Abstract
Cigarette smoking causes airflow limitation with lung hyperinflation being the primary causes of COPD. Fifty chronic smokers (CSs) with no signs of GOLD-adjusted COPD with smoking habit at least ≥10 pack-years (p/yrs) were divided into CS-mild (n = 24) with smoking history from ≥10 to ≤20 p/yrs and CS-heavy groups (n = 26) with smoking history ≥21 p/yrs. Spirometry, plethysmography and diffusing capacity were measured and lung computed tomography (CT) was performed. Residual volume (RV) (L) and RV/TLC (total lung capacity) ratio were significantly increased in CS-heavy when compared to CS-mild (p = 0.001, p = 0.03). A significant reduction of forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratio and airway specific conductance was shown in CS-heavy (p = 0.02, p = 0.03). Lung emphysema signs at CTs were revealed in 17 CSs and ten of them had declined diffusing capacity below 70% of predicted. The percentage of emphysematous lesions inversely and significantly correlated with measured diffusing capacity (p = 0.0009, r = −0.72). Study groups’ smoking intensity inversely correlated the declined airway specific conductance (p = 0.004, r = −0.39) and increase of the RV (L) (p = 0.0004, r = 0.46). Multiple regression analysis determined that smoking intensity regardless of the subjects’ age was significant factor for decline of airway specific conductance and increase of RV (L). Here we conclude that lung function deviation and lung structural changes are present in CSs before the clinical signs of airway obstruction reveal. Body plethysmography and diffusing capacity measurement with routine spirometry can provide valuable information for detection of changes reflecting to the early onset of COPD in CSs.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is progressive and debilitating chronic lung disease with worldwide growing prevalence. Cigarette smoking is a principal risk factor in the development COPD (Citation1). COPD is to a great extent under diagnosed and diagnosis delay causes often situations where the disease at the time of diagnosing is already advanced (Citation2–4).
Tobacco smoking can lead to decline of lung function by the fourth to fifth decade of life. Smoking causes airway inflammation resulting in airflow limitation and lung hyperinflation (Citation5–7). Increased residual capacity (RV) together with increased RV/TLC (total lung capacity ratio) followed by the increase of TLC are the characteristic signs of air trapping and lung hyperinflation that can occur in the chronic smokers` lung (Citation8, 9). Spirometry is the standard tool in diagnosing COPD. According to the Global Initiative for Obstructive Lung Disease (GOLD, 2009) agreement, diagnoses of COPD can be based on the reduction of forced expiratory volume in 1 second (FEV1) and the ratio FEV1/FVC (forced vital capacity) below 70% (Citation1).
This diagnostic approach is valid only in the more advanced COPD leaving under-diagnosed the early onset of lung functions' decline in chronic smokers. Other parameters measured by spirometry like maximal expiratory flow at 50%, 25% and 75% of FVC (MEF50, MEF25, MEF75, accordingly) have also received less attention in evaluating early COPD in chronic smokers. Clinically available technologies, e.g., body plethysmography, allow measuring static lung volumes instead of dynamic which gives more precise information of early lung functional disability in chronic smokers. Residual volume (RV), total lung capacity (TLC), forced residual capacity (FRV), airway specific resistance and conductance (Citation10, 11) are the measures that offer broader view of lung function (Citation11,12).
Although there is no routine recommendation in the current guidelines how to detect the subjects’ susceptibility towards the deleterious effects of smoking on lung function, it is definitely worth to find out at the earliest perspective, as smoking cessation is still the only proven tool to stop lung function decline and as several studies have shown especially in the early quitters (Citation13–15).
The aim of this study was to analyze dynamic and static lung function parameters in chronic symptomatic smokers with no GOLD guideline based signs of COPD to show the relationship between smoking history, early lung function decline and onset of COPD. Smokers were divided into two groups according to the severity of the smoking habit; mild chronic smokers with smoking history from ≥ 10 to ≤ 20 pack-years (p/yrs) (CS)-mild and, CS-heavy with smoking history ≥21 p/yrs. We show here that lung function deviation and structural changes in the lung are present in CSs although GOLD guideline adopted COPD signs are not met.
