Abstract
Background: There is little data about the combined effects of COPD and obesity. We compared dyspnea, health-related quality of life (HRQoL), exacerbations, and inhaled medication use among patients who are overweight and obese to those of normal weight with COPD. Methods: We performed secondary data analysis on 364 Veterans with COPD. We categorized subjects by body mass index (BMI). We assessed dyspnea using the Medical Research Council (MRC) dyspnea scale and HRQoL using the St. George's Respiratory Questionnaire. We identified treatment for an exacerbation and inhaled medication use in the past year. We used multiple logistic and linear regression models as appropriate, with adjustment for age, COPD severity, smoking status, and co-morbidities. Results: The majority of our population was male (n = 355, 98%) and either overweight (n = 115, 32%) or obese (n = 138, 38%). Obese and overweight subjects had better lung function (obese: mean FEV1 55.4% ±19.9% predicted, overweight: mean FEV1 50.0% ±20.4% predicted) than normal weight subjects (mean FEV1 44.2% ±19.4% predicted), yet obese subjects reported increased dyspnea [adjusted OR of MRC score ≥2 = 4.91 (95% CI 1.80, 13.39], poorer HRQoL, and were prescribed more inhaled medications than normal weight subjects. There was no difference in any outcome between overweight and normal weight patients. Conclusions: Despite having less severe lung disease, obese patients reported increased dyspnea and poorer HRQoL than normal weight patients. The greater number of inhaled medications prescribed for obese patients may represent overuse. Obese patients with COPD likely need alternative strategies for symptom control in addition to those currently recommended.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death in the United States, affecting an estimated 23.6 million adults with a prevalence that is predicted to increase (Citation1, 2). At the same time, obesity has reached epidemic proportions, with around one-third of adults in the United States classified as obese based on the World Health Organization (WHO) criteria of a body mass index (BMI) ≥ 30 kg/m2 (Citation3, 4). Despite the increasing number of people affected by both these conditions, relatively little is known regarding the associations between obesity and symptoms or resource utilization among patients with COPD.
Being overweight or obese has been associated with a decreased mortality among patients with COPD; a phenomenon commonly referred to as the “obesity paradox” (Citation5, 6). Despite this, there is some evidence to suggest that obese subjects with a diagnosis of COPD have decreased health-related quality of life (HRQoL) and increased physical limitations due to their respiratory symptoms (Citation7). Obesity is associated with factors such as decreased thoracic compliance, increased airway resistance, and increased work of breathing, that can worsen symptoms of dyspnea and wheezing attributed to airflow obstruction (Citation8, 9).
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend step-wise escalation of inhaled medications for patients with uncontrolled respiratory symptoms (Citation10). Among overweight and obese patients with COPD, this could lead to increased use of inhaled medications to treat symptoms caused by factors other than obstructive lung disease. In addition to providing ineffective symptom control, excessive usage could result in an increase in adverse medication events in overweight and obese patients who may already be at increased risk for such occurrences because of co-morbid cardiac disease (Citation11–15).
We hypothesized that, irrespective of the severity of airflow obstruction, overweight and obese patients with COPD would have increased dyspnea, more frequent treatment for exacerbations, greater impairment of HRQoL, and increased use of inhaled medications than patients of normal weight.
METHODS
Study design and subjects
We performed a cross-sectional study using data collected in a randomized trial designed to improve the quality and occurrence of end-of-life communication between patients with COPD and their providers (Citation16). Three hundred seventy-six patients with spirometric confirmation of airflow obstruction and a diagnosis of COPD, receiving care at the Department of Veterans Affairs Puget Sound Health Care System (VAPSHCS), were recruited between November 2004 and December 2007. The University of Washington and VAPSHCS Institutional Review Boards approved all study protocols and analyses.
