Abstract
A few previous studies have reported that the patients with chronic obstructive pulmonary disease (COPD) have a 29.1% to 36.8% frequency of restless legs syndrome (RLS). In this study, we observed RLS symptoms in patients experiencing COPD exacerbation to better understand the relationship between the many clinical parameters of COPD and the presence of RLS and to attract the attention of specialists on the association between the two conditions. Twenty-two male patients in COPD exacerbation; 17 healthy individuals were evaluated in this study. The patients were evaluated using the 2003 RLS symptom criteria outlined by the International Restless Legs Syndrome Study Groups (IRLSSG). The Pittsburgh Sleep Quality Index and Epworth daytime sleepiness scale were used to assess the sleep quality of patients. The RLS symptoms were correlated with blood levels of laboratory and clinical parameters. Statistical analyses were performed using SPSS 17.0 statistical software packet. The Pittsburgh Sleep Quality Index and Epworth daytime sleepiness scale scores were increased in COPD patients and correlated significantly with RLS symptoms. It was found that 54.5% of COPD patients with acute exacerbations were observed to have RLS symptoms. The Pittsburgh Sleep Quality Index was significantly higher in COPD patients with RLS symptoms compared to COPD patients without RLS symptoms (p < 0.05). We did not observe any significant difference in the previously reported metabolic and clinical parameters associated with RLS in COPD patients with and without RLS. RLS symptoms increase during COPD exacerbation and lead to decreased sleep quality.
Keywords: :
INTRODUCTION
Restless legs syndrome is a common neurological movement disorder that may be undiagnosed and untreated. There have been several prevalence studies of RLS in different populations. The estimated affected adult population is reported to be 7.2–11.5% in France, 9.71% in some geographic areas of Anatolia, 10% in the USA and 5–12% in Europe and North America (Citation1–4).
Restless legs syndrome may occur as an idiopathic, often hereditary, condition (primary RLS), or in association with several medical conditions (secondary RLS) such as nutritional deficiencies (iron, vitamin B12, folate), end stage renal diseases, pregnancy, rheumatologic disorders, diabetes as well as neurological conditions such as Parkinson's disease, spinal cord lesions, multiple sclerosis, and polyneuropathy (Citation5–14).
The current understanding of the pathophysiology of RLS suggests that there is involvement of three interrelated components: dopaminergic dysfunction, impaired iron hemostasis and genetic mechanisms. Enhanced circadian variations in dopamine activity have been demonstrated in those with RLS compared with control patients. Iron content in the substantia nigra and putamen were lower in patients with RLS than in control patients. Alterations in iron metabolism can influence dopamine signaling. For example, iron is a cofactor in tyrosine hydroxylase that can limit the rate of the enzymatic conversion of tyrosine to dopamine (Citation5, 6, Citation15–17).
One of the main causes of RLS may be an imbalance between the dopaminergic agonist system and thyroid hormones (Citation18). Thyroid hormone levels are related to dopamine levels. The negative regulatory effect of dopamine on thyroid hormones was shown to be related to the appearance of RLS symptoms (Citation19). Thyroid-releasing hormone (TRH) regulates TSH synthesis by stimulating the transcription and translation of the TSH-β-subunit gene, and dopamine inhibits this process (Citation20). Anti-dopaminergic drugs aggravate the symptoms of RLS, while dopamine agonists diminish RLS symptoms (Citation21, 22). Reduced iron levels decrease dopamine and aggravate RLS symptoms. There is a relationship between thyroid hormones and lack of sleep and RLS (Citation23).
An increase in thyroid hormones during sleep causes sleep deprivation and sleep deprivation increases RLS, which can itself, contribute to sleeplessness (Citation23–25). Another mechanism by which thyroid hormone is modulated by dopamine system is via increased activity of the cytochrome P450 enzymes. These enzymes are important for the degradation of thyroid hormones. P450 enzymes are diminished by low levels of iron (Citation18). Thyroid function impairments can be detected in conditions involving extra-thyroidal dysfunction, particularly in COPD with acute respiratory failure.
