Abstract
Abstract: Indacaterol is a novel, inhaled once-daily ultra long-acting β2-agonist for the treatment of COPD. This randomised, double-blind, placebo-controlled, two-period crossover study evaluated the effect of two-week treatment with indacaterol 300 μg on peak and isotime exercise inspiratory capacity (IC) in patients with COPD. Patients (40–80 years) with post-bronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) <70%, percent predicted FEV1 ≥40% and ≤80%, smoking history ≥20 pack-years and functional residual capacity >120% of predicted normal were randomised to receive indacaterol 300 μg or placebo once-daily via a single-dose dry powder inhaler. Following 14 days of treatment, IC at peak and isotime during constant-load (80% of maximum workload) cycle ergometry was analysed using linear mixed-effects models. Safety and tolerability were also monitored. Twenty-seven patients (67% male; mean age, 61.3 years) were randomised; 24 completed the study. On Day 14, indacaterol showed statistically significant improvements over placebo in peak (317 mL [95% CI: 118–517]; p < 0.01) and isotime IC (268 mL [95% CI: 104–432]; p < 0.01). Statistically significant improvements were observed with indacaterol versus placebo on Day 14 for the following secondary endpoints: resting IC, trough FEV1, dyspnoea (BDI/TDI and Borg CR10 scale at isotime) and exercise endurance time. Indacaterol was well tolerated, with no serious adverse events or deaths. In conclusion, indacaterol 300 μg administered once-daily showed a clinically relevant increase in IC after 14 days of treatment, reflecting a reduction in dynamic hyperinflation.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a chronic progressive lung disorder resulting in symptoms of increasing dyspnoea on exertion. These symptoms impair exercise tolerance and result in limitation or avoidance of activity. The underlying pathophysiology is peripheral airway obstruction and loss of elastic recoil of alveolar attachments, which results in progressive air trapping during expiration, leading to lung hyperinflation. Hyperinflation is reflected by a decrease in inspiratory capacity (IC), and an increase in end-expiratory lung volume, particularly during exercise, which results in dyspnoea and exercise intolerance (Citation1).
Current clinical practice guidelines recommend the use of bronchodilators for the symptomatic management of COPD (Citation1). Bronchodilators, such as inhaled β2-agonists and anticholinergics, act on peripheral airways and reduce air trapping, thereby reducing lung volumes, improving symptoms and increasing exercise capacity (Citation1).
Indacaterol is a novel, inhaled once-daily ultra long-acting β2-agonist (LABA) (Citation2) that has been approved in a number of countries, including throughout the European Union, for the maintenance treatment of moderate to severe COPD. In previous studies, indacaterol has demonstrated 24-h bronchodilation (Citation3–4), with no loss in efficacy on once daily dosing for up to a year (Citation4), and a good overall safety profile (Citation4). Further, in a previous study, a single 300 μg dose of indacaterol had a greater effect on resting IC than formoterol 12 μg administered twice daily (Citation5). The aim of the present study was to evaluate the effect of indacaterol on dynamic and static lung hyperinflation, breathlessness, and health status in patients with moderate-to-severe COPD.
MATERIALS AND METHODS
Study design
This was a four-centre, double-blind, randomised, placebo-controlled, two-way crossover study designed to examine the effect of two weeks of treatment with indacaterol 300 μg administered once-daily via a single-dose dry powder inhaler (SDDPI). The study was conducted in accordance with the Declaration of Helsinki (1989). Institutional review board or independent ethics committee approval was obtained from each participating study centre, and all patients provided written informed consent prior to taking part in the study.
Study participants
Patients of either sex, aged 40–80 years, with a diagnosis of COPD (according to the Global Initiative of Chronic Obstructive Lung Disease 2005 guideline) (Citation6) and a smoking history of at least 20 pack years were eligible for enrolment in the study if they demonstrated a post-bronchodilator forced expiratory volume in 1 s (FEV1) ≥40% but ≤80% of the predicted normal value, a post-bronchodilator FEV1/forced vital capacity (FVC) <70% and a plethysmographic functional residual capacity (FRC) >120% of the predicted normal value.
Patients were excluded from the study if they had significant concomitant pulmonary disease or a history of asthma. Patients were also excluded if they experienced oxygen desaturation to <80% during cycle exercise while breathing room air.