Data collection
Height and weight were measured at entry into the parent study. The diagnosis of COPD was confirmed using pulmonary function testing and was defined as a ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) less than 0.70 (Citation10). All subjects had spirometry performed by study staff or by respiratory therapists in accordance with American Thoracic Society/European Respiratory Society Task Force guidelines, with the best of three acceptable efforts recorded for both FEV1 and FVC (Citation17, 18). Bronchodilator response was assessed with repeat spirometry after 2 puffs of albuterol. Post-bronchodilator FEV1 values were used to determine severity of obstructive lung disease (Citation10).
Upon enrollment, participants completed interviewer administered questionnaires. Health-related quality of life was evaluated using the American version of the St. George's Respiratory Questionnaire (SGRQ)(Citation19). Severity of dyspnea related to activity level was assessed using the Medical Research Council (MRC) dyspnea scale (Citation20). Sociodemographic information, co-morbid illness, current and past tobacco use, and treatment for COPD exacerbations in the preceding year were ascertained by questionnaire at study entry. Subjects were asked questions about their tobacco exposure, including age at initiation and time since quitting.
They also were asked how many packs were smoked on a typical day, with possible answers including: less than ½ pack, more than ½ pack but less than 1 pack, 1 to less than 2 packs, 2 to less than 3 packs, 3 or more packs. Due to small numbers of subjects at each extreme, we collapsed these categories to a dichotomous variable (< 2 packs vs. ≥ 2 packs) for this analysis. Additional information about co-morbid illness and inhaled medication use as of the enrollment date was obtained utilizing the VA clinical data warehouse.
Exposure and outcome measures
The primary exposure was body mass index (BMI), calculated as the ratio of weight in kilograms to height in meters squared. Patients were categorized by World Health Organization (WHO) criteria: normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2) and obese (BMI ≥ 30.0 kg/m2) (Citation21). Underweight patients (BMI < 18.5 kg/m2) and those with no measure of BMI were excluded. Dyspnea was characterized using the 5-point MRC dyspnea scale to indicate breathlessness related to various activity levels (Citation20). Subjects were dichotomized into two categories: 1) mild dyspnea (MRC score of 1) and 2) moderate to severe dyspnea (MRC score ≥ 2).
As a marker of disease severity, information regarding exacerbations treated with prednisone and/or antibiotics in the year prior was obtained based on self-report. Health-related quality of life was ascertained using the total score and three sub-scores of the SGRQ (symptom, impact, and activity). Higher scores indicate worse HRQoL, and a difference of 4 points on the 100-point scale is considered clinically significant (Citation22). Prescriptions for short-acting inhaled medications (beta agonists and/or ipratropium bromide), long-acting beta-agonists (LABA), and inhaled corticosteroids (ICS) in the year prior to study entry were determined using pharmacy records.
Statistical analysis
We performed all statistical analyses using STATA SE-10.0 (StataCorp, College Station, TX). We used multivariable logistic regression models to estimate associations of BMI with dichotomous outcomes (Citation23), and multivariable linear regression to model associations between BMI category and continuous outcomes (Citation24). A priori, we decided to adjust for scientifically plausible confounders of the association of BMI and our outcomes of interest, including: current tobacco use, age, severity of airflow obstruction based on post-bronchodilator FEV1, and co-morbidities that could result in cardiac symptoms (hypertension, hyperlipidemia, congestive heart failure, diabetes, and coronary artery disease). Huber-white variance estimation was used in all models to conservatively estimate variance where not all traditional regression assumptions for variance may have been met (Citation25). A two-tailed p-value of <0.05 was considered statistically significant.
For all multivariable models, we calculated adjusted predicted values for each subject from the estimated regression equation, using the adjusted β-coefficients and numerical values for each variable included in the model. For selected outcomes, the resulting adjusted probabilities (logistic regression models) or adjusted linear outcomes (linear regression models) for each subject were then plotted against FEV1% predicted, separated by BMI category.