Euthyroid sick syndrome is observed in a variety of clinical conditions such as COPD, cardiovascular disease, diabetic ketoacidosis, renal failure, liver diseases, sepsis etc. There is a strong correlation between the TT3/TT4 ratio and Pa02. Hypoxemia seems to be a determinant of the peripheral metabolism of thyroid hormones (Citation26, 27). Despite complaints of poor sleep being common in patients with COPD, there are few reports related to RLS symptoms. The studies that do exist reported that RLS was significantly more common in COPD patients than the controls (36.8% vs. 11%; p<0.001). There was increased frequency in late stage COPD patients (Citation28, 29).
COPD is a systemic disorder in which sleep disorders, hypoxic and hypermetabolic processes may effect extra-pulmonary organs, particularly during COPD exacerbations. The current understanding of RLS symptoms in COPD patients and the underlying mechanisms are obscure. The aim of this study is to investigate the RLS symptoms in COPD patients during an exacerbation period. RLS criteria are based on the diagnostic standards of the International Restless Legs Syndrome Study Group (Citation30). We would like to emphasize the association of the two conditions and to present to the attention of specialists.
SUBJECTS AND METHODS
This retrospective study was done in the Istanbul Yedikule Thoracic Diseases and Surgery Center in 2011. A written informed consent was obtained from all individuals and the study is conducted in compliance with the approval of the institutional ethical committee. Twenty-two male patients that were hospitalized with a diagnosis of acute COPD exacerbation that were in their 3rd–5th days of stay in the hospital are included in the study. A thorough physical examination was performed prior to the assessment of arterial gases for all patients by expert pulmonologist physicians trained in sleep disorders.
All included COPD patients were evaluated using the GOLD guide and were diagnosed with very severe stage (stage 4-FEV1 30% < or 50% < plus chronic respiratory failure; mean Forced Expiratory Volume 1 (FEV1) was 39.38 ± 9.97%). COPD exacerbation was identified according to Anthonisen's Winnipeg criteria, which define an acute exacerbation as a sustained, worsening dyspnea, cough or sputum production leading to an increased use of maintenance medications or the addition of supplemental drugs, usually for at least 2 consecutive days (Citation31, 32). These subjects were able to answer all study questions and did not exhibit any complications during the treatment. In relevant questions, some of the answers were also confirmed by the sleeping partners where applicable.
All of the patients were receiving inhaled steroid, beta agonist, parenteral theophylline as well as long-term oxygen and non-invasive mechanic ventilation treatment according to their disease status. Patients that had good response to treatment and who were able to answer study questions were included in the study.
All other conditions that may be associated with other causes of RLS are ruled out. Patients with diabetes, renal insufficiency, neurological disorders, periferic vascular disease, connective tissue disorders, secondary causes of anemia, endocrine and psychiatric disorders as well as the subjects that had seek for professional help due to complaints associated with RLS syndrome before COPD are excluded from the study. Also, those patients who were not able to answer the questions due to COPD symptoms or mental problems were excluded.
The healthy control group consisted of age-matched non-smoker voluntary men admitting to the internal medicine outpatient clinic for general check-up purposes without any complaints or diagnosis. The above-mentioned exclusion criteria were also implied to the controls, validating with lab studies where applicable. These selective criteria restricted the size of the subject group.
The diagnosis of the restless legs syndrome was made according to International Restless Legs syndrome Study Groups (IRLSSG), 2003 criteria:
Urge to move legs usually accompanied or caused by uncomfortable or unpleasant sensations in the legs (urge to move may not be accompanied by uncomfortable sensations, and arms or other body parts may be involved);
The urge to move or unpleasant sensation begins or worsens during periods of rest or activity;
The urge to move or unpleasant sensation is partially or totally relieved by movement (such as walking or stretching) as long as the activity continues;
The urge to move or unpleasant sensation worsens in the evening or at night; in very severe cases, the worsening at night may not be noticeable, but must have been previously present (Citation33).
