Abstract
Quadriceps weakness is associated with a poor prognosis in COPD. Several data suggest that immobility and muscle wasting may be related through up-regulation of the p38 Mitogen associated protein kinase (MAPK) signalling pathway. We therefore hypothesised that this might occur in the quadriceps of COPD patients. We studied 105 stable COPD outpatients (mean FEV1 43.9% predicted) and 27 age and gender matched controls. We measured fat-free mass, quadriceps strength and daily physical activity using triaxial accelerometry. A quadriceps biopsy was obtained in which components of the p38-MAPK signalling pathway were examined. Patients had reduced fat-free mass index (16.1 (2.2) kg/m2 vs 17.2 (2.2) kg/m2, p = 0.02) and exhibited quadriceps weakness (mean (SD) maximal voluntary contraction force 72.1% (18.7) predicted and 82.3% (7.1) predicted for patients and controls respectively, p = 0.01). Physical activity was significantly reduced in the patient group; in particular mean (SD) locomotion time was 101 (Citation) minutes/12 hours in controls and 48 (Citation) minutes in patients (p < 0.0001). However, in biopsies obtained from these patients, no differences were observed for total or phosphorylated HSP27 or p38 MAPK protein or p38 MAPK mRNA (MAPK14); of the downstream products both GADD45β and c-jun mRNA were reduced in COPD patients while c-myc was not different from controls. No parameter correlated with physiological data within the patient group. We conclude that, despite the presence of reduced fat-free mass, quadriceps weakness and inactivity, p38 MAPK signalling was not up-regulated in skeletal muscle of stable out-patients with COPD.
Introduction
Skeletal muscle wasting is a common feature of chronic obstructive pulmonary disease (COPD) (Citation1) and is an important predictor of mortality, independent of impairment in lung function (Citation2, 3), but the molecular mechanisms that contribute to the wasting remain to be identified.
Activation of p38 MAPK is a potential candidate mechanism underlying skeletal muscle weakness in COPD for three reasons. First p38 MAPK is believed to be up-regulated in airways disease in general, thus recent studies showed that activation of p38 MAPK occurs in lung parenchyma of patients with asthma (Citation4), and that there was increased expression and activation of p38 MAPK in alveolar macrophages in COPD patients (Citation5). Intriguingly Liu et al. recently reported that cigarette smoke induces muscle atrophy through up-regulation of p38 MAP kinase signalling as well as the downstream mediators JNK and ERK (Citation6).
Second, patients with COPD are well recognised to be more inactive than age matched control subjects (Citation7, 8) and it is known, in skeletal muscle, that immobility can activate p38-MAPK signalling. For example, the expression and activity of p38 MAPK (marked by the abundance of phosphorylated p38 MAPK) increases after 10 days to 3 weeks of immobilisation in a rat hind limb model of acute muscle wasting (Citation9, 10), and, of note the recovery associated with remobilisation was attenuated in aged (compared with younger rats) (Citation11).
Similarly, Machida and Booth (Citation12), also using a hindlimb suspension model in rodents were able to show that acute and complete immobilisation resulted in increases in p38 MAPK phosphorylation and of increased expression of downstream targets such as Growth-arrest and DNA damage-inducible gene (GADD) 45β. Consistent with the ability of these factors to signal to the atrophy machinery in muscle cells in vitro studies have reported that in the muscle cell line (C2C12), TNFα increases the expression of the ubiquitin ligase atrogin/MAFbx via a p38 MAPK dependent pathway (Citation13).
Finally, p38 MAPK is a stress activated kinase and responds to a range of stimuli, including oxidative stress (Citation14), a factor that is recognised to be present in skeletal muscle of patients with COPD (Citation15, 16), perhaps also as a result of immobility (Citation17).
Few data exist regarding p38 MAP kinase pathway signalling in the quadriceps of patients with COPD, but recent data from a small cohort presented in abstract form (Citation18) suggested that this pathway is related to quadriceps bulk since the highest levels of phospho p38 and JNK were observed in the patients with the lowest mid-thigh cross-sectional area as measured by computerised tomography (CT). We therefore compared the expression of target genes of the p38 MAPK signalling pathway and the relative levels of phospho-p38 MAPK and phospho-HSP27 in patients with stable COPD and age matched controls. We considered this question particularly timely given that p38 MAPK inhibitors are now beginning to enter clinical trials (19–22).
