Abstract
Background: The prevalence of Chronic Obstructive Pulmonary Disease (COPD) in Cyprus is largely unknown. The aim of the study was to estimate the prevalence of COPD in Cyprus through a spirometry population- based program and to identify certain disease characteristics in the Cypriot population. Methods: The study was performed in 1,233 randomly selected individuals covering representative urban and rural areas. Inclusion criteria were: age ≥ 35 years old and lifetime smoking history of at least 100 cigarettes. Participants answered a detailed questionnaire and underwent spirometry before and after the inhalation of 200 μg of salbutamol. COPD diagnosis and severity were based on criteria developed by the Global Initiative for Chronic Obstructive Lung Diseases. Results: The overall prevalence of spirometry diagnosed COPD subjects was 4.9% (5.1% in men vs 3.5% in women). Mild COPD was found in 33.3% of COPD individuals, moderate in 45%, severe and very severe COPD was found in 20% and 1.7%, respectively. Physician diagnosis was reported in 48.3% of spirometry diagnosed COPD subjects, whereas 55.9% were asymptomatic. Age (p = 0.000), increased tobacco consumption (p = 0.001) and cough with phlegm (p = 0.048) were found to have a synergistic effect on the diagnosis of COPD. Conclusions: Results suggest that COPD is an important health problem in Cyprus. Programs that raise public awareness focusing on prevention, early detection and treatment are needed. Under-diagnosis of COPD raises the need for spirometry screening programs in high risk individuals and guideline implementation for the management of the disease.
Keywords: :
| Abbreviations | ||
| COPD | = | hronic Obstructive Pulmonary Disease |
| FEV1 | = | Forced expiratory volume at the first second of expiration |
| FVC | = | Forced Vital Capacity |
| GOLD | = | Global Initiative for Chronic Obstructive Lung Disease |
| BMI | = | Body Mass Index |
| ERS | = | European Respiratory Society |
| ATS | = | American Thoracic Society |
| PYS | = | Pack-years |
| IHD | = | Ischemic heart disease |
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by chronic airflow limitation, a range of pathological changes in the lung and important co-morbidities that may contribute to the severity of the disease in individual patients (Citation1). Within the next 20 years, COPD will become the third-leading cause in terms of morbidity and mortality worldwide (Citation2). The growing burden of COPD is partly due to the continued use of tobacco and partly due to the ageing of the world's population (Citation3).
Over the last few years, there has been an increased awareness about these alarming figures and as a result many population-based studies have been conducted in different countries. Not surprisingly, there has been a great variability of the global COPD prevalence because of disagreements on diagnostic criteria and study designs (Citation4–8). In addition, COPD is diagnosed late in its natural history resulting in increased direct and indirect costs (Citation9). In the National Health and Nutrition Examination Survey (NHANES) III, 11.7% of subjects with a mild airflow limitation were undiagnosed, whereas only half of those with moderate to severe airflow limitation were diagnosed and treated (Citation10). Early detection of the disease along with smoking cessation and treatment remain the most effective ways of reducing disease progression (Citation1, Citation11).
In Cyprus so far, there have not been any epidemiological studies on the prevalence of COPD. The aim of the present study was to measure the prevalence of COPD in Cyprus and to identify the main characteristics of the disease in the Cypriot population.
Materials and Methods
This population-based cross-sectional study was performed in urban and rural areas, covering a wide geographical range and a total population of more than 400,000 Greek-Cypriots. [The area under the effective control of the Republic of Cyprus comprises around 59% of the island's total area]. Representative urban and rural regions were selected according to the geographical distribution of the population stated in the annual demographic report of the Statistical Service of Cyprus (Citation12) (Appendix 1). Subjects were recruited using a stratified two-staged random sampling method, in which the first stage was the stratification of survey region. Strata used were: location; urban areas (population > 30,000), rural areas (population < 30,000) and age.
During the second step of the sampling process, individuals were randomly selected using the telephone directory of Cyprus, which represents more than 90% of households. Using random-digit telephone dialing, 3,000 phone calls were attempted in order to define the final number of participants. In case of no answer on first attempt, a second call was made immediately to avoid bias against hard-to-reach people. Phone calls were made between 9 am to 9 pm, 7 days a week to prevent bias against older people and housewives. Eligible subjects were interviewed and a home visit was scheduled within 2 weeks.
