Abstract
Identifying chronic obstructive disease (COPD) cases is required to estimate COPD prevalence, to enroll COPD cohorts and to estimate air pollution health effects. Administrative health data are frequently used to identify COPD cases, though their validity has not been satisfactorily assessed. This paper aims to assess the contribution of pharmaceutical data in detecting COPD cases and to estimate the reliability of hospital/mortality databases in detecting COPD cases. Prevalent COPD cases among 35-plus-year-olds were estimated in four Italian areas in 2006 from hospital/mortality registries and adding pharmaceutical data. Age-specific and age-standardized prevalence rates were calculated in each area. Internal validity of COPD diagnoses from hospital and mortality databases was assessed. Pharmaceutical database was used to confirm the hospital/mortality COPD cases and to examine the selection and misclassification of hospitalized cases. Possible misclassification between COPD and asthma cases was estimated using hospital data. Prevalent COPD cases were 77,098 from hospital/mortality registries, 172,357 when respiratory prescriptions were added. Prevalence ranged from 4.0%–6.7%. Only 22.7% of pharmaceutical COPD cases were hospitalized or died and only 37.2% of hospital/mortality cases consumed respiratory medicines; this last proportion increased to 64.5% among the older cases with a principal diagnosis. COPD cases with a contemporary asthma diagnosis were 3.1%. We found that pharmaceutical data increases COPD prevalence estimates 2.2–2.5 times. Hospitalization does not necessarily indicate COPD severity, COPD as a principal diagnosis confirmed with medicine prescription more likely represented true cases. Misclassification affects asthma cases to greater extent than COPD cases.
Introduction
Chronic obstructive pulmonary disease (COPD) is a considerable health problem with a worldwide prevalence of 10% (Citation1), the fifth-leading cause of death in developed countries, and among the top 10 causes of disability in several countries (Citation2).
Given this background, COPD prevalence is an important indicator to evaluate the health status of a population, to plan interventions and evaluate their impact. Unfortunately, detecting COPD cases is challenging because the onset of symptoms is often neglected (Citation3) and the diagnosis is rarely supported by spirometry. Moreover, household health surveys to estimate COPD frequency may be affected by important methodological problems in sampling. A meta-analysis (Citation4) reported prevalence estimates ranging between 6% in the United States and 16% in Sweden. A multi-centre study using the same survey methods (Citation1), reported estimates ranging between 6% in Germany and 19% in South Africa.
The increasing availability of current health databases, particularly cause mortality registers (CMR) and hospital discharge registers (HDR), is a strong encouragement to use administrative data to estimate COPD prevalence, but also to assess COPD treatment outcomes (Citation5,6) and the air pollution effects (Citation7–10). Although less frequently, even prescription data have been used to estimate COPD prevalence (Citation11) and to enroll COPD cohorts (Citation12). Nevertheless, the validity of the administrative sources has not been satisfactorily assessed. On the other hands, different study designs need different characteristics of validity; estimating prevalence requires high sensitivity, enrolling a cohort requires high specificity and to analyze the effects of air pollution one should avoid misclassifying cases.
The sensitivity and positive predictive value (PPV) of hospital and mortality datasets in detecting COPD cases have been estimated with indirect methods (Citation13–16). Algorithms have been developed to improve the use of administrative data sets; those based on multiple sources and taking a longitudinal approach (which includes subjects who received a COPD diagnosis in the previous years) are suggested to increase the sensitivity of COPD prevalence (Citation16,17). Data from general practitioners and specialists were the most frequent additional sources, contributing up to 76% of the total prevalent COPD cases (Citation18). Data from general practitioners have been used also to test the specificity of COPD hospital and dead cases (Citation19,20).
We carried out a study 1) to estimate the reliability of hospital and pharmaceutical data to assess prevalent cases of COPD and 2) to analyze the possible selection and misclassification of COPD cases from administrative databases.
Methods
COPD cases were obtained from four Italian areas with different sized populations and different administrative profiles: the Friuli-Venetia-Giulia (FVG) region, two health districts: Modena and Rovigo, and the city of Rome. HDR and CMR were used as sources of data for the period 2002–2006; pharmaceutical data for 2005–2007.