Methods
Subjects
One hundred and five patients with stable Grade I to IV COPD according to the Global Initiative in Obstructive Lung Disease guidelines (Citation23) were recruited from respiratory clinic at the Royal Brompton Hospital. Patients were excluded if they had a diagnosis of heart, renal or liver failure, a systemic inflammatory disorder or metabolic disorder or had experienced a severe exacerbation (i.e., requiring antibiotics, oral steroids, or hospitalisation) in the last 4 weeks. Then, 27 healthy age-matched controls were recruited by advertisement. All subjects gave written informed consent and the protocol was approved by the Royal Brompton & Harefield NHS Trust Research Ethics Committee (Study numbers 06/Q0404/35 and 06/Q0410/54).
Lung function
Resting blood gas tensions with the subject breathing air were measured by taking arterialized capillary earlobe blood samples. Lung volumes using plethysmography, diffusing capacity for carbon monoxide using the single breath technique (CompactLab system, Jaeger, Germany) and post-bronchodilator spirometry were measured according to American/European Respiratory Society guidelines.
Fat-free mass (FFM) and quadriceps strength
Global fat-free mass was calculated with the technique of bioelectrical impedance using the Bodystat 1500 device (Bodystat, Isle Of Man, UK) as described previously by Steiner et al (Citation24). FFM index was calculated by dividing FFM in kg by height in metres squared. Quadriceps strength was determined by measuring supine isometric maximal voluntary contraction (MVC) using a modification of the method described by Edwards et al. (Citation25) and expressed as a percent predicted according to the equations of Seymour et al. (Citation1). The unpotentiated quadriceps twitch tension (Tw Q) elicited by supramaximal magnetic stimulation of the femoral nerve was obtained using the method of Polkey and co-workers (Citation26).
Activity assessment
A tri-axial accelerometer (Dynaport Activity Monitor; McRoberts BV) was worn for 12 hours per day, for 2 days when the subject was doing their usual activities, and locomotion time (in minutes per 12 hours) was averaged as described previously by Pitta et al. (Citation7).
Quadriceps muscle biopsy
Percutaneous needle biopsy of the vastus lateralis in the anterior mid-thigh of the right leg under local anaesthesia was performed according to the technique described by Bergstrom (Citation27). Samples were immediately frozen in liquid nitrogen prior to processing for RNA or protein; unfortunately there was insufficient material to do all the analyses on all the samples; numbers on which the data are based are shown in .
Table 1. Patient characteristics.
Table 2. Protein expression levels. Protein was isolated as described in Methods and the levels of total and phospho p38 MAPK and Hsp27 were determined by Luminex assay.
Assessment of protein levels
Samples were homogenised under liquid N2 and the homogenate was resuspended in NP40 lysis buffer (Tris pH 7.4 (50mM), NaCl (250 mM), EDTA (5mM), 1% Nonidet P40 (Roche Applied Science)) supplemented with protease and phosphatase inhibitor cocktails (Sigma). To determine the protein levels of the phosphorylated and unphosphorylated forms of p38-MAPK and the downstream target HSP27, protein supernatants (1 mg/ml) were analysed by fluorescent bead array using p38-MAPK and HSP27 specific beads (Invitogen) on a Luminex 100 analyzer instrument (Luminex Corp.) according to the manufacturer's recommendation.