Inclusion criteria for home contact were: age ≥ 35 years old, smoking history of at least 100 cigarettes in lifetime and willingness for study participation. Subjects were excluded if they reported a history of any other pulmonary disease and presence of co-morbidities that precluded spirometry or use of bronchodilators. During home visit, inclusion criteria were revised; each subject was informed by a pulmonologist and signed a consent form. Participants answered a detailed questionnaire (Appendix 2) that included: demographic information, smoking history, respiratory history and symptoms, COPD risk factors and co-morbidities.
Physician diagnosis of COPD was defined as affirmative answers to the questions: “Has a doctor ever told you that you have: a) chronic bronchitis, b) emphysema, c) chronic obstructive pulmonary disease?” A clinical diagnosis of COPD was defined as a positive answer to the question: “Do you usually have cough and phlegm most days in periods of at least three months during at least two successive years?” Occupational dust, gas exposure and air pollution were defined as affirmative answers to the questions: “Are you being exposed to dust at your work place?”, “Do you use gas for cooking or heating?”, “Do you live near a polluted area?” Smoking status was measured by pack-years (PYS), defined as the average number of cigarettes smoked per day divided by 20 times the duration of smoking in years. For the study purpose we included current and former smokers [those quitting smoking at least one year before the date of spirometry]. Individuals were classified as heavy smokers if smoking intensity was ≥ 15 pack-years.
After an eligibility evaluation for spirometry, anthropometric measurements were taken. Weight was measured with a portable stadiometer (SECA 700 Mechanical Scale, GMBH & co.kg) and height was measured with a height rod (SECA Height Rod, GMBH & co.kg). Body mass index (BMI) was calculated and subjects were categorized into four groups: <20 kg/m2, 20–24.9 kg/m2, 25–29.9 kg/m2 and ≥ 30 kg/m2.
Finally, each individual underwent spirometry before and 15 minutes after the inhalation of 200 μg of salbutamol (Ventolin; GlaxoSmithKline, MiddleSex, UK) using a portable spirometer (Spirolab II, Medical International Research, Rome, Italy) according to the American Thoracic Society recommendations13. Participants performed up to eight forced expiratory maneuvers so as to achieve three technically acceptable spirograms with the two best values of FEV1 and FVC within 150 ml (Citation13). Calibration of spirometers was verified to be accurate within calibration limits of ± 3.0% using a 3-L syringe at the beginning of each testing day. Two independent pulmonologists evaluated daily spirometric data. Ninety-seven percent of all calibration checks were within 50 ml of the of the 3-L standard according to the ATS recommendations (Citation13). Overall 94% of the tests reached quality criteria (i.e., tests with three acceptable maneuvers and reproducibility of FEV1 and FVC to 150 ml) (Citation13).
COPD was defined as a FEV1/FVC < 70% after bronchodilation and a reversibility test result of <12% and < 200 ml improvement in FEV1 compared to pre-bronchodilator FEV1 (Citation1). Following GOLD classification1, COPD severity was assessed according to post-bronchodilator values of FEV1 (percentage of predicted) as follows: mild obstruction: FEV1≥ 80%, moderate: 50 ≤ FEV1< 80%, severe: 30 ≤ FEV1< 50%, very severe: FEV1 < 30% or FEV1< 50% plus chronic respiratory failure. The study protocol was approved by the national ethics committee of Cyprus.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences SPSS 17.0 (SPSS Co Chicago, IL, USA). Continuous variables were expressed as means (SD) and prevalence rates as crude gender-adjusted, community and age-adjusted values. Student t-test was used to compare continuous variables and chi-square (χ2) test was used to compare proportions. The difference in COPD prevalence between variables was determined by the χ2-test. A multiple logistic regression model was constructed to identify independent risk factors for COPD diagnosis. Independent variables were: gender, age, BMI, PYS, educational level, occupational exposure and respiratory symptoms (dyspnea, wheezing, cough), with COPD diagnosis being the dependent variable. Odds ratios were presented with 95% confidence intervals (CI). P-values < 0.05 were considered statistically significant.
Results
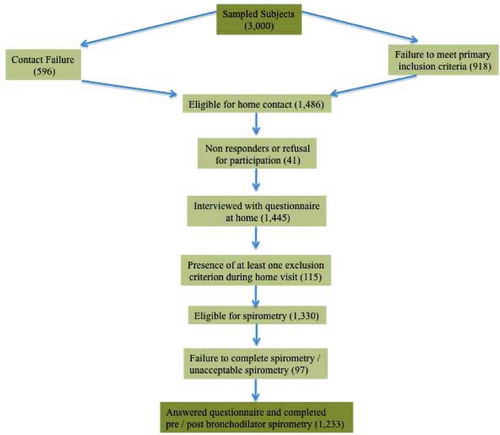
According to the sampling process, it was estimated that 3,000 individuals should have been contacted primarily by telephone (). 1,514 persons (50.5%) had not either answered the telephone call, or did not meet inclusion criteria. Of the remaining 1,486 individuals, 41 (2.7%) were also excluded because were absent at scheduled home visit or because they declined participation. Another 115 (7.9%) were excluded during the completion of the questionnaire, because they met at least one exclusion criterion (appendix 2). From the remaining 1,330 individuals, 15 (1.1%) refused spirometry and 82 (6.2%) had unacceptable spirometry (i.e., cough, inadequate effort, early termination) thus leading to a final number of 1,233 participants. The study was performed between February and June 2008.