RESULTS
Patient characteristics
Of the original cohort of 376 veterans, 12 subjects were excluded from this analysis (4 for being underweight and 8 for having no recorded measurement of BMI). Of the remaining 364 subjects, 111 (30.5%) were normal weight, 115 (31.6%) were overweight and 138 (37.9%) were obese (). The majority of patients were white (85.9%) and male (97.5%). The mean age of the population was 69.0 years (±9.9 years); obese patients were younger than normal weight patients (67.4 ± 9.3 vs. 69.2 ± 11.1 years).
Table 1 Patient characteristics by BMI category
The majority of subjects had moderate or severe obstructive lung disease (n = 269 patients, 73.9%) based on GOLD Stage criteria (Citation10); 17.9% (n = 65) of subjects had very severe disease (FEV1 < 30% predicted). BMI was inversely associated with documented severity of airflow obstruction: obese patients had a mean post-bronchodilator FEV1 of 1.85 ± 0.69 (55.4% ± 19.9% of predicted), while normal weight patients had a mean FEV1 of 1.45 ± 0.63 L (44.2% ± 19.4% of predicted). Hypertension, hyperlipidemia, and diabetes mellitus were all more common among overweight and obese subjects compared to normal weight subjects.
Normal weight patients were more likely to report on-going tobacco use than those who were overweight or obese (37.3%, 22.6%, and 20.5%, respectively). All participants reported to have started smoking before the age of 30 years old. No difference existed in the age that patients started smoking by BMI category (p-value = 0.78), with 76.8% of normal weight patients, 74.0% of overweight patients and 77.1% of obese patients starting between the ages of 10 and 19 years of age. Among former smokers, a greater number of overweight (55.3%) and obese (43.0%) than normal weight subjects (36.7%) reported quitting smoking more than 15 years ago, however this difference was not statistically significant (p-value = 0.08).
Table 2 Associations of patient-reported dyspnea and exacerbations with BMI category
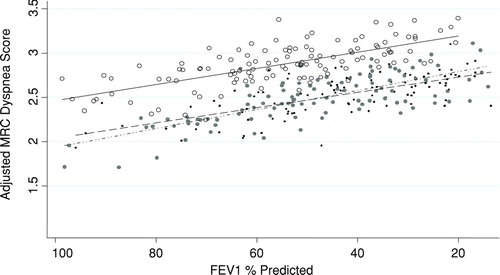
Figure 1 For every given severity of airflow obstruction, obese patients are more dyspneic than normal weight patients. We calculated the adjusted predicted MRC score from the multivariable linear model containing BMI category, FEV1% predicted, age, smoking, diabetes mellitus, hypertension, hyperlipidemia, coronary artery disease and congestive heart failure and plotted it for each subject by BMI category. Regression lines for each BMI category also shown. (Normal weight: ,–––; Overweight: , –ċċ–; Obese: , —).

Table 3 Association of health-related quality of life with BMI category*
Among former smokers, 38.2% of overweight and 41.3% of obese patients compared to 23.7% of normal weight patients reported smoking 2 or more packs on a typical day (non-significant p-value = 0.08). Among current smokers, no differences were found in the amount of tobacco smoked on a typical day by BMI category, with the majority of all patients smoking less than 2 packs a day (p-value = 0.80).
Symptoms of dyspnea
There was no difference in the odds of moderate or severe dyspnea (MRC score ≥ 2) between overweight and normal weight subjects () However, obese subjects were almost 5 times as likely as normal weight subjects to experience moderate or severe dyspnea in adjusted analyses (Adj. OR 4.91 (95% CI 1.80, 13.39, p-value of 0.002). shows the adjusted MRC score for each subject vs. FEV1% predicted and regression lines for each BMI category. For a given severity of lung disease, obese subjects had a consistently higher adjusted mean MRC score than normal weight subjects.
Treatment for exacerbations
In adjusted analyses, there were no differences in odds of treatment for an exacerbation of COPD in the last year between either overweight or obese patients relative to normal weight patients ().