MATERIAL AND METHODS
Study groups
Fifty subjects with self-reported smoking experience at least 10 pack-years (p/yrs) who showed FEV1 > 80% of predicted and the FEV1/FVC ratio >70% were randomly recruited into the study from the subjects pointed for consultancies by the family doctors due to chronic cough to the North Estonia Medical Centre Pulmonology Department. None of the subjects had diagnosed COPD according to the GOLD guideline (Citation1). Subjects over 70 years old were excluded from study, together with subjects with asthma, other etiologies of chronic cough or chronic airway diseases in subjects’ medical history. Collected patients with complaint to chronic cough were divided into 2 study groups according to subject's smoking history and intensity. Twenty-four mild chronic smokers (CS-mild, n = 24) with smoking history from ≥10 to ≤ 20 p/yrs and 26 heavy smokers (CS-heavy, n = 26) with smoking history of ≥21 p/yrs. Subject's characteristics are presented in .
Table 1. Subjects’ demographics.
Pulmonary function tests
Dynamic lung function tests
Forced expiratory flow measurments (FEV1, FVC, vital capacity (VC) and maximal expiratory flow at 50% of forced vital capacity (MEF50) were performed with Jaeger spirograph (Masterscreen Pneumo “Jaeger” version Citation4.Citation6, Cardinal Health, www.cardinal.com) according to ATS/ERS recommendations (Citation16). Predicted normal values by the European Respiratory Society equations were used (Citation17). Readings were performed in triplicate, with the highest FEV1, FVC, VC and MEF50 used in further analysis.
Static lung function volume measurements
Static lung volumes ((functional residual capacity (FRC), residual volume (RV), total lung capacity (TLC), ratio RV/TLC)) and airway resistance (Raw) with airway specific conductance (sGaw) were measured in a constant volume plethysmograph (Masterscreen Body „Jaeger” version 4.6, Cardinal Health, http://www.cardinal.com) according to ATS/ERS recommendations (Citation18, 19). A minimum of two attempts with a reproducible FRC within 5% was made for each patient and the highest measurements were used. Lung volume reference values were taken from the European Respiratory Society (Citation17). Air trapping was defined as increase of RV/TLC ratio >40% and presence of lung hyperinflation was defined as RV/TLC ratio >0.4 and RV >140% of predicted (Citation20, 21, Citation22).
Diffusing capacity measurement
The diffusing capacity measurements were performed by Jaeger Masterscreen Diffusion (The Masterscreen Diffusion “Jaeger” version 4.6, Cardinal Health, www.cardinal.com) using Single Breath (SB) method. The Single Breath method determines the transfer factor for carbon monoxide (DLCOSB). Degree of severity of decrease in DLCOSB based on ATS/ERS recommendation (Citation16). Subjects remained seated at rest for 5 min before the DLCOSB measurement began. The subject was seated in upright position wearing a nose clip. After 3–4 tidal breathing the subject asked to perform maximal expiration and then requested to rapidly inhale a mixture of gas. The inspiratory gas contained a mixture of 0.26% carbon monoxide with 9.4% helium in synthetic air (Cardinal Health). The subject was instructed to perform the breath holding maneuver for 10 sec and then rapid exhalation. The expired gas was collected and DLCOSB was calculated.There was a 4-min break between the trials. All lung function tests were performed by highly trained and experienced pulmonary function technicans.
Lung Computed Tomography (CT)
A low-dose CT chest scans were performed in supine position at suspended full inspiration with or without administration of intravenous contrast by use of General Electric 16 multi-slice scanner with 0.625 or 1.25 mm contiguous slice thickness. Exposure settings were 120 kV peak and Auto mA with minimum value of 80 mA and maximum 250 mA. Images were reconstructed in the low spatial frequency or soft reconstruction algorithms according to manufactures recommendations (General Electric Company). Images were reconstructed and archived with a 512 × 512 matrix.