As there was no validated Turkish RLS diagnostic questionnaire, a questionnaire based on the above IRLSSG, 2003 criteria were prepared. The exact description of the unpleasant symptoms is asked from each subject. Modifications are included specific questioning for the ruling out of other conditions that may mimic RLS; numbness was queried further for further characterization and peripheral neuropathy, radiculopathy, arthritic pain, periodic cramps and such diseases in exclusion criteria such as diabetes, renal insufficiency are further questioned. The initiation and the aggregation of the symptoms are asked. Although prevalence studies are lacking in the Turkish population, the questionnaire was also applied to the healthy controls group for assess- ment.
Table 1 Comparison of Healthy Controls and COPD patients with exacerbation
Table 2 Other features of COPD patients
Individuals in both the COPD and control groups that answered “yes” to all RLS criteria (RLS 1, 2, 3, 4) were diagnosed with RLS. In addition, the relationship between all RLS criteria and the relationship between RLS criteria and other variables were further investigated. After the diagnosis of Restless Legs Syndrome, the Pittsburgh Sleep Quality Index for sleep quality and disturbances (PSQI) and Epworth Sleepiness Scale (ESS) for the level of daytime sleepiness were given to patients and the healthy controls. The stability of these scales is validated by others (Citation34).
The patients that are admitted to the study are evaluated for the arterial blood gas levels (PaO2, PaCO2, and saturated O2) and for the clinal chemistry tests such as glucose, urea, creatinine, hematocrit, hemoglobin, iron, iron binding, ferritin, folic acid, vitamin B12, fT3, fT4, TSH. In addition, on the day of discharge from the hospital, a pulmonary function test was administered to determine the clinical stage according to GOLD.
Statistical evaluation
The values were presented as mean±SD, median (IQR), frequency and percentage. Normal distribution was assessed using the Shapiro-Wilk test, leaf & steam and by drawing histograms. When comparing two groups, variables that were normally distributed were compared using an independent sample t-test, while non-normally distributed variables was examined using a Mann–Whitney U-test. Nominal variable values were evaluated using the Chi-square test, and correlations were determined using the Spearman correlation test. Analyses were performed using SPSS 17.0 statistical software. The statistical significance value was set at p < 0.05.
RESULTS
The mean age of the COPD patient group was 58.13±10.41 and the body mass index (BMI) was 23.44 ± 5.78 (n = 22), while the mean age of the control group was 54.35 ± 7.33 and the BMI was 26.42±4.58 (n = 17). The BMI of the control group was significantly higher than that of the COPD patient group (p < 0.001) (). The control group consisted of non-smokers. All of the individuals experiencing acute exacerbation of COPD were ex-smokers. The mean time period since smoking cessation was 5.38±7.99 years, while the mean duration of smoking was 48.30±42.86 years. The mean time since the first complaint of dyspnea (disease duration) was 17.36 ± 18.09 years (). The first initiation of RLS symptoms more than 1 month was denied by the most of the patients. The only, 18.1% of patients had rarely RLS symptoms more than one months (n = 4).