Characteristics of the study population
Distribution of participants’ characteristics is shown in . The vast majority of the study population was men (83.6%). Mean age ± SD was 59.9 ± 11.4 years, with a range of 35 to 88 years. Two thirds of participants lived in urban areas (60.6%) and 38.2% had only completed primary school. A total of 671 subjects were current smokers (54.6%). Men smoked significantly more than women (mean PYS: 56.4 ± 45.8 vs 29.6 ± 27.3, p = 0.000) as well as older subjects (≥ 71 years) as compared to younger ones (35–70 years), according to . Participants living in rural areas were significantly heavier smokers than those living in urban areas (mean PYS: 56.9 ± 50.1 vs 49 ± 40.1, p = 0.004), as well as less-educated individuals in comparison to those with higher education (p = 0.000), ().
Table 1. Characteristics of the study population (N = 1,233)
Table 2. Mean pack-years by gender, age, community setting and educational level for the study population N = 1,233)
COPD Prevalence
The overall prevalence of COPD according to GOLD spirometric criteria (Citation1) was 4.9%. Although COPD was more prevalent in men (5.1%) as compared to women (3.5%) it was not statistically significant (p = 0.337). COPD prevalence was significantly higher in the age group of 51–70 years as compared to ages between 35–50 years and ≥ 71 years (60% vs 1.7% vs 38.3% respectively, p = 0.000).
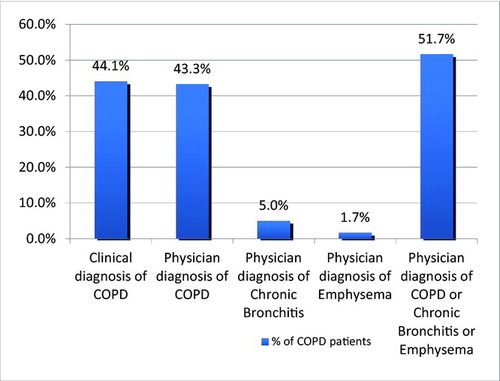
Mild COPD1 was found in 33.3% of spirometry defined COPD subjects, moderate in 45%, severe and very severe COPD was found in 20% and 1.7%, respectively. As shown in , COPD prevalence for stages II-IV was 1.3% vs 0.6% among current and former smokers respectively, in the age group of 51–70 years old. COPD was more prevalent among current smokers of all ages as compared to former smokers, a finding of statistical significance (3% vs. 1.9%, p = 0.032) Only 48.3% and 44.1% of spirometry-diagnosed COPD individuals had a previous physician diagnosis or a clinical diagnosis of COPD, () as defined by the presence of cough and phlegm (most days for 3 or more months in 2 consecutive years) (Citation1).
Figure 2. COPD prevalence stratified by age and smoking status according to GOLD severity classification criteria (Citation1). CS, current smokers; ES, ex-smokers.
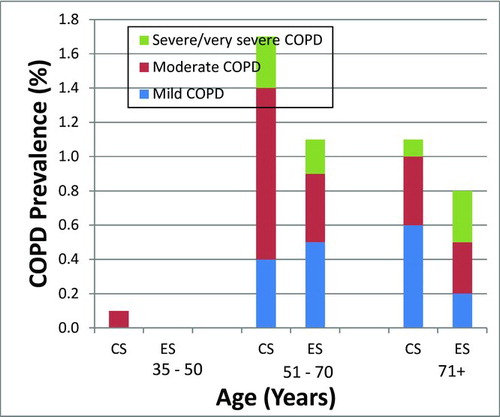
Figure 3. Clinical and physician diagnosis of COPD in spirometry defined COPD subjects. Clinical diagnosis of COPD: affirmative answer to the question: “Do you usually have cough and phlegm most days in periods of at least three months during at least two successive years?” Physician diagnosis of COPD: affirmative answer to the question: “Has a doctor ever told you that you have: a) chronic bronchitis, b) emphysema, c) chronic obstructive pulmonary disease?”
