Abstract
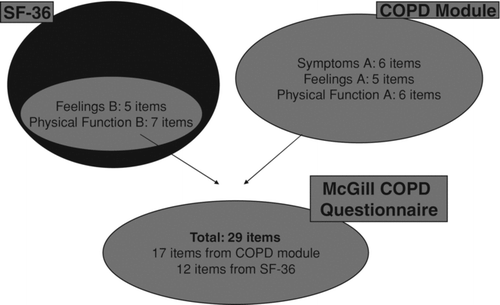
Presently, a generic and a disease-specific questionnaire are often co-administered to capture the different domains of quality of life in chronic obstructive pulmonary disease (COPD) subjects. A health-related-quality of life (HRQL) questionnaire in COPD combining both generic and disease-specific properties is needed. Objective: To develop a new, hybrid-HRQL questionnaire, the McGill-COPD-questionnaire, with qualities of both generic and disease-specific instruments. Using pre-defined criteria, we selected items from the SF-36 to complement the items from a COPD-specific-module to create the new hybrid-HRQL-questionnaire. Domains were identified via confirmatory factor analysis. The McGill COPD questionnaire is available in English and French; it assesses three domains: symptoms, physical-function and feelings, has 29 items: 17 from the COPD-specific-module and 12 from the SF-36. The symptom sub-scale has 6 items, all from the COPD-specific-module; the feelings sub-scale has 10 items, 5 each from COPD-specific-module and SF-36 and the physical-function sub-scale has 13 items, 6 from COPD-specific-module and 7 from SF-36. The McGill COPD questionnaire was developed using a novel method of combining items from the SF-36 and a COPD-specific-module. Thus, this new questionnaire has items from a generic-questionnaire and a disease-specific-module and, hence, is promising to be a stand alone quality-of-life questionnaire for COPD subjects.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a slowly progressive disease and a major cause of chronic morbidity and mortality throughout the world (1). Stratification of disease severity is usually done with the help of post-bronchodilator FEV1 (forced expiratory volume at 1 second). Although FEV1 measurement is necessary for diagnostic purposes and for disease follow-up, FEV1 correlates poorly with symptom intensity, exercise capacity and HRQL in COPD (Citation2–4). Thus, evaluation within the domains of impairment, activity limitation and participation restriction (5) captured by HRQL is essential for characterization of COPD. Moreover, physicians rarely rely on FEV1 thresholds to make therapeutic decisions. It is very well accepted and recommended in guidelines that treatment effectiveness should be based on assessment of patient-perceived outcomes rather than on spirometry alone (Citation6). Therefore, measurement of HRQL is important, both in clinical practice and research.
Patients with COPD often have co-morbid conditions, and may also suffer from adverse effects of treatment. Hence, it is important to assess not only the respiratory consequences of the disease but also the associated extra-pulmonary manifestations. Because they assess different aspects of the disease, generic and disease-specific questionnaires often have to be administered together (Citation7). Although generic questionnaires allow cross-condition comparison, one of their limitations is that they may be insensitive in detecting small changes related to a specific disease (Citation8). Alternatively, the disease-specific questionnaires focus on relevant aspects of the disease and can, detect small changes in the subject's condition. Today, no instrument incorporates both generic and disease specific contents for use with COPD patients. The administration of two separate questionnaires is time consuming, cumbersome, expensive and can increase non-participation.
The most commonly used generic quality of life questionnaire is the Medical Outcomes Study Short Form-36 (SF-36). It has been translated into more than 80 languages, takes 10 minutes to complete, and results can be easily compared with existing population norms (Citation9–11). Disease specific quality of life questionnaires commonly used in COPD are the Chronic Respiratory Questionnaire (CRQ) (Citation12) and the St. George's Respiratory Questionnaire (SGRQ)(Citation13), though there are many others available (Citation14). The CRQ is an individualized, labor-intensive instrument requiring 20–30 minutes of staff time for each administration. The SGRQ is the most commonly used instrument and has 76 items. However, the majority (80%) of the questions in SGRQ have dichotomous (yes/no) responses and, hence, the instrument may have problems with sensitivity to change (Citation15). Importantly, the 2-point scale is less reliable, less interesting and more ambiguous to respondents (Citation16–18) than scales with more categories.