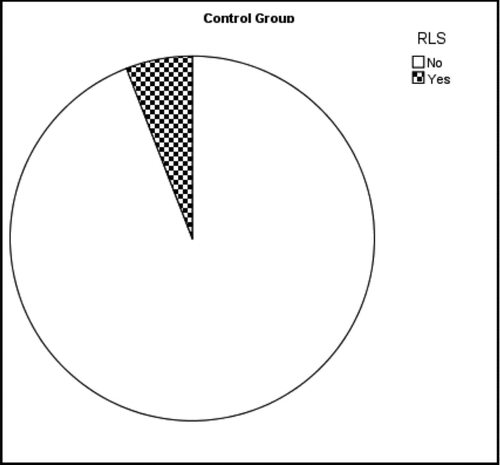
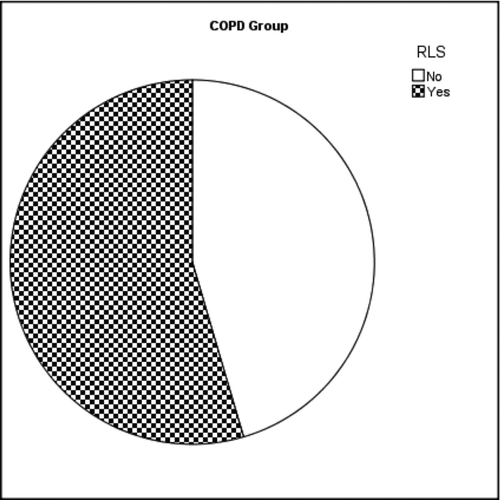
The patient group with COPD exacerbation had significantly higher RLS symptoms compared to the control group (54.5% in COPD patient group (n = 12) and 5.9% in control group (p = 0.001) (Graphs , ). There was a positive correlation among all of the RLS symptom criteria (Significances in binary comparison vary between rs = 0.911, p = 0.01 and rs = 1, p = 0.01). When RLS symptoms criteria were compared with other parameters surprisingly a correlation was observed between the time passed since the observation of first dyspnea symptoms (disease duration) and presence of RLS symptoms (rs = 0.643, p = 0.001).
When the clinical chemistry results were analyzed, no significant difference was observed in serum iron, iron binding capacity, ferritin and vitamin B12 levels between COPD exacerbation patients and the control group (p > 0.05). Although folate levels in the COPD exacerbation patient group were within the normal range, they were lower than the control group (p < 0.001). Likewise, when thyroid function test results were analyzed, levels of fT3 and TSH were observed to be lower in COPD exacerbation patients compared to the control group (p = 0.001 and p = 0.026, respectively).
Conversely, the levels of fT4 in COPD exacerbation patients were higher than that of the control group (p = 0.003). The fT4 levels were negatively correlated with the Epworth sleepiness scale (ESS) (rs = -0.481, p = 0.043) (). When the parameters of the COPD exacerbation patients with RLS symptoms (RLS (+)) were compared to those without RLS symptoms (RLS (-)) as a whole no significant difference could be detected in iron metabolism, thyroid function and arterial blood gas. However, the Pittsburgh Sleep Quality Index was higher in patients with RLS symptoms compared to patients without RLS symptoms (COPD RLS (+): 7.76 ± 3.74, COPD RLS (-): 3.44 ± 2.18, p < 0.05). The average PSQI was 1.47±1.46 in the control group.
DISCUSSION
There is a known association between sleep quality deterioration in COPD and various sleep disorders. Although, the prevalence of the obstructive sleep apnea is 11.9% in COPD patients (Citation35), it is hard to diagnose RLS unless it is not specifically questioned especially in patients that are experiencing exacerbation of COPD. There are limited studies reported in literature concerning RLS in COPD patients and particularly in exacerbated COPD. Recently a prevalence study performed on the general population in Sweden and Iceland points to the association between RLS and various health parameters. In this study, a higher prevalence of respiratory symptoms and airway obstruction was reported in individuals with RLS symptoms (Citation36). Lo Coco et al., reported higher frequency of RLS in COPD patients (36.8%) than in the control group (11%), (p < 0.001) (Citation28). Compared to these studies, we determined a higher frequency of RLS symptoms in COPD patients with acute exacerbations (54.5%).