Randomisation and interventions
The study consisted of a 21-day screening period (patients attended two screening visits within this period), one baseline visit, two 14-day treatment periods (washout of 4 to 21 days between periods), and a study completion visit between 14 and 21 days after the second treatment period. Eligible patients were randomised to one of two treatment sequences using a validated system. In both treatment sequences, indacaterol 300 μg or matching placebo was administered once daily via an SDDPI for 2 weeks. Treatments were concealed from patients, investigators, clinic staff performing the assessments and data analysts from the time of randomisation to database lock.
Rescue medication (short-acting β2-agonists) was prescribed to the study participants for the duration of the study; however, patients were advised to avoid taking rescue medication 6 h prior to the first assessment on study visit days. Patients had to discontinue LABA monotherapy at least 48 h prior to screening, and were not permitted any LABA at any time during the study. The use of inhaled corticosteroids (ICSs) was permitted at a stable dose and regimen throughout the study, providing patients had been using that dose and regimen for at least a month prior to the screening visit. Patients using a fixed-dose combination of a LABA and an ICS were to discontinue the LABA component prior to the screening visit, and were to continue the equivalent ICS monotherapy for the duration of the trial.
Outcomes and assessments
Cardiopulmonary exercise testing was performed using incremental and submaximal cycle ergometry (Citation7). At screening, patients underwent an incremental exercise test to determine their maximum workload (Wmax), defined as the greatest workload achieved for a continuous 30-s period (maintaining at least 40 revolutions/min). The incremental exercise test procedure was as follows: 3 min resting, followed by 3 min unloaded pedalling, followed by exercise with loaded pedalling (commencing with 10 W workload at 60 revolutions/min, increasing by 10 W/min until exhaustion, i.e., the patient was unable to continue).
During the treatment periods, dynamic IC measurements were made during a constant-load, submaximal (80% of Wmax) cycle ergometry test, as follows: 3 min resting, followed by 1 min unloaded pedalling, followed by exercise with loaded pedalling (at 80% Wmax) until exhaustion. During the submaximal cycle ergometry tests, IC was assessed over the final 30 s epoch of each 2 min interval using a hand-held spirometer (Viasys Masterscope) mounted to a mobile stand whilst the patient remained seated on the bike.
Quality assurance methods put in place to ensure good quality exercise endurance tests and data included: all sites used the same make and model of hand-held spirometry; spirometers were calibrated on every study visit; all investigators received detailed training on the exercise endurance test methodology (with the training including practice runs of the exercise endurance tests); at site initiation visits, practice runs were conducted for pulmonary function and exercise tests to ensure that the site staff were all familiar with (and competent in) the tests; all spirometry data were over-read by the same person to ensure a consistent approach; and all data entered into the database were verified against source materials at site by the same monitor.
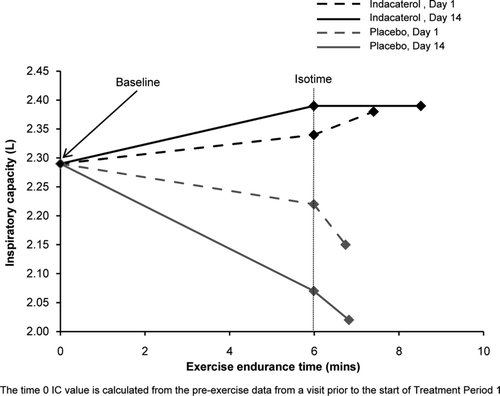
The primary efficacy variable was IC at peak and isotime on Day 14 during constant-load (80% Wmax) cycle ergometry. Peak IC was defined as the last IC measurement taken in each exercise period before exhaustion. Isotime IC was defined as the IC assessed at the last time point that the patient was still exercising during the individual shortest submaximal exercise test in the study (Citation8).
The secondary efficacy variables included exercise endurance time, static (resting) IC, dyspnoea (transition dyspnoea index [TDI] and Borg CR10) and trough FEV1 (mean of the measurements at 23 h 30 min and 24 h post-dose). Exercise endurance time was defined as the exercise time on submaximal load and was measured during submaximal cycle ergometry. Static (resting) IC, FRC, TLC and RV were assessed predose on Days 1 and 14 assessed using whole body plethysmography. Dyspnoea was measured at baseline (predose on Day 1 of each treatment period) using the baseline dyspnoea index (BDI) and during the treatment periods using the TDI (capturing changes from baseline). A TDI total score of 1 is considered to be a clinically significant improvement from baseline (Citation9–11). The Borg CR10 scale is a 12-point rating scale that was used to measure the level of dyspnoea before and during the submaximal exercise tests (Citation12). Spirometry was performed to assess FEV1, FVC, percent predicted FEV1, and FEV1/FVC. Spirometry equipment and the testing procedure were in accordance with the American Thoracic Society standards (Citation13–15).