Real-time quantitative PCR
RNA was extracted from muscle biopsies using the Qiagen RNeasy® kit (Qiagen, UK), the concentration of RNA was quantified by spectrophotometer (Nanodrop (ND1000, Wilmington USA) and first strand cDNA generated using Superscript® II Reverse Transcriptase (Invitrogen). Primers were designed over intron-exon boundaries to prevent amplification of genomic DNA and to generate a PCR product of 150–200bp. The qPCR analysis was carried out in duplicate on each cDNA sample for every target gene and for the reference genes RPLPO using a 10 μl reaction of SYBR® Green Quantitative RT-PCR Kit (Sigma Aldrich, UK) and the primer pair (2pmol/μl) in 96 well plates (MicroAmp, Fast optical 96-well reaction plate (0.1 ml) (Applied Biosystems, UK), covered by an optical plate cover (MicroAmp, Optical adhesive film (PCR compatible), Applied Biosystems, UK). The qPCR reactions were run on the 7500 Fast Real-Time PCR System (Applied Biosystems, UK), with the following cycle program: 95°C for 10 minutes, then 40 cycles of 95°C for 15 seconds, 60°C for 30 seconds. The annealing temperature was optimised for each individual pair of primers. The PCR products were run on a 2% agarose gel to confirm the size of the correct base pair size. The Primer Sequences used are as follows: c-myc Forward, CTGCGACGAGGAGGAGGACT, c-myc Reverse GGCAGCAGCTCGAATTTCTT; c-jun Forward AGATGCCCGGCGAGACA C CG, c-jun Reverse AGCCCCCGACGGTCTCTCTT; GADD45 Forward CGGTGGAGGAGCTT TGGTG, GADD45 Reverse CACCCGCACGATGTTGATGT; RPLPO Forward TCTACAACCC TGAAGTGCTTGATATC,PRLPO Reverse GCAGACAGACACTGGCAACATT; MAPK14 Forward TGAAATGACAGGCTACGTGG, MAPK14 Reverse CTATAAGGAGGTCCCTGCTTT C.
Statistical analysis
The patient characteristics data are presented as mean ± SD and all other data were presented as median and interquartile range since analysis showed that the data were not normally distributed. Statistical analysis of group differences was performed using unpaired t-test and Mann–Whitney U-Test depending on the normality of data distribution. Chi squared tests were used for categorical variables (i.e., gender). A significance of p < 0.01 was accepted in view of the multiple comparisons made for the muscle biopsy pathway data which were potentially interrelated, and p < 0.05 elsewhere.
Results
The physiological characteristics of the patients are summarised in . There was no significant difference in age, gender, BMI, weight, or PaCO2 (mean 5.2 kPa in both patients and controls) but as expected significant differences between patients and controls were observed in FEV1, TLCO, fat-free mass index, PaO2, locomotion time, and quadriceps strength measured as maximal voluntary contraction force. Quadriceps measured as twitch tension failed to achieve a significant difference between patients and controls (p = 0.07) (). Thirteen of the patients and none of the controls were taking oral corticosteroids. As expected the patients had a significantly greater tobacco history (mean 50.3 vs 11.7 pack-years, p < 0.0001) but the proportion of current smokers did not differ between patients and controls (p = 0.16, Fishers Exact test)
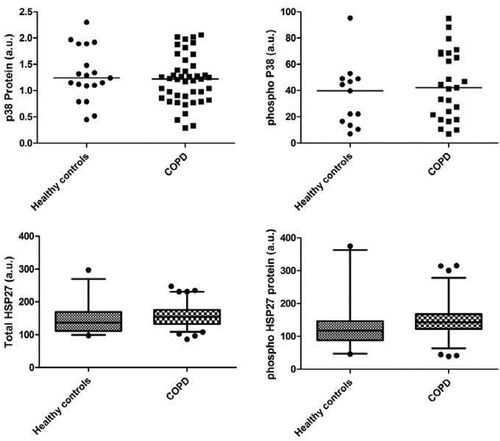
The primary readout was considered to be levels of total and phosphorylated p38 MAPK and especially the downstream target HSP27, which were assessed by Multiplex fluorescent bead array using p38 MAPK and HSP27 specific beads. The results for the protein expression are summarised in and . Total and phosphorylated p38 MAPK protein levels were not different between controls and COPD patients. Total and phosphorylated HSP27 protein levels were also not different between controls and COPD patients. Again there were no correlations between the physiological parameters and the levels of phospho-p38 MAPK or phospho-HSP27 (data not shown). In subsidiary analyses we sought differences when the participants were split by GOLD stage (Citation23), by FFMI using the criteria of Franssen and co-workers (Citation28), 6-minute walk using the threshold of 350m suggested both by Cote and colleagues and Spruit and co-workers (Citation29, 30) or as a ratio of phospho/total p38 and HSP 27. All of these analyses proved negative (Figures E1–4).