Health-related quality of life
There was no difference in the total scores or in any of the sub-scores on the SGRQ among overweight patients compared to normal weight patients (). However, after adjusting for our a priori confounders, obese subjects reported significantly worse HRQoL as measured by the higher total SGRQ score (adjusted β-coefficient +5.93 (95% CI +0.97,+10.88), impact (adjusted β-coefficient +5.37 (95% CI +0.11,+10.63) and activity (adjusted β-coefficient +8.16 (95% CI +1.93,+14.40) sub-scores, with higher scores indicating greater impairment.
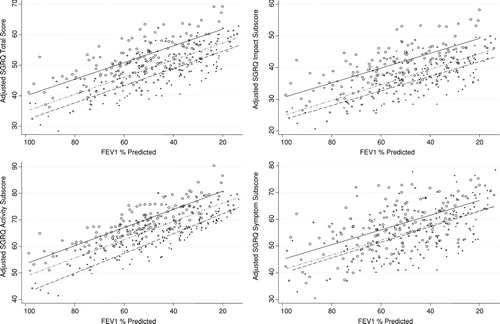
shows the adjusted SGRQ score and sub-scores (estimated from the multiple regression model) for each subject plotted against the FEV1% predicted and the resulting best-fit least squares regression line for each BMI category. For a given severity of lung disease, obese subjects had consistently higher (worse) mean adjusted total, impact and activity scores on the SGRQ.
Medication use
The great majority of patients in each BMI category were prescribed at least one short-acting inhaler (91.0% of normal weight vs. 91.3% of overweight and 92.8% of obese subjects). There were no differences in the odds of prescription for a short-acting inhaler across BMI categories (). Similarly, there were no differences between overweight and normal weight patients in the odds of prescription of either a LABA or ICS. However, in adjusted analyses, obese patients had a more than two-fold higher odds of being prescribed a long-acting inhaler than their normal weight counterparts (OR 2.21 (95% CI 1.14, 4.31) p-value = 0.019 for LABA and OR 2.34 (95% CI 1.25, 4.40) p-value = 0.008 for ICS).
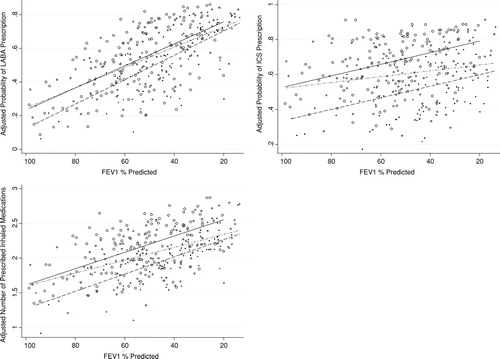
In adjusted analyses, obese patients also were prescribed a significantly greater number of classes of inhaled medications when compared to normal weight patients (). illustrates the estimated adjusted probability of prescription for LABA, prescription for ICS and the estimated adjusted number of medication classes prescribed, for a given FEV1% predicted, separated by BMI categories.
Figure 2 Obese patients report worse health-related quality of life than normal weight patients. We calculated the adjusted predicted SGRQ score from the multivariable linear model containing BMI, FEV1% predicted, age, smoking, diabetes mellitus, hypertension, hyperlipidemia, coronary artery disease and congestive heart failure and plotted it for each subject by BMI category. Regression lines for each BMI category shown. . (Normal weight: ,–––; Overweight: , –ċċ–; Obese: , —).

Exploratory analysis
Both LABA and ICS have been shown to reduce exacerbations and improve dyspnea and HRQoL among patients with COPD (Citation26–29). We therefore performed exploratory analyses to test whether there was effect modification of the associations of BMI with dyspnea (as defined as MRC score ≥ 2), HRQoL (as defined by total score on SGRQ), and the occurrence of treated exacerbations by the use of long-acting inhalers. For each of these models, we found the interaction term of BMI and long-acting inhaler use to be non-significant (data not shown).
DISCUSSION
This study demonstrates that among patients with COPD, obese patients reported increased severity of dyspnea and overall poorer health-related quality of life. Although obese subjects were no more likely to be treated for a COPD exacerbation with either antibiotics or prednisone, they were more likely to be prescribed either a LABA or ICS. On average, obese subjects were also treated with a greater number of different inhaled medications than normal weight patients with COPD.
