006; 100(4):754–759.
- Hill K, Goldstein RS, Guyatt GH, Blouin M, Tan WC, Davis LL, Heels-Ansdell DM, Erak M, Bragaglia PJ, Tamari IE, Hodder R, Stanbrook MB. Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. CMAJ 2010 April 20; 182(7):673–678.
- Buist AS, Vollmer WM, Sullivan SD, Weiss KB, Lee TA, Menezes AM, Crapo RO, Jensen RL, Burney PG. The Burden of Obstructive Lung Disease Initiative (BOLD): Rationale and design. COPD 2005 June; 2(2):277–283.
- Decramer M, Nici L, Nardini S, Reardon J, Rochester CL, Sanguinetti CM, Troosters T. Targeting the COPD exacerbation. Respir Med 2008 June; 102 Suppl 1:S3–15.
- Toy EL, Gallagher KF, Stanley EL, Swensen AR, Duh MS. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD 2010 June; 7(3):214–228.
- Oostenbrink JB, Rutten-Van Molken MPMH. Resource use and risk factors in high-cost exacerbations of COPD. Respir Med 2004; 98(9):883–891.
- Mittmann N, Kuramoto L, Seung SJ, Haddon JM, Bradley-Kennedy C, Fitzgerald JM. The cost of moderate and severe COPD exacerbations to the Canadian healthcare system. Respir Med 2008 March; 102(3):413–421.
- O'Donnell DE, Aaron S, Bourbeau J, Hernandez P, Marciniuk D, Balter M, Ford G, Gervais A, Goldstein R, Hodder R, Maltais F, Road J, McKay V, Schenkel J, Ariel A, Day A, Lacasse Y, Levy R, Lien D, Miller J, Rocker G, Sinuff T, Stewart P, Voduc N, Abboud R, Ariel A, Becklake M, Borycki E, Brooks D, Bryan S, Calcutt L, Chapman K, Choudry N, Couet A, Coyle S, Craig A, Crawford I, Dean M, Grossman R, Haffner J, Heyland D, Hogg D, Holroyde M, Kaplan A, Kayser J, Lein D, Lowry J, McDonald L, MacFarlane A, McIvor A, Rea J, Reid D, Rouleau M, Samis L, Sin D, Vandemheen K, Wedzicha JA, Weiss K. State of the art compendium: Canadian Thoracic Society recommendations for the management of chronic obstructive pulmonary disease. Can Respir J 2004 July; 11 Suppl B:7–59B.
- McIvor RA. Tiotropium and chronic obstructive pulmonary disease. BMJ 2010; 340:c833.
- American Thoracic Society, European Thoracic Society. American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med 2003; 168(7):818–900.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Bethesda (MD): National Heart, Lung and Blood Institute; 2008.
- O'Donnell DE, Aaron S, Bourbeau J, Hernandez P, Marciniuk DD, Balter M, Ford G, Gervais A, Goldstein R, Hodder R, Kaplan A, Keenan S, Lacasse Y, Maltais F, Road J, Rocker G, Sin D, Sinuff T, Voduc N. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease – 2007 update. Can Respir J 2007; 14(Suppl B):5–32B.
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992 June; 145(6):1321–1327.
- Quirk FH, Jones PW. Patients’ perception of distress due to symptoms and effects of asthma on daily living and an investigation of possible influential factors. Clin Sci (Lond) 1990 July; 79(1):17–21.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996 February;17(1):1–12.
- Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. Lancet 2002 February 16; 359(9306):614–618.
- Fang LZ, Liang X, Zhang JQ, Liu L, Fu WP, Zhao ZH, Dai LM. Combination of inhaled salmeterol/fluticasone and tiotropium in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial [in Chinese]. Zhonghua Jie He He Hu Xi Za Zhi 2008; 31(11):811–814.
- Cazzola M, Ando F, Santus P, Ruggeri P, Di Marco F, Sanduzzi A, D'Amato M. A pilot study to assess the effects of combining fluticasone propionate/salmeterol and tiotropium on the airflow obstruction of patients with severe-to-very severe COPD. Pulm Pharmacol Ther 2007; 20(5):556–61.
- Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T, Kessler R. Efficacy and tolerability of budesonide/formoterol added to tiotropium in COPD patients. Am J Respir Crit Care Med 2009 August 13; 180:741–750.
- Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, Balter M, O'Donnell D, McIvor A, Sharma S, Bishop G, Anthony J, Cowie R, Field S, Hirsch A, Hernandez P, Rivington R, Road J, Hoffstein V, Hodder R, Marciniuk D, McCormack D, Fox G, Cox G, Prins HB, Ford G, Bleskie D, Doucette S, Mayers I, Chapman K, Zamel N, FitzGerald M. Tiotropium in combination with placebo, salmeterol, or fluticasone- salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2007; 146(8):545–555.
- Donaldson GC, Seemungal TA, Patel IS, Lloyd-Owen SJ, Wilkinson TM, Wedzicha JA. Longitudinal changes in the nature, severity and frequency of COPD exacerbations. Eur Respir J 2003 December; 22(6):931–936.
- Andersson F, Borg S, Jansson S-A, Jonsson A-C, Ericsson A, Prutz C, Ronmark E, Lundback B. The costs of exacerbations in chronic obstructive pulmonary disease (COPD). Respir Med 2002; 96(9):700–708.
- Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J 2002 March; 19(3):398–404.
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008 October 9; 359(15):1543–1554.
- Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, Brusasco V, Burge PS, Calverley PM, Celli BR, Jones PW, Mahler DA, Make B, Miravitlles M, Page CP, Palange P, Parr D, Pistolesi M, Rennard SI, Rutten-van Molken MP, Stockley R, Sullivan SD, Wedzicha JA, Wouters EF. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J 2008 February; 31(2):416–469.
- LaCroix AZ, Lipson S, Miles TP, White L. Prospective study of pneumonia hospitalizations and mortality of U.S. older people: the role of chronic conditions, health behaviors, and nutritional status. Public Health Rep 1989 July; 104(4):350–360.
- Mannino DM, Davis KJ, Kiri VA. Chronic obstructive pulmonary disease and hospitalizations for pneumonia in a US cohort. Respir Med 2009 February; 103(2):224–229.
- O'Meara ES, White M, Siscovick DS, Lyles MF, Kuller LH. Hospitalization for pneumonia in the Cardiovascular Health Study: incidence, mortality, and influence on longer-term survival. J Am Geriatr Soc 2005 July; 53(7):1108–16.