Figure 1. Levels of total (left panels) andphosphorylated (right panels) of p38 MAPK (upper panels) and HSP27 (lower panels) protein. Upper panels show individual data; lower panels, because of number of participants, show medians with 5th and 95th percentiles.
To identify any changes in the activity of the MAPK signalling pathway we measured the expression of p38 MAPK (MAPK14) and the expression of genes known to be activated by the p38 MAPK signalling pathway, (c- myc (Citation31), c-jun (Citation32, 33) and Gadd45β (Citation34)) in the biopsies of quadriceps muscles from COPD patients and in controls. These target genes were chosen as they have also previously been associated with increased MAPK signalling in muscle wasting in humans (Citation35). Gene expression was normalised to RPLPO and expressed as arbitrary units (au). The results for the QPCR candidate genes are summarised in . Unexpectedly, highly significant reductions in c-jun and Gadd45β were observed ().
Figure 2. Expression of 2 downstream components of the p38 pathway, (upper) c-jun and (lower) Gadd45β, which were reduced in COPD patients. Data show the median values with bars at the 5th and 95th percentile values.
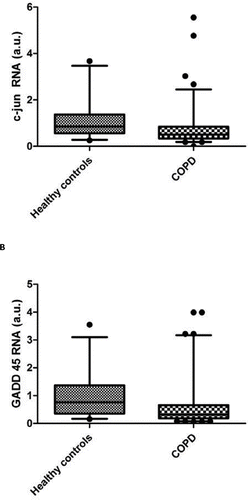
Table 3. Gene expression.
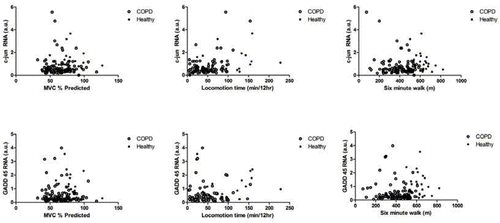
To determine whether changes in expression correlated with physiological parameters we compared the expression of each gene with MVC, Twitch strength, BMI, FFM and physical activity measured in two 12-hour periods immediately prior to the biopsy. This analysis failed to demonstrate any correlation with these markers of the MAPK signalling pathway (data for c-jun and GADD45 β data as a function of quadriceps MVC, locomotion time and 6-minute walk distance are shown in ; remaining data not shown.
Discussion
The main finding of the present study is that, in a cohort of 105 stable COPD out-patients and objectively documented reduced physical activity and exercise capacity as well as quadriceps weakness, neither RNA nor protein expression of p38 MAPK or its downstream products was significantly different from a group of age and gender matched controls. The ratio of phospho-p38 MAPK protein to total p38 MAPK protein was similar between patients and controls suggesting that we have not overlooked differences in activation. Consistent with this suggestion and supportive of it was the observation that neither RNA nor protein expression of p38 MAPK was related to either indices of quadriceps strength or physical activity levels of the patients. These data suggest that the degree of inactivity displayed by the patients in our cohort is insufficient to increase p38 MAPK signalling, and moreover that it is not increased by any other candidate mechanism, for example inflammation.
Critique of the method
We failed to find evidence of p38 MAPK activation in quadriceps biopsies taken from patients with COPD. The strength and significance of this conclusion rests on three considerations. First, was the hypothesis reasonable? We have discussed in the introduction that immobility is associated with activation of p38 MAPK in a rodent immobility model and that aged rats are less able than younger rats to reverse these changes on remobilisation (Citation11) as well as a potential mechanistic link through oxidative stress known to be present both in skeletal muscle from COPD patients as well as other respiratory disease (Citation36). P38 MAPK activity is also known to be increased in lung tissue and alveolar macrophages of patients with COPD; importantly recent data directly addressing the same question has claimed that p38-MAPK signalling is up-regulated in COPD patients with skeletal muscle atrophy (Citation18).