The ideal questionnaire for COPD should have the strengths of generic and disease-specific questionnaires to capture the extent of COPD manifestations, while being sensitive to change. Our objective was to develop a hybrid questionnaire combining items from a generic questionnaire, the SF-36, and a respiratory disease specific module developed earlier by our group (Citation19). We selected items from the SF-36-version 2 because of its brevity, relevance and availability in many languages and cultures (permitting its utilization in international trials). The resulting tool will have the ability to detect changes in quality of life related to COPD, while retaining the ability to compare this population with other patient populations and healthy subjects. This new questionnaire will also be of practical value, reducing the time required to evaluate health related quality of life.
METHODS
Overview
This study used data collected as part of a large, pulmonary rehabilitation cohort study in COPD patients from 4 hospitals in the province of Québec, Canada with a 3-year longitudinal follow-up. The project was approved by each institutional research ethics board and patients signed a written consent. Of the 246 subjects in the cohort, 142 had completed the COPD-specific module. Eligibility criteria were: 1) clinical diagnosis of COPD with incompletely reversible airflow obstruction (post-bronchodilator FEV1/forced vital capacity (FVC) <70%); 2) stable disease state (i.e., no prior exacerbation of their disease in the preceding month); 3) no asthma as a primary diagnosis; 4) no clinically significant co-morbidities such as heart failure, dementia or unstable psychological conditions; 5) no participation in a pulmonary rehabilitation program in the past 12 months. Subjects were required to have completed the baseline evaluation (before rehabilitation) and at least one evaluation after rehabilitation (immediately after and/or within 2 months).
Our team had previously developed a COPD-specific module19 to be used in conjunction with the SF-36. The module development consisted of three phases: 1) content development and scaling; 2) item selection and; 3) preliminary testing of the module's psychometric properties. The content development process selected items from multiple sources of information, including COPD subjects and their significant others, as well as experts in the fields of COPD and quality of life research who participated in item generation for the module.
The COPD-specific module was developed in two languages, English and French. It was divided into three sub-scales: Symptoms, Physical Function and Feelings. The COPD-specific module was originally comprised of 17 items. From the initial factor analysis previously carried out on the COPD module items alone, the item “fatigue” did not group with any of the other items and had a very low extraction factor (low variance in all the variables accounted for by the item “fatigue”). From 17 items, the module was further reduced to 16 items. Previously testing the psychometric properties of this COPD-specific measure used a cross-sectional data base of COPD subjects (Citation19). The present work is a continuation of this project to develop a new quality of life questionnaire using the COPD module and the SF-36. The McGill COPD questionnaire is designed to be similar to the SF-36 in that higher scores indicate better health-related quality of life.
Item selection of the McGill COPD questionnaire
Item selection of the McGill COPD questionnaire consisted of combining items of the COPD module and the SF-36. The COPD module consisted of the previously selected 16 items and the addition of the item “fatigue”, i.e., 17 items. The item “fatigue” was reintroduced as it is well recognized to be important in COPD as the disease progresses (Citation20–22); this item merits further evaluation.
To select items from the SF-36, we established pre-defined criteria and applied them as follows: The first 2 questions of the SF-36 (self-assessed general health question was too general and the health transition question was not appropriate) were excluded.
A correlation matrix with the remaining items from the SF-36 and all the items from the COPD-specific module was then created. Items from the SF-36 with a correlation greater than 0.6 with any item in the module were excluded, to avoid redundancy.