Table 4 Association of Inhaled Medication Use with BMI category
As recommended by the GOLD guidelines, increased prescription of inhaled medications may reflect an effort on the part of providers to ameliorate symptoms mistakenly attributed to airflow obstruction (Citation10). It could be postulated that obese patients demonstrate a more severe clinical phenotype of COPD necessitating more aggressive inhaled therapy. However, we found no evidence to support this theory, as there were no differences in treatment for COPD exacerbations with either prednisone or antibiotics across BMI categories. Instead, among patients whose shortness of breath may result from obesity-related factors, our results imply that this escalation in inhaled therapy may be less effective than intended. Attribution of symptoms to obstructive lung disease may lead to lost opportunities to focus on lifestyle and surgical interventions to address dyspnea due to excessive weight.
Providing medications of limited effectiveness by definition exposes patients to potential risks while providing little benefit. In this situation, even rare events may cause the balance of risk and benefit to be unfavorable. Medications commonly used for the treatment of COPD including inhaled corticosteroids, long-acting beta-agonists and anti-cholinergics have all been implicated to have potential untoward effects including elevated serum glucose (Citation30), increased risk of pneumonia (Citation31), and more frequent myocardial infarction and cardiac-related death (Citation11–15). Although these untoward effects continue to be debated (Citation32), careful consideration of which populations benefit most or may be at elevated risk of adverse events need further exploration.
We found obese subjects to have better lung function than the normal weight subjects. There are a number of possible explanations for this finding. For each degree of severity of lung disease, obese subjects reported increased dyspnea and decreased quality-of-life than their normal weight counterparts. Such symptoms could prompt them to come to medical attention earlier, allowing them to be diagnosed at an earlier stage of their disease.
Another possible explanation is that obesity is a marker for less cumulative tobacco exposure, as we know that patients who quit smoking gain more weight over time than those who continue tobacco use (Citation33). Last, chronic malnourishment has been associated with “emphysema-like” changes in lung parenchyma (Citation34). It is, therefore, possible that obesity could somehow be protective against the development of more severe emphysema among patients with similar amounts of tobacco exposure.
Few studies have examined difference in outcomes associated with obesity among patients with COPD. A recent study of 36 patients examining the combined effects of obesity and COPD on exercise tolerance showed that obese patients did not have greater dyspnea or exercise limitation than normal weight patients with similar FEV1 (Citation35). However, uncontrolled confounding may explain the null results and difference with findings from the current study, as the small sample size resulted in low statistical power and limited adjustment for potential confounding factors. In contrast, our findings are supported by another recent study examining outcomes of obese patients referred for pulmonary rehabilitation (Citation36).
The investigators found that while obese patients with COPD were referred to pulmonary rehabilitation at an earlier spirometric stage of their disease, they had poorer exercise performance, greater degree of functional impairment and increased fatigue when compared to their non-obese counterparts. Furthermore, in one study, overweight or obese patients with severe COPD who maintained or reduced their weight over five years had significantly better survival than subjects who gained weight over the same time period (Citation37). It remains to be seen whether weight reduction, typically thought of as a poor prognostic sign among patients with COPD (Citation6), leads to improved COPD related outcomes among obese patients.
This study has limitations. First, the results for our largely male VA patient population may not generalize to other populations, particularly those with large proportions of women. Women tend to have more peripheral fat distribution than men with the same BMI, who instead deposit fat more centrally in their thorax and abdomen (Citation38). Second, we do not have other measures of body weight distribution available, such as waist-hip ratio, waist circumference, or fat-free mass to make comparisons in outcomes.