All of the CT scans were evaluated at the Department of Radiology in the North Estonia Medical Centre by senior radiologists. Quantitative assessment of lung volumes and the percentage of lung CT voxels below the threshold of −950 Hounsfield Units (as a representative value of the presence of lung emphysema) were performed by use of the Thoracic VCAR software (General Electric Company) and advanced workstation AW 4.5 (General Electric Company).
Statistical analysis
Spearman's rank correlation analysis was used to relate the subject's smoking history (smoking pack-years), age (years), spirometry, whole body plethysmography and diffusing capacity results with the percentage of emphysematous lesions on CT scans. Linear and multiple regression modeling was performed to evaluate the relationship between lung function values and other independent variables including smoking history (smoking pack-years) and chronic smokers’ age. The non-parametric Mann-Whitney U-test was used to analyze data for individual variables from the study groups. Confidence intervals were determined using the Astute software package. A p-value of < 0.05 is considered significant.
RESULTS
Study group's demography
Study groups demographic profiles are presented in . Subjects in both groups were in the middle age, however, subjects in the CS-heavy (a mean age 56.3 yrs with age range 36–69 yrs) were older than subjects in the CS-mild (a mean age 48.9 yrs with age range 34–65 yrs in CS-mild, p = 0.003). Smokers in both groups were overweight (a BMI 26.7 (range 20.8–34.9) in CS-heavy and a BMI 25.7 (range 20–33.2) in CS-mild, respectively).
Dynamic and static lung volume measurements
The results of dynamic and static lung function measurements are presented in . FEV1 (L) and FEV1 (% of predicted) values did not differ when compared to the CS-heavy (FEV1(L) 95% CI 2.97–3.66 and FEV1% of predicted 95% CI 90.12–111.00) to CS-mild (FEV1(L) 95% CI 2.94–3.75 and FEV1% of predicted 95% CI 95, 27–107.87).
Table 2. Lung function parameters
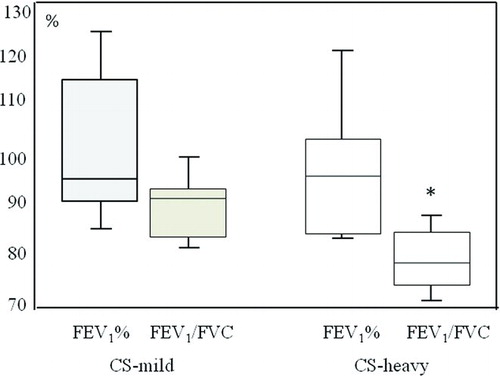
FEV1/FVC ratio was significantly lower (p = 0.02) in CS-heavy (mean 79.92; 95% CI 77.46 – 82.36) when compared to CS-mild (mean 84.65, 95% CI 81.84–87.45) (). A mean MEF50 values expressed as % of predicted were lower in CS-heavy (95% CI 64.96– 85.86 as compared to CS-mild (95% CI 70.76–96.0, p = 0.2).
Figure 1. FEV1% of predicted and FEV1/FVC ratio in CS-mild and CS-heavy. P< 0.05 compared to CS-mild.