One intriguing possibility which the present study leaves unanswered is whether fibre type specific p38 MAPK activation may contribute to quadriceps myopathy in COPD. Machida and Booth, by using different muscles, were able to show that for immobility, phospho-p38 MAPK activation (and downstream GADD45 expression) occurred predominantly in type I fibres. Consistent with this, in a rodent model, muscle contraction stimulated phospho-p38 MAPK selectively in type II fibres(Citation37). Moreover, given that COPD is a disease of the elderly, fibre type differences in p38MAPK expression may also be modulated by ageing (Citation38).
Type I fibres are reduced in COPD (Citation39) and so conceptually our finding of equivalence between COPD and controls could conceal increased p38 activation in a reduced number of type I fibres, potentially even raising the hypothesis that p38 MAPK activation contributes to fibre loss or switching. It is now technically possible to resolve the fibre-specific p38 MAPK activation question by laser capture microdissection (Citation40), but this facility is not available in our institution presently and has not previously been used in COPD for skeletal muscle though it has been applied to the airway (Citation41).
Second, were our patients representative? We believe they were since, on average, they had severe COPD (mean FEV1 43.9% predicted) with quadriceps weakness measured by MVC compared to control subjects. Importantly, from the perspective of our hypothesis, they were also less physically active by approximately 50% (). We accept that strength measured by TwQ and expressed as kg failed to reach statistical difference between patients and controls (p = 0.07) but there are no normative ranges for TwQ except the small series of younger patients described by Hamnegård et al. (Citation42) so we opted not to express these data as percent predicted.
Third, was the sample size big enough? We believe it was since in addition to not identifying any changes in the activity of MAPK we also analysed the expression of specific downstream markers of the p38 MAPK signalling cascade. Specifically c-myc, c-jun, HSP27 and GADD45β, were assessed to determine whether there was activation of p38 MAPK in quadriceps muscles of COPD patients. Gene expression of these target genes was normalised to RPLPO and expressed as arbitrary units (au). This approach is particularly valid since in vivo reduction in HSP 27 occurs in a dose dependent following administration of a MAPK inhibitor (Citation21), and Machida and Booth found increased GADD45 expression in their model. While our data showed a mild increase in HSP 27 and phospho-HSP 27, which allowing for multiple end points, we believe to be neither clinically nor statistically significant, while we saw highly significant decreases in c-Jun and GADD45.
The reason why c-jun and GADD45 should have fallen rather than remained unchanged is unexplained by our data. One explanation (see above) is that the processes driving their expression (whether immobility, inflammation or other) is fibre-type specific and that this fall simply represents the expected fibre-type change in a cohort of this severity (Citation12), however so far as is known this could not explain reduced c-jun(Citation43). Another intriguing possibility is an interaction with Vitamin D. In vitro Vitamin D has is able to cause cell cycle arrest in ovarian cancer models through up-regulation of GADD45 (Citation44). Similarly it has been documented in muscle cell lines that c-jun can be activated by Vitamin D (Citation45). Patients with COPD are frequently Vitamin D deplete (Citation46) and we have previously documented a relationship between Vitamin D receptor gene polymorphism and strength in COPD (Citation47). Conceptually therefore one could hypothesise that reduced GADD45 and c-jun expression was due to either reduced Vitamin D, or to receptor insensitivity.
Finally, we acknowledge that although our data show, at a cross-sectional level, that activation of p38 MAPK signalling pathway is not evident in the quadriceps of COPD patients with quadriceps weakness, this conclusion assumes that wasting occurs in a chronic manner. It is, however, possible that wasting is episodic. It is well known that acute exacerbation is associated with the acute development of muscle weakness (Citation48) and reduced physical activity (Citation49) as well as changes in the inflammatory milieu. In this cross-sectional study it is not possible to rule out the involvement of p38 MAPK signalling in this type of muscle wasting, which may nevertheless be of clinical importance; these factors would not however explain the discrepancy between our findings and those of Lemire and co-workers (Citation18).
Significance of the findings
The main finding of the present study is that, in a cohort of outpatients with severe stable COPD, weakness and immobility, neither RNA nor protein expression of p38 MAPK or selected downstream products was significantly different from a group of age matched controls. Consistent with this and supportive of it was the observation that neither RNA nor protein expression of p38 MAPK was related to either indices of quadriceps strength or physical activity levels of the patients suggesting that inactivity does not result in increased p38 MAPK signalling.