Compared to the previously reported observations which were conducted on COPD patients without exacerbations, this study was focused on patients with exacerbations which was lacking in the literature. In another study on COPD patients by Kaplan et al., the RLS prevalence was found 29.1% and was accompanied by severe hypoxemia/hypercapnia and an increased RLS frequency was observed in late stage patients (Citation28, 29). There was a significant correlation between the time passed since the emerging of the first dyspnea symptoms (disease duration) and the presence of RLS in our study. However, in this study the correlation between hypoxia/hypercapnia and RLS could not be evaluated mainly due that all of the patients were experiencing hypoxia /hypercapnia secondary to the exacerbations.
In a study by Henning et al., the RLS diagnostic criteria had been assessed to be 84% for specificity, 100% for sensitivity. The positive predictive value of the 4 criteria alone was reported to be 76%. They emphasize that the 4 diagnostic criteria is necessary for the first step of diagnosis. This is in line with the aim of our study, which is to accomplish an initial evaluation of the RLS at the respiratory clinics (Citation37). Non-standardized electrodiagnostic investigations such as polysomnogram or suggested immobilization test may provide additional objective data (Citation38), but these investigations may not be applicable to hypoxic patients during COPD exacerbations.
The selection of the healthy voluntaries as the control population, aimed to compensate for the lack of the population-specific, large-scale validation of the 4 diagnostic criteria. Questions are added to the query that defines specific symptoms such as numbness for the clarification of any potential neuropathies. Further electrophysiological studies could not be performed due to the dyspneic condition of the patients.
The further ruling out of any primary neuropathies was done depending on the queries. Although Nineb et al. found that a patient complaining with RLS also frequently presents symptoms suggestive of peripheral neuropathy, Kaplan et al. also demonstrated that polyneuropathy is common in COPD patients and is the strongest predictor of severe RLS (Citation29, Citation39). Further differential diagnosis of any other underlying cause is required in severe RLS cases where medical treatments are to be considered.
In the two previous reports of the Sweden-Iceland (Citation36) study and Lo Coco et al., it was shown in COPD patients that the ESS score was higher in cases with RLS symptoms (Citation28, Citation30). Similarly, our study confirms the correlation between the RLS symptoms and ESS scores. Moreover, COPD patients with RLS had marked deterioration in sleep quality. The Pittsburgh sleep quality index (PSQI) score was significantly higher in COPD patients with RLS symptoms when compared to the ones without RLS. PSQI and ESS scores were also correlated. It was previously shown that deterioration of sleep quality is accompanied by worsening of the quality of life in COPD patients (Citation40).
In the current study, the COPD group had a higher PSQI score than the control group. The PSQI is consisted of 7 scores. Each score is ranged from 0 (no difficulty) to 3 (severe difficulty). A PSQI global score >5 is considered to be suggestive of significant sleep disturbance (Citation41) while the control group exhibited a score mean 1.47±1.46 that is within the normal limits. In addition, COPD patients with RLS symptoms had a higher PSQI score than ones without RLS symptoms. This indicates that RLS is an additional factor involved in sleep quality deterioration in COPD. Furthermore, increased BMI correlated with an increase in PSQI and ESS. In the Sleep Heart Health Study the prevalence of moderate to severe OSA was 3-folds higher in the highest quartile of body mass index (BMI), relative to the lowest (Citation42).
In terms of the clinical chemistry, free iron, folate and vitB12 are suspected factors with a possible influence on RLS (Citation43). Free iron and vitB12 levels exhibited no difference between COPD patients with or without RLS or controls. Interestingly, we observed significantly lower folate levels in the COPD patients compared to controls; however the measurements were within the reference range. No difference was observed between COPD patients with or without RLS.
Hypermetabolism is frequently observed in COPD patients; increased energy consumption is seen at rest and during physical exercise that in some cases it may exhibit as cachexia (Citation44). Previous studies demonstrated lower folate levels in COPD patients. Additionally, an association between lower folate intake and severe dyspnea was previously established (Citation45). However, all of the patients enrolled in our study were within normal BMI range. Future directed studies are needed on selected groups of COPD patients with different BMI and various clinical presentations to further elucidate the role of folate in COPD with RLS.