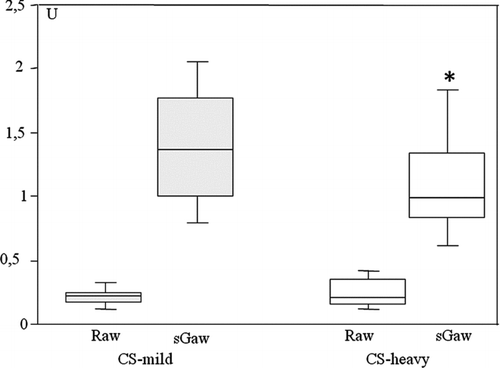
A significant decline of airway specific conductance (sGaw; p = 0.03) was found in CS-heavy (a mean 1.10, 95% CI 0.92 – 1.27 in CS-heavy and a mean 1.39, 95% CI 1.17–1.61 in CS-mild, ). A mean RV/TLC ratio was significantly higher (p = 0.03) in CS-heavy (a mean 41.63%, 95% CI 38.57–44.68) as compared to CS-mild (a mean 37.57%, 95% CI 34.16–40.96 in CS-mild). RV/TLC ratio > 40% as a sign of air trapping was revealed in 14 subjects in CS-heavy (53%) and in 7 subjects in CS-mild (29%). Presence of lung hyperinflation (RV/TLC ratio >40% and RV >140% of predicted) was shown in 7 subjects in CS-heavy and in 3 CS-mild subjects. RV (L) was significantly (p = 0.001) increased in CS-heavy (a mean 3.03 L 95% CI 2.77 – 3.27 in CS-heavy) as compared to CS-mild (a mean 2.48 L, 95% CI 2.20–2.75 in CS-mild). RV and FRC (% of predicted) values were higher but not statistically different in CS-heavy when compared to CS-mild. Inspiratory capacity (IC (L) and% of predicted) values did not differ between two studied group.
Pulmonary diffusing capacity
Lung diffusion capacity (DLCOSB) presented as a percentage of predicted value was not significantly different between two studied groups (95% CI 73.63–84.21 in CS-heavy and 95% CI 77.44–88.13 in CS-mild). Decline of DLCOSB below 70% of predicted value was shown in 7 cases in the CS-heavy and 3 cases in the CS-mild group. Signs of lung emphysema at CT-scans were revealed in all CSs who had DLCOSB below 70% of predicted (n = 10).
Correlations between smoking intensity (in pack-years) and lung function decline
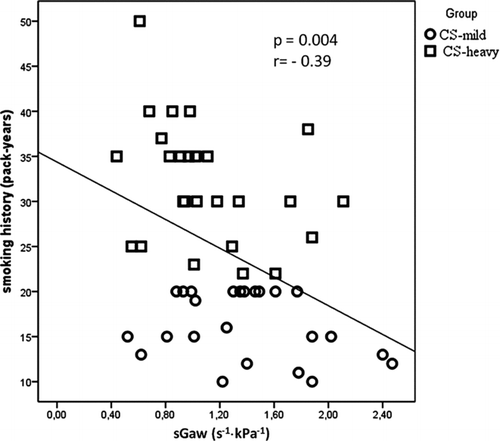
Whole study group smoking intensity showed an inverse significant correlation with the FEV1/FVC ratio (p = 0.01; r = – 0.33), and with the decline of airway specific conductance (p = 0.004, r = – 0.39, ). Importantly, smoking history correlated significantly with the increase of the RV (L) (p = 0.0004, r = 0.46) and the RV/TLC ratio (p = 0.03, r = 0.29, respectively).
Relationship between lung function values, smoking history and subjects’ age evaluated by the linear and multiple regression analysis
To determine smoking intensity (pack-years) related lung function limitation we used linear and multiple regression analysis where smoking history (pack-years) and subject age were independent parameters. Results of linear and multiple regression analysis are summarized in . Linear and multiple regression analysis showed that smoking history regardless of the chronic smokers’ age was a significant factor in declining the airway specific conductance (p = 0.0003, p = 0.02, respectively) and increasing RV (L) (p = 0.002 and p = 0.01, respectively). To date, smoking history together with chronic smokers’ age was significant predictor for FEV1 (L), FVC (L) and TLC (L) change.