Differences among COPD and non-COPD individuals
Spirometry-defined COPD individuals were significantly older than normal subjects (mean age 67.5 ± 7.0 vs 59.6 ± 11.4, p = 0.000) as shown in . Mean post-bronchodilator FEV1, FVC and FEV1/FVC values were significantly lower in COPD as compared to non COPD subjects with respective p-values of 0.000, 0.003 and 0.000 (). Furthermore, 56.7% of COPD individuals had completed only primary education as compared to 37.4% of non-COPD subjects, a finding of statistical significance (p = 0.019). However, outdoor air pollution, gas exposure and occupational dust exposure did not vary significantly among normal spirometry and COPD individuals (p = 0.447, p = 0.127, p = 0.147, respectively).
Table 3. Comparison between COPD and non COPD participants
In regard to co-morbidities, 65% and 57.8% of COPD and non COPD subjects reported at least one co-morbid condition. As shown in , 57 subjects self-reported a previous asthma diagnosis (affirmative answer to the question: “Have you ever had asthma?”). Asthma was more prevalent in spirometry defined COPD subjects in comparison to normal individuals (11.7% vs 4.3%), a finding of statistical significance (p = 0.008). Although ischemic heart disease (IHD) and depression were more common among COPD individuals, they were not statistically significant (p = 0.307 and 0.378).
Table 4. Distribution of specific chronic conditions among the study population
Discussion
This was the first population-based cross-sectional epidemiological study on COPD prevalence performed in Cyprus. It also provides the first national estimate of the extent of under-diagnosis and awareness of the disease. Study results have shown that COPD prevalence in Cyprus was 4.9% among individuals of ≥ 35 years old with a lifetime smoking history of ≥ 5 PYS. The European Health Survey (EU-HS), which was conducted by the Statistical Service of Cyprus (Citation14) in a representative sample of the entire population showed that 4.1% of the population ≥ 35 years old reported suffering from COPD, a finding close to the results of our study. Results from previous COPD prevalence estimates employing spirometric testing ranged from 4–10% (Citation10), which are comparable with the 5% of our study. However, when compared with a similar methodological study performed in Greece by Tzanakis et al. (Citation15), COPD prevalence found in our study was substantially lower (5% vs 8.4%, respectively).
In two other Greek studies, COPD prevalence was 5.6% (Citation16) and 18.4% (Citation17), respectively. Several Mediterranean spirometry-based studies identified prevalence rates ranging from 6.9% in Turkey (Citation18) to 7.5% in France (Citation19) and from 9.1% in Spain (Citation20), to 11% in Italy (Citation21). According to the results of PLATINO study (Citation22) performed in individuals of ≥ 40 years old, COPD prevalence ranged from 7.8% in Mexico City to 19.7% in Montevideo. Data analyzed from 14 sites of the BOLD study in subjects of ≥ 40 years old for GOLD stages 2–4 have shown prevalence rates ranging from 5% in Germany (Hanover) to 16.3% in South Africa (Cape Town) (Citation23). In most of the aforementioned studies (Citation22–27) COPD definition was based solely on the FEV1/FVC ratio of < 70% and no reversibility test applied. Further analysis of our study data in the population of 40 years and older and without applying the reversibility test showed that the prevalence of COPD in Cyprus rose from 4.9% to 6.7% (79 individuals), a finding that is comparable with the results of the above studies. As supported by several authors (Citation6, Citation8, Citation21, Citation26, Citation28), COPD prevalence displays wide variations due to differences in epidemiologic methodology, proportions of age and gender, response rate and diagnostic criteria of COPD (ATS (Citation29), ERS (Citation29), GOLD (Citation1), BTS (Citation30)).
Another finding of our study was that men smoked significantly more than women (mean PYS: 56 vs 30), which probably contributes to the increased prevalence found in men as compared to women (5.1% vs 3.5%). Respective percentages in Greece (Citation15) were 11.6% for men and 4.8% for women. In the PLATINO study (Citation22), COPD prevalence ranged from 11.4% in men and 6.5% in women in Mexico City to 24.2% vs 12.1% in Santiago. Miravitlles (Citation31) reported a prevalence of 15.1% in men and 5.6% in women in Spain. Studies (Citation32, 33) have shown, that male gender comprises a risk factor for COPD due to earlier and heavier smoking habit and increased occupational exposure. However, in our study we did not find a significant gender difference in COPD prevalence (p = 0.337) possibly because of the low participation of women (16.1%).