There is no consensus in the literature on the status of the thyroid functions in COPD patients. Controversial observations report solely increased T4 or T3 levels or alterations in T3, T4 and TSH (Citation27, Citation44). In our study, we found lower levels of fT3 and TSH in COPD exacerbation patients. Conversely, fT4 levels were higher in COPD. However no difference was observed during COPD exacerbation with and without RLS patients.
The change in thyroid hormone levels in COPD might play a role in development of RLS. The relationship between thyroid hormone and RLS was shown by Tan et al. L-thyroxin replacement treatment in hypothyroid patients may be complicated by RLS, which is related to low iron levels. In another study by Tan et al., no significant differences were found with regards to RLS when comparing patients with thyroid diseases to a healthy control group. In cases where RLS symptoms were detected, the symptoms of RLS were decreased when thyroid disorders were treated (Citation46, 47). However, there are no studies addressing thyroid hormone levels in COPD patients with RLS symptoms. We demonstrated that the fT4 levels were negatively correlated with the Epworth sleepiness scale (ESS).
The dopamine pathway is known to influence RLS symptoms and there is evidence showing that dopamine depresses the thyroid axis. Pereira et al proposed that there is a similarity between the daily circadian rhythm of TSH and the rhythms of RLS. Deviations in the thyroid axis might lead to RLS as well as other sleep disorders (Citation18). Future studies are needed to investigate the association between the daily thyroid circadian rhythm and RLS symptoms in COPD patients.
The overall therapeuticals that are administered in COPD treatment should also be considered in regards of RLS symptoms. Although no study addresses the relationship of COPD medications and RLS. However, the anxiety (nervousness), restlessness sensation and sleepness are among the known adverse effects of theophylline. The effects of the many medications used in treatment of COPD should also be considered. Pharmacologically induced/exacerbated restless legs syndrome (RLS) are increasingly recognized in clinical sleep medicine, especially associated with antidepressant medications (Citation48).
In this study we show that the RLS symptoms during COPD exacerbation was higher than controls, and the frequency was also higher than the stable COPD cases reported previously. RLS can be an additional factor involved in sleep quality deterioration in COPD. Patients in severe and late-stage COPD have more risk for RLS. Future studies on RLS in COPD should focus on the relationship of daily levels and the rhythm of thyroid hormones, folate levels and the effects of the medications administered to the patients. We believe this study will aid to increase the awareness of the RLS in COPD exacerbation.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Allen RP, Walters, AS, Montplaisir J, Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med 2005; 165:1286–1292.
- Ghorayeb I, Tison F. Epidemiology of restless legs syndrome. Rev Neurol (Paris) 2009; 165:641–649.
- Erer S, Karli N, Zarifoglu M, Ozcakir M, Yildiz D. The prevalence and clinical features of restless legs syndrome: a door to door study in Orhangazi, Bursa in Turkey. Neurol India 2009; 57:729–733.
- Philips B, Hening W, Britz P, Mannino D. Prevalence and correlates of restless legs syndrome: result from 2005 National Sleep Foundation Poll. Chest 2006; 129:76–80.
- Brindani F, Vitetta F, Gemignani F. Restless legs syndrome: differential diagnosis and management with pramipexole. Clin Intervent Aging 2009; 4:305–313.
- Early CJ, Connor JR, Beard JL, Malecki EA, Epstein DK, Allen RP. Abnormalities in CSF concentrations of ferritin and transferring in restless legs syndrome. Neurology 2000; 54:1698–1700.
- Lee KA, Zaffke ME, Baratte-Beebe K. Restless legs syndrome and sleep disturbances during pregnancy: the role of folate and iron. J Womens Health Gen Based Med 2001; 10:335–341.
- Manconi M, Govoni V, De Vito A, Restless legs syndrome and pregnancy. Neurology 2004; 63:1065–1069.