Table 3. Relationships between lung function values, smoking history and age in smokers
Emphysematous lesions on CT scans
Signs of lung emphysema were evaluated on CT-scans and confirmed by radiologists. Centrilobular, paraseptal and mixed type of lung emphysematous lesions were diagnosed. Emphysematous lesions were found in 17 subjects (11/26 in CS-heavy and 6/24 in CS-mild, respectively). Centrilobular emphysematous changes in upper part of lungs were presented in 3 subjects in CS-heavy and 4 subjects in CS-mild. Mixed type of emphysema was diagnosed in 6 cases in CS-heavy and one case in CS-mild. Moreover, paraseptal emphysematous changes were diagnosed in 2 subjects in CS-heavy and one subject in CS-mild. The percentage of lung emphysematous lesion and the decrease of DLCOSB showed significant and inverse correlation (p = 0.0009, r = −0.72). The data of emphysema lesion on CT scans (the threshold of -950 Hounsfield Units) are summarized in .
Table 4. Characteristics of emphysematous lesions in chronic smokers
DISCUSSION
The primary findings in this study showed that lung malfunction and structural changes can be present in chronic smokers who did not fulfill the diagnostic criteria of COPD (Citation1). In CT scans, remarkable structural changes were found in the study group smokers’ lung with no GOLD guideline-directed signs of COPD. Chronic smokers in current study groups were in middle age with complaints to chronic cough. Although COPD was the primarily suspected disease for all these subjects, according to the GOLD guideline (Citation1) no COPD could not be diagnosed in these patients. Nevertheless, the 4th to 5th decade of life is critical age for smokers to development of airway obstruction due to accelerated decline in lung function (Citation13–15).
Spirometry is ubiquitous clinical diagnostic routine applied to evaluate lung function. Although the heavy smokers’ patient group was older than mild smokers group, the routine spirometry measures showed significantly lower FEV1/FVC ratio in CS-heavy than in the CS-mild group. Association between subjects’ age, airway trapping (RV/TLC ratio) and resistance (Raw) was shown which indicates the overall influence of aging on airway function limitation (). However, no relationships were found between smokers’ age and diffusing capacity (DLCOSB).
Ten chronic smokers from the study group had signs of emphysema on CT-scans with decrease of diffusing capacity below 70% of predicted. On the other hand, seven chronic smokers with emphysematous lesions on CT showed normal diffusing capacity values. Also Parr et al. (Citation23) have shown that emphysema often presents before the FEV1 declines below normal values. No relationship between smokers’ age residual volume (RV) and airway specific conductance (sGaw) measures was found. Evidently in chronic smokers at 4th to 5th decade of their life both either smoking-directed or age-associated processes determining lung mal-functionality and possible start–up of chronic obstructive disease.
Previous studies have shown that smoking can increase lung RV and FRC (Citation9). Fifty three percent of heavy smokers and 29% of mild showed the presence of air trapping (RV/TLC) in the bronchi measured by body plethysmography in current study, whereas RV/TLC was significantly higher in CS-heavy than in CS-mild subjects the smokers' condition of bronchi developed air trapping in both smokers' group. This shows in one hand the hidden potential of cigarette smoking to early onset of chronic airway obstruction also in modest smoking intensity. Long-term smoking in the other hand can cause lung hyperinflation with the decline of airway conductance and latter, increase of airway resistance (Citation10, Citation12) with the air trapping that all can develop in the smoking affected bronchial environment.
Presence of lung hyperinflation (RV/TLC >40% and RV>140% of predicted) was more prevailed in the CS-heavy than in CS-mild group. Lung hyperinflation is indicating an increase of functional residual capacity or also of residual volume and total lung capacity (Citation24). In patients with chronic airway obstruction the lung hyperinflation may result either from loss of lung elastic recoil (static hyperinflation) or decreased expiratory flow (dynamic hyperinflation). Although narrowing of airway is the common abnormal sign found always in patients with COPD, the early sign of chronic smoking affection is associated with the lung hyperinflation (Citation8).