As the aim was to create a responsive instrument, changes in the remaining SF-36 items, from pre- to post- pulmonary rehabilitation were examined in two small subsets of subjects. One was a randomly selected sample of 20 patients with COPD from our COPD pulmonary rehabilitation cohort database and another was a sample of patients who showed a good improvement in their functional capacity (improvement equivalent to the minimal important difference on either the 6-minute walk test or the constant work rate cycle endurance test) (Citation23) after completing a pulmonary rehabilitation program (n = 22). Items were removed if there was no change or only a very insignificant improvement in the mean change score after undergoing pulmonary rehabilitation (post-rehabilitation score minus pre-rehabilitation score less than 0.25).
Domain identification of the McGill COPD questionnaire
To identify the domains in the new, hybrid, McGill COPD questionnaire, confirmatory factor analysis was performed on data from 142 COPD patients, using all the items. Principal Components Analysis using eigenvalue-one procedure with varimax rotation was employed to rotate factors to a simple structure. The fit was done by optimizing the log likelihood assuming multivariate normality over the uniquenesses (Citation24). Items with a loading of greater than 0.4 were assigned to a specific factor (Citation25). Data were analyzed using R (2.7.1) (26).
RESULTS
A total of 142 COPD subjects from the original pulmonary rehabilitation cohort completed the questionnaire. The baseline sociodemographic and clinical characteristics of subjects used in the study are described in . Seventy percent of subjects had moderate-to-severe COPD as per FEV1 and SGRQ scores. The baseline characteristics of the original cohort (n = 246) were similar to the study cohort (data not shown).
Table 1 Baseline sociodemographic and clinical characteristics of the study population (n = 142)
Item selection
Items from the SF-36 with a correlation greater than 0.6 with any item in the COPD module were excluded. Twenty-five items were maintained from the SF-36 questionnaire, after excluding the first 2 items (the general health question and the health transition question) and those highly correlated. Based on the mean change score, additional items were removed as they showed no or a very small response to pulmonary-rehabilitation (). Twelve items were finally selected from the SF-36. The 17 items from the COPD-specific module and 12 items from the SF-36 made up the new, hybrid McGill COPD questionnaire with a total of 29 items.
Table 2 Additional item selection from SF-36 based on face/content validity, item-to-item correlation between SF-36 and COPD module items or response to pulmonary rehabilitation
Domain identification
Baseline data of the 142 subjects were used for the confirmatory factor analysis. Test of the hypothesis that 3 factors are sufficient was checked and the chi square statistic was 1093.15 on 592 degrees of freedom with the p-value of <0.0001. Factor I had eigenvalue of 12.21 accounting for 32.1% of total variance, factor II had eigenvalue of 5.4 accounting for 14.1% of total variance and factor III had eigenvalue of 4.0 accounting for 10.6% of total variance. All 17 items from the COPD-specific module were grouped under a single domain, factor I. The items from the SF-36 were grouped under two separate domains other than the COPD-specific module items in the factor analysis, factors II and III ().
Table 3 Confirmatory Factor Analysis (subjects = 142)
Instrument format
Based on their face and content validity, items from COPD-specific module and SF-36 were split in 3 separate sub-scales: Symptoms, Feelings and Physical Function. Sub-scales with suffix A and B were created to differentiate items from the COPD-specific module in sub-scale A and items from the SF-36 in sub-scale B. shows the composition of the new questionnaire. Complete questionnaire is available in the appendix.
DISCUSSION
The new, hybrid, McGill COPD quality of life questionnaire, based on a novel concept of combining items from the SF-36 and from a COPD-specific module, was designed to measure health-related quality of life in COPD patients. The strategy of administering a disease-specific module along with a generic questionnaire is widely used, especially in the field of cancer (Citation27). The strategy of combining items from a generic questionnaire with a disease-specific module has, to the best of our knowledge, not been used before.
The simultaneous development of English and French versions by a bilingual team avoided issues related to direct item translation. Use of a 5-point Likert scale in a health-related quality of life questionnaire is favored by experts in the field (Citation28,29). However, the most commonly used disease-specific questionnaire for COPD, the SGRQ, has primarily dichotomous items. The new questionnaire has all items anchored on 5-point Likert scale. This feature should enhance responsiveness, but this property remains to be tested. This improvement with use of a 5-point Likert scale required changing some SF-36 items.