Safety assessments included recording of adverse events (AEs) and serious AEs (SAEs), electrocardiograms (ECGs), monitoring of vital signs and standard clinical laboratory evaluations (haematology, blood chemistry and urinalysis).
Table 1 Demographic summary and baseline clinical characteristics (safety population)
Table 2 Exercise-related assessments on Day 14
Statistical analysis
The analysis of the primary endpoints, Day 14 isotime and peak IC, was performed at a two-sided 5% significance level. As this was an exploratory study, all other efficacy endpoints were analysed using a two-sided 10% significance level. No adjustment for multiple comparisons was performed. It was calculated that a sample size of 21 patients would have 80% power to detect a difference of 200 mL in treatment means in IC at isotime on Day 14 (the predefined clinically relevant difference), assuming a standard deviation (SD) of 310 mL and a two-sided significance level of 5%. Assuming a dropout rate of approximately 10%, it was calculated that a total of 24 patients would need to be randomised. All randomised patients who received at least one dose of study medication and had at least one post-baseline assessment for the primary efficacy variable were included in the analyses of efficacy data; patients who received at least one dose of study medication were included in the safety analyses.
The primary endpoints, isotime and peak IC, were analysed using linear mixed effects models, with the baseline isotime or peak measurement as a covariate (assessed prior to the start of Treatment Period 1), treatment, sequence and period as fixed effects, and subject as a random effect. The secondary efficacy endpoints were analysed using similar models (note that for some, e.g. BDI, baseline was assessed at the start of each treatment period). The results are presented as least squares means with the exception of FRC, TLC and RV results, which are presented as raw means.
RESULTS
Patient disposition and demographics
A total of 27 patients with COPD were enrolled, of whom three discontinued prior to completing the study (two due to administrative reasons, and one as a result of an adverse event whilst receiving placebo). The baseline demographics and clinical characteristics of these patients are shown in . All 27 patients enrolled were included in the analyses. One patient withdrew during the first treatment period (for administrative reasons), and so overall 26 patients received both indacaterol and placebo. The other two patients withdrew during their second treatment period.
Efficacy
Indacaterol–placebo differences for mean peak and isotime IC on Day 14 (n = 25) were 317 mL (95% CI: 118–517; p < 0.01) and 268 mL (95% CI: 104–432; p < 0.01), respectively (, with raw mean values in ). Statistically significant differences were also noted on Day 1 (n = 26), with an indacaterol–placebo difference of 250 mL for mean peak IC (p < 0.001) and 148 mL for mean isotime IC (p < 0.03). Mean resting IC on Day 14 with indacaterol showed an improvement of 182 mL (90% CI: 70–294; p < 0.05) versus placebo. In addition, there was a greater reduction from baseline in raw mean resting FRC and RV with indacaterol than placebo.
TLC hardly changed from baseline with indacaterol, compared with a slight decrease in the placebo group (). Exercise endurance time in patients treated with indacaterol was statistically higher than in those treated with placebo on Day 14 (an increase of 1.46 min; 90% CI: 0.70–2.22; p < 0.01; ; the difference on Day 1 was 0.53 min; 90% CI: −0.23–1.29; p = NS). For Borg CR10, a significant decrease (i.e., improvement) was observed between indacaterol and placebo at isotime on Day 14, with a non-significant decrease at peak time on Day 14 (), and with no statistically significant difference on Day 1 between indacaterol and placebo for either endpoint.
Table 3 Whole body plethysmography on Day 14
The mean TDI at Day 14 with indacaterol was higher than with placebo (). In relation to spirometric results, the mean trough FEV1 on Day 14 was higher for indacaterol than placebo (), while the mean trough FVC with indacaterol was also higher than with placebo (difference of 273 mL; 90% CI: 159−388 mL; p < 0.001).
Safety and tolerability
The overall incidence of AEs was 35% in both the indacaterol and placebo treatment groups. Most of the AEs reported were mild or moderate in severity, and most were not suspected of being study drug related. There were no deaths, SAEs, or significant AEs during this study. One patient withdrew from the study because of an AE (ST depression during exercise) following placebo treatment. Changes in vital signs were assessed as being not clinically significant by the investigators. No clinically relevant effects were seen in ECG and physical examinations, and no patient had any corrected QT interval values that were considered to be prolonged.