Although we had been primarily concerned with the relationship between reduced mobility and p38 MAPK signalling some data from the literature had raised the possibility that inflammation might also up-regulate p38 MAPK signalling (Citation13), although specific data that this could happen in skeletal muscle was sparse (Citation50). We found the p38 MAPK signalling pathway not to be activated in the skeletal muscle of the quadriceps implying negligible activity of the pro-inflammatory cytokines (e.g., TNFα) in this tissue. This conclusion is consistent with previous studies showing no change in pro-inflammatory cytokines in quadriceps muscles of COPD patients (Citation16, Citation51). Equally the fact that p38 MAPK signalling was not up-regulated also excludes the possibility that it is up-regulated as a result of another mechanism whether presently postulated (for example oxidative stress) or indeed as yet unknown.
The discrepancy between our findings and those of Lemire et al. is perhaps fortuitous since, with the development of p38 MAPkinase inhibitors the issue may in the future be settled in the best way, following a prospective RCT. The optimal design for such a study must needs await the appearance of their full manuscript but our data suggest that screening either with a 6MW or physical activity monitoring would not be the best way to stratify patients for such a study where muscle specific outcomes were desired. We suspect instead that an index of muscle bulk could be preferable. Although CT-derived mid-thigh cross-sectional area (CSA) would be one possibility, ultrasound measurement of the rectus femoris RFCSA has a close relationship with CT derived data (Citation52) and, being radiation free, could be used to screen participants and to make recurrent measurements through the study.
We conclude that activation of p38 MAP Kinase is not a feature of immobility or muscle weakness in out-patients with stable COPD; while alternative pathways should be explored in the search for a solution for this common clinical problem we suggest that future studies of quadriceps dysfunction in COPD should attempt where possible to assess signalling pathways separately in Type I and Type II fibres.
Declaration of interest statement:
JRC was funded by a research grant from GlaxoSmithKline (GSK). This project was undertaken at the NIHR Biomedical Research Unit in Advanced Lung Disease at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London; MIP's salary is part funded by the Biomedical Research Unit. The authors gratefully acknowledge the assistance of Ruth Mayer and Susan Boyce of GSK in the luminex assays. RTS is a GSK Employee and Shareholder. SA Natanek (née Sathyapala) is in receipt of a Wellcome Trust Clinical fellowship #079686. The authors alone are responsible for the content and writing of the paper.
References
- Seymour JM, Spruit MA, Hopkinson NS, The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J 2010; 36(1):81–88.
- Marquis K, Debigare R, Lacasse Y, Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 166(6):809–813.
- Swallow EB, Reyes D, Hopkinson NS, Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007; 62(2):115–120.
- Liu W, Liang Q, Balzar S, Cell-specific activation profile of extracellular signal-regulated kinase 1/2, Jun N-terminal kinase, and p38 mitogen-activated protein kinases in asthmatic airways. J Allergy Clin Immunol 2008; 121(4):893–902 e2.
- Renda T, Baraldo S, Pelaia G, Increased activation of p38 MAPK in COPD. Eur Respir J 2008; 31(1):62–69
- Liu Q, Xu WG, Luo Y, Cigarette smoke-induced skeletal muscle atrophy is associated with up-regulation of USP-19 via p38 and ERK MAPKs. J Cell Biochem 2011; in press.
- Pitta F, Troosters T, Spruit MA, Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171(9):972–977.
- Watz H, Waschki B, Meyer T, Physical activity in patients with COPD. Eur Respir J 2009; 33(2):262–272.
- Choi I, Lee K, Kim M, Differential activation of stress-responsive signalling proteins associated with altered loading in a rat skeletal muscle. J Cell Biochem 2005; 96(6):1231–1243.
- Childs TE, Spangenburg EE, Vyas DR, Temporal alterations in protein signaling cascades during recovery from muscle atrophy. Am J Physiol Cell Physiol 2003; 285(2):C391–98.
- Morris RT, Spangenburg EE, Booth FW. Responsiveness of cell signaling pathways during the failed 15-day regrowth of aged skeletal muscle. J Appl Physiol 2004; 96(1):398–404.