Although we used pre-defined criteria to reduce items, there is no established science behind the process of item reduction. Furthermore, there are no guidelines to choose items to create a responsive scale. As we were developing an evaluative measure, it was decided to use item responsiveness in small samples of COPD patients as a guide. The face validity of the items and their importance, as stated by the focus groups participating in the first phase of this study, were also used in addition to pre-defined criteria. With the addition of 17 disease-specific questions to the selected core questions from the SF-36, it is possible to tap both generic and disease specific components of health-related quality of life.
However, it remains to be determined if we can use the new questionnaire and compare its score in different diseases as is possible with the generic questionnaires e.g., SF-36. Moreover, the new questionnaire has ‘COPD’-specific items, but these symptoms are well described in patients with other pulmonary pathologies such as asthma, interstitial lung diseases etc. Thus, this new questionnaire could be potentially useful in other respiratory diseases as well.
Factor analysis suggested that all items from the COPD-specific module could form a single domain, but the decision was taken to divide them into three sub-scales based upon their face and content validity. Although we labeled questions related to dyspnea and fatigue ‘symptoms’, in fact, they involved physical functioning. Moreover the ‘feelings’ sub-scale items involved breathing problems. Consequently, for subjects with moderate-to-severe COPD, symptoms, physical activities, social activities, and emotional reactions all are intertwined. Although we refer to the domains being ‘separate’, all arise largely from the common symptoms of COPD, i.e., exertional dyspnea and limitations in physical function due to dyspnea.
We have designed a novel questionnaire which is self-sufficient, short, self-administered, easily understood and available in English and French. The idea of developing a hybrid questionnaire using the well-established tool, the SF-36, is the major innovation in this study. The originality of this approach in the development of the new questionnaire is also important. This questionnaire needs to be validated in different COPD populations, mild-to-severe, males and females, and different ethnicities. Moreover, its psychometric properties need to be carefully examined and the ‘minimal important difference’ needs to be ascertained before it can be used in clinical practice or research.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
ACKNOWLEDGMENTS
Completion of this study was possible because of the dedication of the study personnel of each participating centre: Hôpital Laval: Marthe Bélanger, Brigitte Jean, Josée Picard, Montreal Chest Institute: Hanèn M'Kaouar and Palmina Mancino, Centre Hospitalier Universitaire associé de Québec: Ghyslaine Dubé, Louise Pagé, and Mont-Sinai Hospital: Jennie-Laure Sully, Benoît Major, Mira Mierzwinski, Maria Stathatos, Michelle Houde, Julie Bouchard. The authors also thank Yvan Fortier, Danielle Picard, Denise Légaré and Micheline Paquin for their work on the database and Serge Simard for his statistical assistance. Most importantly, the authors thank Dr. John Ware for permission to include and study items used in the SF-36 Health Survey.

REFERENCES
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: GOLD Executive Summary. Am J Respir Crit Care Med 2007; 176: 532–555.
- Weiss ST, DeMeo DL, Postma DS. COPD: Problems in diagnosis and measurement. Eur Respir J 2003; 21:4S–12.
- Rennard S, Decramer M, Calverley PMA, Pride NB, Soriano JB, Vermeire PA, Vestbo J. Impact of COPD in North America and Europe in 2000: Subjects’ perspective of Confronting COPD International Survey. Eur Respir J 2002; 20:799–805.
- Hajiro T, Nishimura K, Tsukino M, Ikeda A, Koyama H, Izumi T. Comparison of discriminative properties among disease-specific questionnaires for measuring health-related quality of life in patients with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 1998; 157:785–790.
- World Health Organization. International Classification of Functioning, Disability and Health: ICF. Geneva, WHO; 2001.