Lung volumes, but no airflow measures, correlate sufficiently with the early impairment of smokers’ functional capability. Therefore lung volume measures can be utilized as predicting values for lung functionality. Also, understanding the mechanisms how hyperinflation occurs in COPD lung can provide better insight to future therapy approaches of patients. Static hyperinflation is caused by a decrease in elasticity of the lung due to emphysema. The ability to fully exhale depends on the degree of airflow limitation and the time available for exhalation. These can both vary, causing greater hyperinflation during exacerbations or increased respiratory demand, such as during exercise. The primary cause of airway obstruction, regardless of etiology, is increased airway resistance (Raw). Raw is the reciprocal process to airway conductance (Gaw). Previous studies of Borrill et al. (Citation12) showed lower airway conductance values in chronic smokers than in non-smokers.
Our current study showed that a significant decline of airway specific conductance occur in smokers who have been smoking more than 20 pack-years, however, with no significant increase of airway resistance. We also found a significant association between subjects smoking history (in pack-years), decline of airway specific conductance and increased RV, which strongly indicates to the airway pathophysiology changes with decline of lung function that develops in chronic smokers far earlier than COPD can usually diagnose according to current guideline values (Citation1).
It is critical to enlarge the variable options to measure airway obstruction and evaluated smokers status concerning COPD. Unfortunately, the parameters of airway resistance/conductance are not well understood and accordingly, underutilized in the clinical practice nowadays. Body plethysmography measurements instead of routine spirometry alone are more reliable to detect early pathophysiological changes in CSs. Spirometry does not always give sufficient information to diagnose respiratory obstruction because a fixed FEV1/FVC ratio of 0.7 often misses the airway obstruction in younger CSs (Citation25, 26).
Moreover, plethysmography remains as “the gold standard” to measure airway resistance and conductance (Citation10). Plethysmography measures are performed in the tidal breathing, which is the largest practical advantage of the method. There are, however, some additional needs in the clinical practice; plethysmography measures require experienced medical stuff, also are measurements time-consuming (Citation10) which restricts the broader use of this technology. Nevertheless, our current study results showed that by use of plethysmography is possible to select early-phase COPD patients from chronic smoker's cohort. This advantage of the technology definitely overcomes tentative difficulties.
Unexpectedly, some subjects in both groups were overweight with BMI >24.9. According to previous studies being overweight could be a protective factor towards the development of COPD (Citation27, 28). However, a negative consequence of being overweight increases the risk of cardiovascular diseases and metabolic syndrome development (Citation29, 30). Recent studies have shown that the obesity is a remarkable problem in COPD patients (Citation31, 32).
In conclusion, current results highlighted that FEV1 and FEV1/FVC ratio are not sensitive markers to detect early changes in lung pathophysiology or onset of COPD in chronic cigarette smokers. Lung function deviation and lung structural changes are present in CSs before the clinical signs of airway obstruction reveal. In addition to clinical routine spirometry, the body plethysmography and diffusing capacity measures are strongly recommended to apply upon smokers with even minor complaints related to smoking-associated diseases especially COPD. As a preventive approach, all CSs should be consulted for smoking cessation which to date, is the only intervention influencing upon smokers lung health.
ACKNOWLEDGMENTS
We thank Professor Thomas Fehniger and Aleksei Baburin for his kind expert assistance.
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease Available from www.goldcopd.com (2009 update).
- Celli BR. Update on the management of COPD. Chest 2008; 133;1451–1462.
- Mannino DM, Buist AS. Global burden of COPD: Risk factors, prevalence, and future trends. Lancet 2007; 370:765–773.
- Soriano JB, Zielinski J, Price D. Screening for and early detection of chronic obstructive pulmonary disease. Lancet 2009; 374:721–732.
- Lee G, Walser TC, Dubinett SM. Chronic inflammation, chronic obstructive pulmonary disease, and lung cancer. Curr Opin Pulm Med 2009; 15:303–307.
- Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev 2007; 87:1047–1082.
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Paré PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004; 350:2645– 2653.
- Stanescu D. Small airways obstruction syndrome. Chest 1999; 116: 231–233.
- O'Donnell DE, Laveneziana P. Lung hyperinflation in COPD: The impact of pharmacotherapy Eur Respir Rev 2006; 15:85–89.