Figure 3 Obese patients are prescribed more long-acting inhaled medications than normal weight patients. We calculated the adjusted probability of long-acting beta-agonist (LABA) and inhaled corticosteroid (ICS) prescription from the multivariable logistic model containing BMI, FEV1% predicted, age, smoking, diabetes mellitus, hypertension, hyperlipidemia, coronary artery disease and congestive heart failure and plotted it for each subject by BMI category. The adjusted number of medication classes was similarly calculated from the multivariable linear regression model. Regression lines for each BMI category also shown. (Normal weight: ,–––; Overweight: , –ċċ–; Obese: , —).

Although these measures might be more directly associated with pulmonary symptoms, BMI is routinely measured in practice and therefore more clinically relevant for providers. Third, we do not have additional outcome measures available, such as deaths in this cohort, or hospitalization for COPD exacerbation or cardiac related causes. Lastly, BMI was measured upon enrollment and assumed to reflect that present during the previous year, when medication use was assessed. Although we recognize that small weight fluctuations could occur in some patients resulting in misclassification of exposure, this is likely to be non-differential in nature with respect to BMI category and affect only a small number of subjects.
CONCLUSIONS
In summary, among patients with COPD, obesity was associated with less severe airflow obstruction but increased symptoms of dyspnea, worse health-related quality of life, and increased prescription of inhaled medications. This increased prescription for inhaled medications occurred in the absence of differences in treatment for COPD exacerbations. Our results suggest that inhaled medications traditionally given to treat COPD may be less effective among obese and overweight patients after accounting for severity of airflow limitation.
This raises the possibility that the risk-benefit ratio for medication use among obese patients may be less favorable than for non-obese patients with COPD. Most importantly, awareness of these findings should alert clinicians to consider alternative strategies to treat dyspnea in obese patients with COPD, including placing a greater emphasis on weight reduction and pulmonary rehabilitation rather than escalation in inhaler therapies. Studies to evaluate the effect of such interventions will become increasingly important as obesity continues to become more prevalent worldwide among patients with COPD.
DECLARATION OF INTEREST
This material is based upon work supported by a Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development grant [IIR 02–292]. It is also supported in part by the Office of Research and Development Cooperative Studies Program, Department of Veterans Affairs. Dr. Cecere was previously supported by an NIH institutional training grant [2T32HL007287–31], and is currently supported by a Veterans Affairs HSR&D Fellowship [TPM 61–037]. Dr. Slatore is supported by a Veterans Affairs HSR&D Career Development Award and resources from the Portland VA Medical Center, Portland, OR. Dr. Littman was supported by a VA Rehabilitation Research and Development Career Development Award (#6982). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. An earlier version of this manuscript was presented in abstract form at the American Thoracic Society International Conference, May 2009.
Drs. Au and Bryson are co-investigators on a grant from Giliad Sciences for work which is unrelated to this manuscript. Dr. Boyko has consulted for Ely Lily, Inc. and Seventh Sense Biosystems. Dr. Au sits on the medical editorial board for Nexcura and is a research consultant for Bosch LLC. None of the other authors have any potential conflicts of interest to report.
REFERENCES
- Mannino DM, Braman S. The epidemiology and economics of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2007; 4(7):502–506.
- Minino AM, Xu J, Kochanek KD. Deaths: Preliminary data for 2008. Natl Vital Stat Rep 2010; 59(2):1–71.
- Koepsell TD, Littman AJ, Forsberg CW. Obesity, Overweight, and Their Life Course Trajectories in Veterans and Non-Veterans. Obesity (Silver Spring). 2011 Feb 3 [epub ahead of print].
- Ogden C, Carroll M, Curtin L, Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006; 295(13):1549–1555.
- Franssen F, O'Donnell D, Goossens G, Obesity and the lung: 5. Obesity and COPD. Thorax 2008; 63(12):1110–1117.
- Landbo C, Prescott E, Lange P, Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 160(6):1856–1861.
- Arterburn D, McDonell M, Hedrick S, Association of body weight with condition-specific quality of life in male veterans. Am J Med 2004; 117(10):738–746.
- Naimark A, Cherniack R. Compliance of the respiratory system and its components in health and obesity. J Appl Physiol 1960; 15:377–382.