- Celli BR, MacNee W, Agusti A, Anzueto A, Berg B, Buist AS, Calverley PMA, Chavannes N, Dillard T, Fahy B, Fein A, Heffner J, Lareau S, Meek P, Martinez F, McNicholas W, Muris J, Austegard E, Pauwels R, Rennard S, Rossi A, Siafakas N, Tiep B, Vestbo J, Wouters E, ZuWallack R. Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. Eur Respir J 2004; 23:932–946.
- Mahler DA. How should health-related quality of life be assessed in patients with COPD? Chest 2000; 117:54S–57S.
- Guyatt GH, King DR, Feeny DH, Stubbing D, Goldstein RS. Generic and Specific measurement of health-related quality of life in a clinical trial of respiratory rehabilitation. J Clin Epidemiol 1999; 52:187–192.
- Ware JE Jr, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36): I. Conceptual Framework and Item Selection. Med Care 1992; 30:473–483.
- Martin M, Kosinski M, Bjorner JB, Ware JEJ, MacLean R, Li T. Item response theory methods can improve the measurement of physical function by combining the Modified Health Assessment Questionnaire and the SF-36 Physical Function Scale. [Article]. Qual Life Res 2007; 16:647–660.
- McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993; 31:247–263.
- Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987; 42:773–778.
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am.Rev.Respir Dis 1992; 145:1321–1327.
- Lacasse Y, Wong E, Guyatt GH, Goldstein RS. Health status measurement instruments in chronic obstructive pulmonary disease. Can Respir J 1997; 4:152–164.
- Kirshner B, Guyatt G. A methodological framework for assessing health indices. J Chronic Dis 1985; 38:27–36.
- Preston CC, Colman AM. Optimal number of response categories in rating scales: reliability, validity, discriminating power, and respondent preferences. Acta Psychol 2000; 104:1–15.
- Miller GA. The magical number seven, plus or minus two: some limits on our capacity for processing information. Psycho Rev 1956; 63:81–97.
- Jones RR. Differences in response consistency and subjects’ preferences for three personality inventory response formats. In Proceedings of the 76th Annual Convention of the American Psychological Association, Washington, DC; 1968:247–248.
- Spahija J, Collet J-P, Bourbeau J, Ducruet T, Robinson A, Wood-Dauphinee S. A new module for measuring quality of life in COPD patients. Am J Respir Crit Care Med 2000; A823.
- Theander K, Unosson M. Issues and innovations in nursing practice: Fatigue in patients with chronic obstructive pulmonary disease. J Advan Nurs 2004; 45:172–177.
- Walke LM, Byers AL, Tinetti ME, Dubin JA, McCorkle R, Fried TR. Range and severity of symptoms over time among older adults with chronic obstructive pulmonary disease and heart failure. Arch Intern Med 2007; 167:2503–2508.
- Janssen DJA, Spruit MA, Wouters EFM, Schols JMGA. Daily symptom burden in end-stage chronic organ failure: A systematic review. Palliative Med 2008; 22:938–948.
- Laviolette L, Bourbeau J, Bernard S, Lacasse Y, Pepin V, Breton MJ, Baltzan M, Rouleau M, Maltais F. Assessing the impact of pulmonary rehabilitation on functional status in COPD. Thorax 2008; 63:115–121.
- Lawley DN, Maxwell AE. Factor Analysis as a Statistical Method, 2nd edition. Butterworths, London; 1971:27–32.
- Kim JO, Mueller CW. Factor analysis: Statistical methods and practical issues Sage Publications Inc., London; 1978.
- Development Core Team. A Language and Environment for Statistical Computing. Foundation for Statistical Computing, Vienna, Austria; 2008.
- Sprangers MA, Cull A, Bjordal K, Grovenvold M, Aaronson NK. The European Organization for Research and Treatment of Cancer approach to quality of life assessment: Guidelines for developing questionnaire modules. Qual Life Res 1993; 2:287–295.
- Miller GA. The magical number seven, plus or minus two: Some limits on our capacity for processing information. Psycho Rev 1956; 63:81–97.
- Nunnally JC. Psychometric Theory. McGraw-Hill, New York; 1994.