- Blonshine S, Goldman MD. Optimizing performance of respiratory airflow resistance measurements. Chest 2008; 134:1304–1309.
- Clayton N. Review series: Lung function made easy. Assessing lung size. Chronic Respiratory Disease 2007; 4:151–157.
- Borill ZL, Roy K, Vessey RS, Woodcock AA, Singh D. Non-invasive biomarkers and pulmonary function test in smokers. Inter J COPD 2008; 3:171–183.
- Kohansal R, Martinez-Camblor P, Agustí A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited, An analysis of the Framingham offspring cohort. Am J Respir Crit Care Med 2009; 180:3–10.
- Gold PM. The 2007 GOLD Guidelines: A comprehensive care framework. Respir Care 2009; 54:1040–1049.
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ 1977; 1:1645–1648.
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, Van Der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J 2005; 26:948–968.
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party, Standardization of Lung Function Tests“. European Coal and Steel Community. Eur Respir J 1993; 6 (Suppl. 16):5–40.
- Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, Van Der Grinten CPM, Gustafsson P, Hankinson J, Jensen R, Johnson D, MacIntyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes. Series “ATS/ERS Task force: Standardization of lung function testing” Edited by V. Brusasco, R. Crapo and G. Viegi. Eur Respir J 2005; 26:511–522.
- Goldman MD, Smith HJ, Ulmer WT. Whole-body plethysmography. Eur Respir Mon 2005; 31:15–43.
- Albuquerque ALP, Nery LE, Villaça DS, Machado TYS, Oliveira CC, Paes ÂT, Neder JA. Inspiratory fraction and exercise impairment in COPD patients GOLD stages II–III. Eur Respir J 2006; 28:939–944.
- Madama VC. Pulmonary Function Testing and Cardiopulmonary Stress Testing. Second Edition, New York: Delmar Publisher Team; 1998:100–105.
- Irvin CG. Guide to the evaluation of pulmonary function. In. Hamid Q, Joanne Shannon J, Martin J. Physiologic Basis of Pulmonary Disease. Hamilton: BC Decker Inc.; 2005:649–658.
- Parr DG, Stoel BC, Stolk J, Stockley RA. Pattern of emphysema distribution in alpha1-antitrypsin deficiency influences lung function impairment. Am J Respir Crit Care Med 2004; 170:1172–1178.
- Brusasco V. Fitting JW. Lung hyperinflation in airway obstruction. Eur Respir J 1996; 9:2440.
- Miller MR, Quanjer PH, Swanney MP, Ruppel G, Enright PL. Interpreting lung function data using 80 percent of predicted and fixed thresholds misclassifies over 20% of patients. Chest. 2010 [Epub ahead of print]
- Hansen JE, Sun XG, Wasserman K. Spirometric criteria for airway obstruction: Use percentage of FEV1/FVC ratio below the fifth percentile, not < 70%. Chest. 2007; 131:349–355.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructivepulmonary disease. N Engl J Med 2004; 350:1005–1012.
- Vestbo J, Prescott E, Almdal T, Dahl M, Nordestgaard BG, Andersen T, Sørensen TI, Lange P. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: Findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med 2006; 173:79–83.
- Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, Kosiborod M, Portnay EL, Sokol SI, Bader F, Krumholz HM. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med 2005; 165:55–61.
- Poulain M, Doucet M, Drapeau V, Fournier G, Tremblay A, Poirier P, Maltais F. Metabolic and inflammatory profile in obese patients with chronic obstructive pulmonary disease. Chron Respir Dis 2008; 5:35–41.
- ten Hacken NH. Physical inactivity and obesity: relation to asthma and chronic obstructive pulmonary disease? Am Thorac Soc 2009; 6:663–667.
- Ora J, Laveneziana P, Ofir D, Deesomchok A, Webb KA, O'Donnell DE. Combined Effects of Obesity and COPD on Dyspnea and Exercise Tolerance Amer J Respir Crit Care Med 2009; 180:964–971.