- Machida S, Booth FW. Changes in signalling molecule levels in 10-day hindlimb immobilized rat muscles. Acta Physiol Scand 2005; 183(2):171–179.
- Li YP, Chen Y, John J, TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J 2005; 19(3):362–370.
- Obata T, Brown GE, Yaffe MB. MAP kinase pathways activated by stress: the p38 MAPK pathway. Crit Care Med 2000; 28(4 Suppl):N67–77.
- Barreiro E, Gea J, Corominas JM, Nitric oxide synthases and protein oxidation in the quadriceps femoris of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2003; 29(6):771–778.
- Barreiro E, Schols AM, Polkey MI, Cytokine profile in quadriceps muscles of patients with severe COPD. Thorax 2008; 63(2):100–107.
- Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J Appl Physiol 2007; 102(6):2389–2397.
- Lemire BB, Debigaré R, Theriault M, MAPK Signaling In The quadriceps of patients with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2011; 183:A2527.
- Kent LM, Smyth LJ, Plumb J, Inhibition of lipopolysaccharide-stimulated chronic obstructive pulmonary disease macrophage inflammatory gene expression by dexamethasone and the p38 mitogen-activated protein kinase inhibitor N-cyano-N’-(2-{[8-(2,6-difluorophenyl)-4-(4-fluoro-2-methylphenyl)-7-oxo-7,8-dihydropyrido[2,3-d] pyrimidin-2-yl]amino}ethyl)guanidine (SB706504). J Pharmacol Exp Ther 2009; 328(2):458–468.
- Montalban AG, Boman E, Chang CD, KR-003048, a potent, orally active inhibitor of p38 mitogen-activated protein kinase. Eur J Pharmacol 2010; 632(1–3):93–102.
- Singh D, Smyth L, Borrill Z, A randomized, placebo-controlled study of the effects of the p38 MAPK inhibitor SB-681323 on blood biomarkers of inflammation in COPD patients. J Clin Pharmacol 2010; 50(1):94–100.
- Medicherla S, Fitzgerald MF, Spicer D, p38alpha-selective mitogen-activated protein kinase inhibitor SD-282 reduces inflammation in a subchronic model of tobacco smoke-induced airway inflammation. J Pharmacol Exp Ther 2008; 324(3):921–929.
- Rabe KF, Hurd S, Anzueto A, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176(6):532–555.
- Steiner MC, Barton RL, Singh SJ, Bedside methods versus dual energy X-ray absorptiometry for body composition measurement in COPD. Eur Respir J 2002; 19(4):626–631.
- Edwards RHT, Young A, Hosking GP, Human skeletal muscle function: description of tests and normal values. Clin Sci 1977; 52:283–290.
- Polkey MI, Kyroussis D, Hamnegard C-H, Quadriceps strength and fatigue assessed by magnetic stimulation of the femoral nerve in man. Muscle Nerve 1996; 19:549–555.
- Bergstrom L. Muscle electrolytes in man. Determination by neutron activation analysis on needle biopsy specimens. A study on normal subjects, kidney patients, and patients with chronic diarrhea. Scand J Clin Lab Invest 1962; 68:1–110.
- Franssen FM, Broekhuizen R, Janssen PP, Limb muscle dysfunction in COPD: effects of muscle wasting and exercise training. Med Sci Sports Exerc 2005; 37(1):2–9.
- Cote CG, Casanova C, Marin JM, Validation and comparison of reference equations for the 6-min walk distance test. Eur Respir J 2008; 31(3):571–578.
- Spruit MA, Polkey MI, Celli B, Predicting Outcomes from 6-Minute Walk Distance in Chronic Obstructive Pulmonary Disease. J Amer Med Direct Asso 2011; in press.
- Chen S, Qiong Y, Gardner DG. A role for p38 mitogen-activated protein kinase and c-myc in endothelin-dependent rat aortic smooth muscle cell proliferation. Hypertension 2006; 47(2):252–258.
- Weiss C, Faust D, Durk H, TCDD induces c-jun expression via a novel Ah (dioxin) receptor-mediated p38-MAPK-dependent pathway. Oncogene 2005; 24(31):4975–4983.