DISCUSSION
The present study is the first to evaluate the effect of indacaterol on dynamic lung hyperinflation and exercise endurance in patients with COPD. The major findings of this study were that treatment with indacaterol 300 μg once-daily for 2 weeks was associated with statistically significant improvements in IC at peak and isotime, together with statistically significant improvements in exercise endurance time and dyspnoea. The improvements over placebo in both peak and isotime IC on Day 14 exceeded 200 mL (the prespecified level of clinical relevance). The improvement in IC probably occurred as a result of increased tidal expiratory flow rates and improved lung emptying consequent to enhanced airway patency. Thus with increased IC, the required alveolar ventilation could be achieved at lower operating volumes, which in turn might favour muscle function and dynamic mechanics during exercise.
Dynamic hyperinflation is a primary mechanism for exertional dyspnoea and reduced exercise capacity associated with COPD (Citation16-17). O'Donnell et al confirmed that exercise endurance time (during submaximal cycle exercise testing), Borg CR10 dyspnoea ratings and measurements of IC are highly reproducible and responsive to change in patients with COPD (Citation18). In the present study, the exercise endurance time, as measured during submaximal exercise testing, was 1.46 min higher after 14 days of treatment with indacaterol compared with placebo. In hyperinflated patients as recruited in this study (FRC >120%), an improvement in hyperinflation permits greater increases in tidal volume and ventilation during exercise and, consequently, an improved exercise capacity (Citation18-19).
Table 4 Trough FEV1, Borg CR10 scores and TDI total scores on Day 14
In recognition of the importance of dyspnoea as the main symptom experienced by patients with COPD, two measures of dyspnoea were included in the present study. Borg CR10 evaluates exertional dyspnoea whereas the TDI assesses the impact of dyspnoea on activities of daily living. It is noteworthy that indacaterol significantly improved Borg CR10 score at isotime on Day 14 (which was not symptom limited) compared to placebo. Although indacaterol was only associated with modest improvements in the Borg CR10 score at peak, this could be because the duration of the constant load test was symptom limited − in other words patients stopped exercising as a result either of intolerable dyspnoea or of muscle fatigue, both of which would be reflected in the Borg CR10 score.
For the assessment of dyspnoea by TDI scale, the difference between indacaterol and placebo on Day 14 (3.33 units) was in excess of the 1 unit that is recognised as clinically meaningful (Citation20). This improvement in dyspnoea (TDI) was accompanied by an improvement of 182 mL in resting IC after 14 days of treatment with indacaterol compared with placebo, together with significant improvement (p < 0.05) in trough FEV1 of 145 mL. These results are consistent with a previous study that showed that a single dose of indacaterol demonstrated greater improvements in both resting IC and FEV1 compared with twice daily formoterol (Citation5), and confirm the 24-h bronchodilator efficacy of indacaterol in patients with COPD. Likewise, in the indacaterol treatment groups, there were improvements in a range of volume measures including FVC, VC and RV. Moreover, the use of indacaterol was also associated with reductions in FRC and reciprocal increase in resting IC.
The results of our study are also in line with a previous double-blind, placebo-controlled, parallel-group study with tiotropium, a once-daily long-acting anticholinergic, wherein 42 days of treatment with tiotropium was associated with sustained reductions of lung hyperinflation at rest and during exercise (Citation8). The authors of that study hypothesised that increased IC with tiotropium permitted greater expansion of tidal volume and therefore contributed to significant improvements in dyspnoea and exercise endurance (Citation8).
With respect to the safety results, indacaterol was well tolerated with a good overall safety profile, consistent with previous studies (Citation21). The frequency of AEs was the same in both groups, all were mild or moderate in severity, and none of the AEs were significant nor were deemed to be study drug related.
In conclusion, indacaterol provided a sustained (over a 14-day period) improvement compared with placebo in IC at both peak and isotime, with significant improvements in resting spirometry measures, TDI scores, dyspnoea, and exercise endurance.
DECLARATION OF INTEREST
This study was funded by Novartis Pharma AG, Basel, Switzerland. Sanjeev Khindri and Anton Franz Drollmann are employees of Novartis. Kai-Michael Beeh has received compensation for serving on advisory boards for Novartis. He has participated as speaker in scientific meetings or courses organized and financed by various pharmaceutical companies (AstraZeneca, Boehringer, Novartis, Pfizer) in 2005–2010. The institution where Kai-Michael Beeh is currently employed has received compensations for design and performance or participation in single or multi-centre clinical trials in 2005–2010 from several companies (Altana, AstraZeneca, Boehringer Ingelheim, Cytos, Novartis, GSK, Revotar Biopharmaceuticals, Almirall Prodesfarma, Merck Sharp & Dohme, Fujisawa, Pfizer, Medapharma). Frank Wagner has no relevant conflicts of interest or financial disclosure. All authors contributed to the development of the manuscript, and approved the final version for submission.