In the present study, COPD prevalence was significantly higher among less educated individuals, probably because of the increased tobacco consumption identified in this group (mean PYS: 61.7 ± 49.9), a finding of statistical significance (p = 0.000). Moreover, COPD prevalence was higher in rural in comparison to urban areas (5.2% vs 4.7%) a non-statistically significant finding, despite increased smoking intensity in rural areas. In accordance with our findings are the results of the study performed by Tzanakis et al. (Citation15) showing that men smoked significantly more in rural areas in comparison to urban areas (mean PYS: 41 vs 24, p = 0.001). COPD prevalence was found to be higher in rural in comparison to urban areas (9.1% vs 6%) (Citation15). The above findings suggest that smoking is the major determinant of the raised COPD prevalence in rural areas despite the presence of other risk factors in urban areas like air pollution and occupational exposure.
As recommended by GOLD1 and ATS/ERS (Citation29) guidelines COPD diagnosis should be considered in the presence of respiratory symptoms (including cough and sputum production) and a history of exposure to risk factors. This is in accordance with the results of this study showing that age (OR, 1.07; 95% CI, 1.04–1.10) increased tobacco consumption (OR, 1.01; 95% CI, 1.0–1.01) and cough with phlegm (OR, 0.57; 95% CI, 0.33–0.99) are significant risk factors for COPD diagnosis. Similar findings were reported by Minas et al. (Citation16). Miravitlles (Citation31) identified age, increased tobacco consumption and education as main COPD risk factors, whereas according to Lindberg (Citation27) age > 45 years, ever smoking and family history of obstructive lung disease were major risk factors for the disease.
Most subjects with COPD had mild and moderate disease (33.3% vs 45% respectively) whereas 20% had severe disease. According to Tzanakis et al. (Citation15) 57.4% of those with COPD had mild disease, 25.3% had moderate and 16% had severe disease. In another Greek study (Citation16) performed in primary care practices 26.3% of those spirometrically diagnosed with COPD had mild disease, 54.1% and 18.9% had moderate and severe disease respectively, results that are in accordance with those of our study.
Stage II COPD (moderate severity) (Citation1) was found in 47.4% of individuals of ≥40 years old, a finding that is comparable to that of other studies that used GOLD criteria1 for severity classification of the disease with rates ranging from 35.2% to 43.9% (Citation25, Citation34, Citation35). Moreover, COPD prevalence was significantly higher among current smokers as compared to former smokers (3% vs 1.9%, p = 0.032). As shown in the study of Shahab et al. (Citation35) COPD prevalence was significantly higher among current smokers in comparison to ex- smokers (19.3% vs 15.2%), a finding which is in accordance with the results of our study.
Important findings of the study were the considerable under-diagnosis and reduced awareness of the disease. Under-diagnosis was expressed by the low proportion of a previous physician diagnosis (48.3%) in spirometry diagnosed COPD subjects. This emphasizes the need for adequate training of primary care practitioners in the use and interpretation of spirometry in primary care (Citation1). Results of several studies also emphasize underdiagnosis of COPD with rates ranging between 27% to 67% (Citation31, Citation34, Citation36–38). Unawareness of the disease was pronounced by the high proportion of subjects reporting no symptoms (55.9%).
This likely reflects the fact that those with mild COPD may have no symptoms or even if they have symptoms (chronic cough and sputum) these are not perceived as abnormal (Citation1). According to Zhong et al. (Citation24) 35.3% of spirometry diagnosed COPD patients were asymptomatic whereas only 35.1% reported physician diagnosis of the disease. In our study 35.6% of spirometry defined COPD subjects (21/59 individuals) self-reported a previous spirometry test, whereas in the preceding Chinese study (Citation24) respective percentage was much lower (6.5%).
Hypertension and IHD were the most prevalent chronic conditions among COPD subjects (48.3% and 20%, respectively). Results are in accordance with those of other studies with percentages ranging from 18–52% for hypertension (Citation39–42) to 13–65% for IHD (Citation39–43). Asthma coexisted in 11.9% and 4.3% of COPD and non COPD individuals respectively, which was statistically significant. As reported in GOLD (Citation1) asthma is a possible risk factor for the development of COPD and according to the results of a longitudinal study (Citation43), 20% of subjects with asthma developed functional signs of COPD with irreversible airflow limitation. As supported by Silva et al. (Citation45), adults with asthma have a 12-fold higher risk of acquiring COPD than those without asthma, after adjusting for smoking status
The study has several limitations. Strict inclusion criteria were applied (≥ 35 years old instead of 40 years as in most studies), which probably contributed to the lower COPD prevalence as compared to results of other studies. This argument is also supported by the higher prevalence found when we applied analysis of COPD prevalence in the age group of ≥ 40 years old by using only the ratio of post-bronchodilation FEV1/FVC < 70. Moreover, rigorous spirometric definition criteria were used (reversibility test) in order to avoid misclassification of asthma individuals as COPD ones, given the fact that is an overlap between the two diseases (Citation46).