- Winkelmann JW, Chertow GM, Lazarus JM. Restless legs syndrome in end-stage renal disease, Am J Kidney Dis 1996; 28:372–378.
- Quinn C, Uzbeck M, Saleeem I, Cotter P, Ali J, O'Malley G, Gilmartin JJ, O'Keeffe ST. Iron status and chronic kidney disease predict restless legs syndrome in an older population. Sleep Med 2011; 12:295–301.
- Gemignani F, Brindani F, Marbini A. Restless legs syndrome and polyneuropathy in rheumatologic diseases. Semin Arhritis Rheum 2009; 38:55–62.
- Gemignani F, Brindani F, Marbini A. Restless legs syndrome and diabetic neuropathy. Sleep 2008; 31:307.
- Iranzo A, Comella CL, Santamaria J, Oertel W. Restless legs syndrome in Parkinson's disease and neurodegenerative diseases of the central nervous system, Mov Disord 2007; 22 Suppl 18: S424–S430.
- Manconi M, Ferini-Strambi L, Filippi M, Multicenter case-control study on restless legs syndrome in multiple sclerosis: the REMS study. Sleep 2008; 31:944–952.
- Winkelman JW. Considering the causes of RLS. Eur J Neurol 2006; 3:8–14.
- Trotti LM, Bhadriraju S, Rye DB. An update on the pathophysiology and genetics of restless legs syndrome. Curr Neurol Neurosci Rep 2008; 8:281–287.
- Early CJ, Hyland K, Allen RP. Circadian changes in CSF dopaminergic measures in restless legs syndrome. Sleep Med 2006; 7:263–268.
- Preira Jr. JC, Pradella , Hallinan M, Pessoa HL. Imbalance between thyroid hormones and dopaminergic system might be central to the pathophysiology of restless legs syndrome: a hypothesis. Clinics 2010; 65:547–554.
- Scalon MF, Weetman AP, Lewis M, Pourmand M, Rodrigues A, Weightman DR. Dopaminergic modulation of circadian thyrotropin rhythm and thyroid hormone levels in euthyroid subjects. J Clin Endocrinol Metabol 1980; 51:1251–1256.
- Shupnik MA, Greenspan SL, Ridgway EC. Transcriptional regulation of thyrotrophin-releasing hormone and dopamine in pituitary cell culture. J Biol Chem 1986; 261:675–679.
- Wojcikowski J, Gocen Biowska K, Wladystawa AD. Regulation of liver cytochrome P450 in brain dopaminergic system. Biochem Pharmacol 2008, 76:258–267.
- Walters A, Ondo WG, Dreykluft T, Grunstein R, Lee D, Sethi K. Ropinirole is effective in the treatment of restless legs syndrome: TREAT RLS 2: a 12- week, double-blind, randomized, parallel-group, placebo-controlled study. Mov Disord 2004; 19:1414–1423.
- Parker DC, Rossman LG, Pekary AE, Effect of 64-hour sleep deprivation on circadian waveform of thyrotropin (TSH): further evidence of sleep-related inhibition of TSH release. J Clin Endocrinol Metab 1987; 64:157–161.
- Early C. Clinical practice, Restless legs syndrome. N Engl J Med. 2003; 348:2103–2109.
- Allen R. Dopamine and iron in pathophysiology of restless legs syndrome. Sleep Med 2004; 4:385–391.
- Camacho PM, Dwarkanathan AA. Sick euthyroid syndrome. What to do when thyroid function tests are in critically ill patients. Postgrad Med 1999; 105:215–219.
- Karadag F, Ozcan H, Karul AB, Yilmaz M, Cildag O. Correlates of non-thyroidal illness syndrome in chronic obstructive pulmonary disease. Respir Med 2007; 101:1439–1446.
- Lo Coco D, Mattaliano A, Lo Coco A, Randisi B. Increased frequency of restless legs syndrome in chronic obstructive pulmonary disease patients. Sleep Med 2009; 10:572–576.