- Kress J, Pohlman A, Alverdy J, Hall J. The impact of morbid obesity on oxygen cost of breathing (VO2RESP) at rest. Am J Respir Crit Care Med 1999; 160(3):883–886.
- Rabe K, Hurd S, Anzueto A, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176(6):532–555.
- Cazzola M, Imperatore F, Salzillo A, Cardiac effects of formoterol and salmeterol in patients suffering from COPD with preexisting cardiac arrhythmias and hypoxemia. Chest 1998;114(2):411–415.
- Lee T, Pickard A, Au D, Risk for death associated with medications for recently diagnosed chronic obstructive pulmonary disease. Ann Intern Med 2008; 149(6):380–390.
- Anthonisen N, Connett J, Enright P, Hospitalizations and mortality in the Lung Health Study. Am J Respir Crit Care Med 2002; 166(3):333–339.
- Ringbaek T, Viskum K. Is there any association between inhaled ipratropium and mortality in patients with COPD and asthma? Respir Med 2003; 97(3):264–272.
- Singh S, Loke Y, Furberg C. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. JAMA 2008; 300(12):1439–1450.
- Au D, Udris E, Engelberg R, A Randomized Trial to Improve the Occurrence and Quality of Communication about End-of-Life Care among Patients with COPD (submitted 2/2011).
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995; 152(5 Pt 2):S77–121.
- Celli B, MacNee W, Force AET. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23(6):932–946.
- Barr J, Schumacher G, Freeman S, American translation, modification, and validation of the St. George's Respiratory Questionnaire. Clin Ther 2000; 22(9):1121–1145.
- Bestall J, Paul E, Garrod R, Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54(7):581–586.
- World Health Organization. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 1995; 854:1–452.
- Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J 2002; 19(3):398–404.
- Hosmer D, Lemeshow S. Applied Logistic Regression. New York: Wiley; 1989.
- Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression Methods in Biostatistics: Linear, Logistic, Survival and Repeated Measures Models. New York: Springer; 2005.
- Huber P. Robust Statistics. New York: John Wiley & Sons; 1981.
- Mahler DA, Donohue JF, Barbee RA, Efficacy of salmeterol xinafoate in the treatment of COPD. Chest 1999; 115(4):957–965.
- Dahl R, Greefhorst LA, Nowak D, Inhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164(5):778–784.
- Burge PS, Calverley PM, Jones PW, Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000; 320(7245):1297–1303.
- Calverley P, Pauwels R, Vestbo J, Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003; 361(9356):449–456.
- Slatore C, Bryson C, Au D. The association of inhaled corticosteroid use with serum glucose concentration in a large cohort. Am J Med 2009; 122(5):472–478.
- Crim C, Calverley P, Anderson J, Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J 2009; 34(3):641–647.
- Ferguson GT, Funck-Brentano C, Fischer T, Darken P, Reisner C. Cardiovascular safety of salmeterol in COPD. Chest 2003; 123(6):1817–1824.
- O'Hara P, Connett JE, Lee WW, Early and late weight gain following smoking cessation in the Lung Health Study. Am J Epidemiol 1998; 148(9):821–830.
- Coxson HO, Chan IH, Mayo JR, Early emphysema in patients with anorexia nervosa. Am J Respir Crit Care Med 2004; 170(7):748–752.
- Ora J, Laveneziana P, Ofir D, Combined effects of obesity and chronic obstructive pulmonary disease on dyspnea and exercise tolerance. Am J Respir Crit Care Med 2009; 180(10):964–971.
- Ramachandran K, McCusker C, Connors M, The influence of obesity on pulmonary rehabilitation outcomes in patients with COPD. Chron Respir Dis 2008; 5(4):205–209.
- Prescott E, Almdal T, Mikkelsen K, Prognostic value of weight change in chronic obstructive pulmonary disease: results from the Copenhagen City Heart Study. Eur Respir J 2002; 20(3):539–544.
- Power ML, Schulkin J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: possible evolutionary origins. Br J Nutr 2008; 99(5):931–940.