In addition, nonsmokers were excluded from the study as well as those with smoking history < 5 PYS resulting to further underestimation of COPD. Finally, the number of women included in the study was substantially low. This could be explained by the strict inclusion criteria regarding smoking history. The results of the EU-HS (Citation14), support this argument since only 13.6% women ≥ 35 years in Cyprus were identified as current smokers.
In conclusion, this population-based study showed that COPD prevalence in Cyprus is 4.9% in individuals aged ≥ 35 years with a lifetime smoking history of 100 cigarettes. Results of the study have implications on the recognition, prevention and treatment of COPD. Multifaceted approaches are needed so as to reduce the burden of the main risk factors of the disease targeting high risk individuals and health policies to be directed towards the implementation of evidence-based management for COPD.
Declaration of Interests
None of the authors presents any conflicts of interest related to this manuscript.
Acknowledgments
Prof. K. Gourgoulianis and A. Georgiou coordinated the study. Principal investigators were A. G. Zachariades, T. Zachariadou and T. Adamide. Prof. K. Gourgoulianis, A. Georgiou and U. Anagnostopoulou contributed with ideas in the report and data analysis. The article was read and approved by all authors.
The authors would like to acknowledge the pulmonologists of the Respiratory Medicine Department of Nicosia General Hospital (M. Lemesios, M. Oikonomidou, A. Elevtheriou, the General Practitioners G. Athanasiou, Th. Kouppari), the assistant technician (Mrs. N. Agathokleous) and the secretary of the respiratory Medicine Department (Mrs. E. Andreou) for their contribution in the data collection of the study. We also thank Mr. S. Zannetos for the statistical analysis. This study was supported by the Ministry of Health of Cyprus.
References
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease –updated 2008 http://www.goldcopd.org/Guidelines/guideline-global-strategy-for-diagnosis%2c-management%2c-and-prevention-of-copd-%282008-edition%29.html
- Chapman KR, Mannino DM, Soriano JB, Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J 2006; 27:188–207.
- Lopez AD, Shibuya K, Rao Cet al., Chronic obstructive pulmonary disease: Current burden and future projections. Eur Respir J 2006; 27:397–412.
- Atsou K, Chouaid C, Hejblum G. Variability of the chronic obstructive pulmonary disease key epidemiological data in Europe: systematic review. BMC Med 2011; 9:7 Accessed at http://www.biomedcentral.com/17741–7015/9/7.
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370:765–773.
- Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J 2006; 28:523–532.
- Nowak D, Berger K, Lippert B, Kilgert K, Caeser M, Sandtmann R. Epidemiology and health economics of COPD across Europe. A critical analysis. Treat Respir Med 2005; 4(6):381–395.
- Halbert RJ, Isonaka S, George D, Iqbal A. Interpreting COPD prevalence estimates: What is the true burden of disease? Chest 2003; 123:1684–1692.
- Sinn DD, Stafinski T, Ng YC, Bell NR, Jacobs P. The impact of chronic obstructive pulmonary disease on work loss in the United States. Am J Respir Crit Care Med 2002; 165:704–707.
- Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med 2000; 160:1683–1689.
- Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med 2002; 166:675–679.
- Statistical Service of the Republic of Cyprus. Demographic Report 2007 http://www.mof.gov.cy/mof/cystat/statistics.nsf/index_gr/index_gr?OpenDocument.
- ATS Statement. Standardization of spirometry –2005 update. Eur Respir J 2005; 26:319–338.
- Statistical Service of Cyprus. European Health Survey 2008. Health Statist 2008; II:7.
- Tzanakis N, Anagnostopoulou U, Filaditaki V, Christaki P, Siafakas N. Prevalence of COPD in Greece. Chest 2004; 125:892–900.
- Minas M, Hatzoglou C, Karetsi E, Papaioannou Al, Tanou K, Tsaroucha R, Gogou E, Gourgoulianis KI, Kostikas K. COPD prevalence and the differences between newly and previously diagnosed COPD patients in a spirometry program. Prim Care Respir J 2010; 19:363–370.