- Kaplan Y, Inonu H, Yilmaz A, Ocal S. Restless legs syndrome in patients with chronic obstructive pulmonary disease. Can J Neurol Sci 2008; 35:352–357.
- Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless Legs Syndrome Diagnosis and Epidemiology workshop at the National Institutes of Health; International Restless Legs Syndrome Study Group, Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health, Sleep Med 2003; 4:101–119.
- Rabe KF, Hurd S, Anzueto A, at al. Global stratedy for diagnosis, management and prevention of chronic obstructive pulmonary disease: GOLD executive summary, Am J Respir Crit Care Med 2007; 176:532–555.
- Intyre NM, Huang YC. Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proc Amer Thorac Soc 2008; 5:530–535.
- Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med 2003; 4:101–119.
- Knutson KL, Rathouz PJ, Yan LL, Liu K, Lauderdale DS. Stability of Pittsburgh Sleep Quality Index and the Epworth Sleepness Questionnaires over 1 year in early middle-aged adults: the CARDIA study. Sleep 2006; 29:1503–1506.
- O'Brien A, Whitman K. Lack of benefit of continuous positive airway pressure on lung function in patients with overlap syndrome. Lung 2005; 183:389–404.
- Benediktsdottir B, Janson C, Lindeberg E, Arnardottir ES, Olafsson I, Cook E, Thorarinsdottir EH, Gislason T. Prevalence of restless legs syndrome among adults in Iceland and Sweden: Lung function, comorbidity, ferritin, biomarkers and quality of life. Sleep Med 2010; 11:1043–1048.
- Hening W, Allen R, Washburn M, Lessage S, Cristopher JE. The four diagnostic criteria for Restless Legs Syndrome are unable to exclude confounding conditions (“mimics”). Sleep Med 2009; 10:976–978.
- Michaud M, Paquet J, Lavigne G, Desautels A, Montplasir J. Sleep laboratory diagnosis of restless legs syndrome. Eur Neurol 2002; 48:108–113.
- Nineb A, Rosso C, Dumurgier J, Nordine T, Lefaucheur JP, Créange A. Restless legs syndrome is frequently overlooked in patients being evaluated for polyneuropathies. Eur J Neurol 2007; 14:788–792.
- Scharf SM, Maimon N, Bernard-Scharf BJ, Reuveni H, Tarasiuk A. Sleep quality predicts quality of life in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2011; 6:1–12.
- Buysse, DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index (PSQI): A new instrument for psychiatric research and practice. Psych Res 1989; 28:193–213.
- Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, Predictors of sleep-disordered breathing in community dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002; 162:893–900.
- Lyn P. Restless legs syndrome: Pathophysiology and the role of iron folate. Altern Med Rev 2007; 12:101–112.
- Creutzberg EC, Casaburi R. Endocrinological disturbances in chronic obstructive pulmonary disease. Eur Respir J 2003: 22: Suppl. 46:76–80.
- Hirayama F, Lee AH, Terrasawa K, Kagawa Y. Folate intake associated with lung function, breathlessness and the prevalence of chronic obstructive pulmonary disease. Asia Pac J Clin Nutr 2010; 19:103–109.
- Tan EK, Ho SC, Koh L, Pavanni R. An urge to move with L-thyroxine: clinical, biochemical, and polysomnographic correlation. Mov Disord 2004; 19:1365–1367.
- Tan EK, Ho SC, Eng P, Loh LM, Kohl L, Lum SY, Teoh ML, Yih Y, Khoo D. Restless legs symptoms in thyroid disorders. Parkinson Relat Disord 2004; 10:149–151.
- Hoque R, Chesson AL. Pharmacologically induced/exacerbated restless legs syndrome, periodic limb movements of sleep, and REM behaviour disorder/REM Sleep without Atonia: Literature review, qualitative scoring, and comparative analysis. J Clin Sleep Med 2010; 6:79–83.