- Marinissen MJ, Chiariello M, Gutkind JS. Regulation of gene expression by the small GTPase Rho through the ERK6 (p38 gamma) MAP kinase pathway. Genes Dev 2001; 15(5):535–553.
- Sarkar D, Su ZZ, Lebedeva IV, mda-7 (IL-24) Mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc Natl Acad Sci USA 2002; 99(15):10054–10059.
- Di Giovanni S, Molon A, Broccolini A, Constitutive activation of MAPK cascade in acute quadriplegic myopathy. Ann Neurol 2004; 55(2):195–206.
- Swallow EB, Barreiro E, Gosker H, Quadriceps muscle strength in scoliosis. Eur Respir J 2009; 34(6):1429–1435.
- Wretman C, Widegren U, Lionikas A, Differential activation of mitogen-activated protein kinase signalling pathways by isometric contractions in isolated slow- and fast-twitch rat skeletal muscle. Acta Physiol Scand 2000; 170(1):45–49.
- Mylabathula DB, Rice KM, Wang Z, Age-associated changes in MAPK activation in fast- and slow-twitch skeletal muscle of the F344/NNiaHSD X Brown Norway/BiNia rat model. Exp Gerontol 2006; 41(2):205–214.
- Gosker H, Zeegers M, Wouters E, Muscle fibre type shifting in the vastus lateralis of patients with COPD is associated with disease severity: a systematic review and meta-analysis. Thorax 2007; 62(11):944–9.
- Adachi T, Kikuchi N, Yasuda K, Fibre type distribution and gene expression levels of both succinate dehydrogenase and peroxisome proliferator-activated receptor-gamma coactivator-1alpha of fibres in the soleus muscle of Zucker diabetic fatty rats. Exp Physiol 2007; 92(2):449–455.
- Gosselink JV, Hayashi S, Elliott WM, Differential expression of tissue repair genes in the pathogenesis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 181(12):1329–1335.
- Hamnegard CH, Sedler M, Polkey MI, Quadriceps strength assessed by magnetic stimulation of the femoral nerve in normal subjects. Clin Physiol Funct Imag 2004; 24(5):276–280.
- Csukly KJ, Martineau LC, Gardiner PF. Inter- and intra-muscle comparisons of MAPK mechanosensitivity: evidence for the absence of fibre-type dependency. Pflugers Arch 2002; 444(6):732–737.
- Jiang F, Li P, Fornace AJ, Jr., et al. G2/M arrest by 1,25-dihydroxyvitamin D3 in ovarian cancer cells mediated through the induction of GADD45 via an exonic enhancer. J Biol Chem 2003; 278(48):48030–48040.
- Buitrago CG, Ronda AC, de Boland AR, MAP kinases p38 and JNK are activated by the steroid hormone 1alpha,25(OH)2-vitamin D3 in the C2C12 muscle cell line. J Cell Biochem 2006; 97(4):698–708.
- Janssens W, Bouillon R, Claes B, Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 2010; 65(3):215–220.
- Hopkinson NS, Li KW, Kehoe A, Vitamin D receptor genotypes influence quadriceps strength in chronic obstructive pulmonary disease. Am J Clin Nutr 2008; 87(2):385–390.
- Spruit MA, Gosselink R, Troosters T, Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax 2003; 58(9):752–756.
- Pitta F, Troosters T, Probst VS, Physical activity and hospitalization for exacerbation of COPD. Chest 2006; 129(3):536–544.
- Austin RL, Rune A, Bouzakri K, siRNA-mediated reduction of inhibitor of nuclear factor-kappaB kinase prevents tumor necrosis factor-alpha-induced insulin resistance in human skeletal muscle. Diabetes 2008; 57(8):2066–2073.
- Crul T, Spruit MA, Gayan-Ramirez G, Markers of inflammation and disuse in vastus lateralis of chronic obstructive pulmonary disease patients. Eur J Clin Invest 2007; 37(11):897–904.
- Seymour JM, Ward K, Sidhu P, Ultrasound measurement of rectus femoris cross-sectional area and the relationship to quadriceps strength in chronic obstructive pulmonary disease. Thorax 2009; 64:418–423.
- Doucet M, Russell AP, Leger B, Muscle atrophy and hypertrophy signaling in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 176(3):261–269.