ACKNOWLEDGMENTS
The authors thank the patients who took part in the study and the staff at the participating clinical centres. The authors thank Yogeeta Maju professional medical writer (Novartis) and David Young (Novartis) for their assistance in preparing the manuscript.
REFERENCES
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for Diagnosis, Management, and Prevention of COPD (updated 2009). Available at http: //www.goldcopd.com/ (accessed 14 Jan 2010).
- Cazzola M, Matera MG, Lotvall J. Ultra long-acting beta 2-agonists in development for asthma and chronic obstructive pulmonary disease. Expert Opin Investig Drugs 2005; 14: 775–783.
- Feldman G, Siler T, Prasad N, Jack D, Piggott S, Owen R, Higgins M, Kramer B. Efficacy and safety of indacaterol 150 mcg once-daily in COPD: a double-blind, randomised, 12-week study. BMC Pulm Med 2010; 10:11.
- Dahl R, Chung KF, Buhl R, Magnussen H, Nonikov V, Jack D, Bleasdale P, Owen R, Higgins M, Kramer B, on behalf of the INVOLVE (indacaterol: value in COPD pulmonary disease: longer term validation of efficacy and safety) study investigators. Efficacy of a new once-daily LABA, indacaterol, versus the twice-daily LABA, formoterol, in COPD. Thorax 2010; 65:473–479.
- Beier J, Beeh KM, Brookman L, Peachey G, Hmissi A, Pascoe S. Bronchodilator effects of indacaterol and formoterol in patients with COPD. Pulm Pharmacol Ther 2009; 22:492–496.
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease NHLBI/WHO workshop report. Bethseda, National Heart, Lung, and Blood Institute. April 2001(updated 2005). Available at www.gold.copd.com (accessed 14 Jan 2010).
- Roca J, Whipp BJ, Agusti AGN, Anderson SD, Casaburi R, Cotes JE, Donner CF, Estenne M, Folgering H, Higenbottam TW, Killian KJ, Palange P, Patessio A, Prefaut C, Sergysels R, Wagner PD, Weisman I. Clinical exercise testing with reference to lung diseases: indications, standardization and interpretation strategies. ERS Task Force on Standardization of Clinical Exercise Testing. European Respiratory Society. Eur Respir J 1997; 10:2662–2689.
- O'Donnell DE, Fluge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, Make B, Magnussen H. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004; 23:832–840.
- Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest 1984; 85:751–758.
- Witek TJ, Jr., Mahler DA. Meaningful effect size and patterns of response of the transition dyspnea index. J Clin Epidemiol 2003; 56:248–255.
- Witek TJ, Jr., Mahler DA. Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur Respir J 2003; 21:267–272.
- Borg GAV. Psychophysical basis of perceived exertion. Med Sci Sports Exerc 1982; 14:377–381.
- Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J; ATS/ERS Task Force. General considerations for lung function testing. Eur Respir J 2005; 26:153–161.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J; ATS/ERS Task Force. Standardisation of spirometry, Eur Respir J 2005; 26:319–338.
- Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Hankinson J, Jensen R, Johnson D, MacIntyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes Eur Respir J 2005; 26: 511–522.
- Diaz O, Villafranca C, Ghezzo H, Borzone G, Leiva A, Milic-Emil J, Lisboa C. Role of inspiratory capacity on exercise tolerance in COPD patients with and without tidal expiratory flow limitation at rest. Eur Respir J 2000; 16:269–275.
- Belman MJ, Botnick WC, Shin JW. Inhaled bronchodilators reduce dynamic hyperinflation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996; 153:967–975.
- O'Donnell DE, Lam M, Webb KA. Measurements of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 158:1557–1565.
- O'Donnell DE, Sciurba F, Celli B, Mahler DA, Webb KA, Kalberg CJ, Knobil K. Effect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPD. Chest 2006; 130:647–656.
- Mahler DA, Witek TJ Jr. The MCID of the transition dyspnea index is a total score of one unit. COPD 2005; 2:99–103.
- Rennard S, Bantje T, Centanni S, Chanez P, Chuchalin A, D'Urzo A, Kornmann O, Perry S, Jack D, Owen R, Higgins M. A dose-ranging study of indacaterol in obstructive airways disease, with a tiotropium comparison. Respir Med 2008; 102:1033–1044.