- Sichletidis L, Tsiotsios I, Gavriilidis A, Chloros D, Kottakis I, Daskalopoulou E, Konstantinidis T. Prevalence of chronic obstructive pulmonary disease and rhinitis in northern Greece. Respiration 2005; 72:270–277.
- Gunen H, Hacievliyagil SS, Yetkin O, Gulbas G, Mutlu LC, Pehlivan E. Prevalence of COPD: First epidemiological study of a large region in Turkey. Eur J Intern Med 2008; 19:499–504.
- Roche N, Dalmay F, Perez T, Kuntz C, Vergnenegre A, Neukirch F, Giordanella JP, Huchon G. Impact of chronic airflow obstruction in a working population. Eur Respir J 2008; 31:1227–1233.
- Peña VS, Miravitlles M, Gabriel R, Jiménez-Ruiz CA, Villasante C, Masa JF, Viejo JL, Fernández-Fau L. Geographic variations in prevalence and underdiagnosis of COPD: results of the IBERPOC multicentre epidemiological study. Chest 2000; 118:981–989.
- Viegi G, Pedreschi M, Pistelli F, Di Pede F, Baldacci S, Carrozzi L, Giuntini C. Prevalence of airways obstruction in a general population: European Respiratory Society vs American Thoracic Society definition. Chest 2000; 117(Suppl 2):339–345.
- Menezes AM, Perez-Padilla R, Jardim JR, Muino A, Lopez MV, Valdivia G, , for the Platino Team. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet 2005; 366:1875–1881.
- Vollmer WM, Gislason P, Burney P, Enright PL, Gulsvik A, Kocabas A, Buist AS for the BOLD Collaborative Research Group. Comparison of spirometry criteria for the diagnosis of COPD: results from the BOLD study. Eur Respir J 2009; 34:588–597.
- Zhong N, Wang C, Yao W, Chen P, Kang J, Shaoguang H Prevalence of chronic obstructive pulmonary disease in China: a large population-based survey. Am J Respir Crit Care Med 2007; 176:753–760.
- Lindberg A, Bjerg-Backlund A, Rönmark E, Larsson LG, Lundbäck B. Prevalence and under-diagnosis of COPD by disease severity and the attributable fraction of smoking: report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med 2006; 100:264–272.
- Johannessen A, Omenaas ER, Bakke PS, Gulsvik A. Implications of reversibility testing on prevalence and risk factors for chronic obstructive pulmonary disease: A community study. Thorax 2005; 60:842–847.
- Lindberg A, Jonsson AC, Rönmark E, Lundgren R, Larsson LG, Lundbäck B. Prevalence of chronic obstructive pulmonary disease according to BTS, ERS, GOLD and ATS criteria in relation to doctor's diagnosis, symptoms, age, gender and smoking habits. Respiration 2005; 72:471–479.
- Tsoumakidou M, Tzanakis, N, Voulgaraki O, Is there any correlation between the ATS, BTS, ERS, and GOLD COPD's severity scales and the frequency of hospital admissions? Respir Med 2004; 98:178–183.
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23:932–946.
- British Thoracic Society. BTS guidelines for the management of chronic obstructive pulmonary disease. Thorax 1997; 52 (suppl 5):1S–28S.
- Miravitlles M, Soriano JB, Garcia-Rio F, Munoz L, Duran-Tauleria E, Sanchez G, Sobradillo V, Ancochea J. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax 2009; 64:863–868.
- Becklake MR. Occupational exposures: evidence for a causal association with chronic obstructive pulmonary disease. Am Rev Respir Dis 1989; 14:S85–S91.
- Stang P, Lydick E, Silberman C, The prevalence of COPD: using smoking rates to estimates disease frequency in the general population. Chest 2000; 117 (Suppl 2):349S–354S.
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, Menezes AM, Sullivan SD, Lee TA, Weiss KB, Jensen RL, Marks GB, Gulsvik A, Nizankowska-Mogilnicka E. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007; 370:741–750.
- Shahab L, Jarvis MJ, Britton J, West R. Prevalence, diagnosis and relation to tobacco dependence of chronic obstructive pulmonary disease in a nationally representative population sample. Thorax 2006; 61:1043–1047.
- Buffels J, Degryse J, Heyrman J, Decramer M. Office spirometry significantly improves early detection of COPD in genarl practice: the DIDASCO Study. Chest 2004; 125:1394–1399.
- Zielinski J, Bednarek M, Górecka D, Viegi G, Hurd SS, Fukuchi Y, Increasing COPD awareness. Eur Respir J 2006; 27:833.
- Soriano JB, Davis KJ, Coleman B, The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United Kingdom. Chest 2003; 124:474–481.
- Sidney S, Sorel M, Quesenberry CP Jr, DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest 2005; 128:2068–2075.
- Van Manen JG, Bindels PJ, IJzermans CJ, van der Zee JS, Bottema BJ, Shade E. Prevalence of comorbidity in patients with a chronic airway obstruction and controls over the age of 40. J Clin Epidemiol 2001; 54:287–293.
- Mapel DW, Hurley JS, Frost FJ, Petersen HV, Picchi MA, Coultas DB. Health care utilization in chronic obstructive pulmonary disease: a case control study in a health maintenance organization. Arch Intern Med 2000; 160:2653–2658.
- Walsh JW, Thomashow BM. COPD and co-morbidities: Results of COPD Foundation national survey. Paper presented at “COPD and co-morbidities: treating the whole patient,” ATS 2006 San Diego International Conference; 2006 May 19–24; San Diego, CA.
- Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL. Patterns of co-morbidities in newly diagnosed COPD and asthma in primary care. Chest 2005; 128:2099–2107.
- Vonk JM, Jongepier H, Panhuysen CI, Schouten JP, Bleecker ER, Postma DS. Risk factors associated with the presence of irreversible airflow limitation and reduced transfer coefficient in patients with asthma after 26 years of follow up. Thorax 2003; 58:322–327.
- Silva GE, Sherill DL, Guerra S, Barbee RA. Asthma as a risk factor for COPD in a longitudinal study. Chest 2004; 126:59–65.
- Guerra S. Overlap of asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med 2004; 11:7–13.
Appendix 1
Urban and rural areas surveyed
Urban Population: Nicosia, Limassol, Larnaca, Paphos,
Rural Population: Akaki, Augorou, Dali, Geri, Geroskipou, Kampos, Klirou, Kofinou, Ormideia, Paralimni, Polemidia, Pyrgos, Trachoni, Tseri, Ypsonas
Appendix 2
Questionnaire used at home visit
Patient number:
Area number:
Investigator number:
Exclusion criteria
Does any of the following apply for yourself?
a) Active tuberculosis? No Yes
b) History of lung cancer? No Yes
c) History of lung resection? No Yes
d) History of cystic fibrosis? No Yes
e) Participation in other clinical trial at present? No Yes
f) Hemoptysis (blood in sputum)? No Yes
g) PneumoThorax? No Yes
h) Recent myocardial infarction (heart attack) or pulmonary embolism? No Yes
i) Recent Chest or abdomen surgery? No Yes
j) Recent eye surgery (e. g. cataract)? No Yes
k) Nausea or vomiting at present? No Yes
l) Dyspnea at present? No Yes
m) Exacerbation of respiratory symptoms at present? No Yes
Study questionnaire
1. Do you smoke? No Yes
If no, go to question 2, if yes:
1.1. How many cigarettes per day do you smoke on average?
1.2. For how many years do you smoke?
1.3. How old were you when you started smoking?
2. Did you smoke in the past? No Yes
2.1. If yes: How many cigarettes per day did you smoke on average?
2.2. For how many years?
2.3. When did you stop smoking (year)?
3. Have you ever had asthma? No Yes
4. Do you usually have cough and phlegm most days in periods of at least three months during at least two successive years? No Yes
5. Have you ever had a spirometry test? No Yes
6. Are you being exposed to dust in your work place? No Yes
7. Are you being exposed to fumes at your work place? No Yes
8. Do you use gas for cooking or heating at home? No Yes
9. Do you live near a polluted area? No Yes
10. Has a doctor ever told you that you have:
10.1. Chronic bronchitis? No Yes
10.2. Emphysema? No Yes
10.3. Chronic Obstructive Pulmonary Disease? No Yes
11. Do you suffer from any of the following?
11.1. Diabetes mellitus No Yes
11.2. Hypertension No Yes
11.3. Coronary heart disease
(angina, previous myocardial infarction) No Yes
11.4. Depression No Yes
Demographics
Day Month Year
12. What is your birth date?
13. Are you male or female? Male Female
14. What is your marital status?
14.1. Single
14.2. Married
14.3. Widowed
14.4. Separated/ Divorced
15. What is your occupation?
15.1. Public employee
15.2. Private employee
15.3. Housewife
15.4. Retired
16. What is your highest educational level?
16.1. Primary education graduate (0–6 years)
16.2. Lower secondary education graduate (7–9 years)
16.3. Upper secondary education graduate (10–12 years)
16.4. Tertiary education graduate (≥ 13 years)
17. Height (cm) Body weight (kg)