A COPD case was defined as a 35-plus-year-old subject discharged from hospital with a principal or secondary diagnosis of COPD or a subject who died from COPD (underlying cause of death) or a subject who used respiratory medicines; the definition further identifies probable and possible COPD cases based on the length of treatment and the number of packages consumed in one year (Citation11). Medicines were identified with codes of the Anatomical Therapeutic Chemical (ATC) classification, where those specific for COPD complied with the GOLD (Global Initiative for Chronic Obstructive Lung Disease) guidelines (Citation21). More details about the algorithm to detect pharmaceutical COPD cases are reported in supplemental material (Appendix 1). Human subjects did not participate in the study since only administrative databases were used with no involvement of individuals, and under the privacy laws enforced in Italy.
Prevalent cases in 2006 were counted as residents who were hospitalized in 2002–2006 (the first hospitalization was chosen as index) and were still alive on 1st January 2006, according to the longitudinal approach (Citation16,17), plus those who died from COPD in 2006 and were never hospitalized in 2002–2006, and those who took respiratory medicines starting in 2006, were not hospitalized in 2002–2006 and did not die from COPD in 2006. Subjects’ ages as given in discharge records in 2002–2005 period were updated to the same day and month of 2006.
Two algorithms were developed to estimate prevalence; the first included only hospitalizations and deaths (HDR-CMR), the second added pharmaceutical data (HDR-CMR-DRUG). The two algorithms are reported in detail in supplemental material (Appendix 2), together with the codes of International Classification of Diseases, 9th revision (ICD-9) and the ATC codes. Crude and age standardized rates were estimated as the percentage cases in the resident population as of the 30th of June, 2006, with 95% confidence intervals (95% CI), in each area. The 2006 Italian population was used to standardize rates.
To estimate the magnitude of misclassification of the COPD cases when the administrative diagnoses were used, we assumed that having been prescribed respiratory medicines along with a hospital diagnosis of COPD was more likely to represent true COPD than a diagnosis or a prescription alone. Then we estimated the proportion of the pharmaceutical COPD cases who were discharged with a COPD diagnosis in the 5-year period or who died from COPD in 2006 (a/a+b) (sensitivity) and the proportion of hospitalized or died copd cases who took respiratory medicines (a/a+c) (box 1) (PPV).
The year of treatment follow-up for hospital cases started after discharge for those hospitalized in 2006 or at the time the first pharmaceutical prescription was filled in 2006 for those hospitalized in previous years; for those who died, the year that preceded death was used to assess their treatment.
These analyses were carried out on different age groups (35–64 year-olds, 65–84 year-olds and 85-plus- year-olds), using different definitions of hospital COPD cases (both principal and secondary COPD diagnoses, a principal diagnosis of COPD or a secondary diagnosis combined with a principal diagnosis of respiratory failure (ICD-9 Codes = 518.8, 518.5, 786.0) or congestive heart failure (ICD-9 Codes = 428.0)), and excluding COPD cases who also received an asthma diagnosis (ICD-9 Code = 493) in any hospitalization between 2002 and 2006. We did not carry out sensitivity analysis for the less specific codes of COPD (ICD-9 Codes = 490, 494, 496) since they represented 4.6%. The analyses were done in each area separately, then pooled estimates were obtained with a random meta-analysis and the heterogeneity across the areas was estimated.
To test the hypothesis that the most severe cases of COPD would be selected when hospital and mortality data were used, we assumed that having been prescribed respiratory medicines according to probable case definition along with a hospital COPD diagnosis, was more likely to be a severe COPD case than having been prescribed according to possible case definition or not being hospitalized. We restricted the analysis to the pharmaceutical COPD cases classified according to treatment regimens. Then we estimated the proportion of those who took respiratory medicines according to the probable COPD case definition, who were discharged with COPD or who died from COPD (a/a+b) (sensitivity), the proportion of hospitalized or dead copd cases who took medicines according to the probable COPD case definition (a/a+c) (PPV). In this case, we could add the proportion of those taking respiratory medicines according to the possible COPD case definition who were not hospitalized or did not receive hospital diagnoses of COPD, respiratory failure or heart failure in the 5-year period (d/c+d) (specificity) (box 2).
To estimate the magnitude of misclassification between COPD and asthma, we detected the 35-plus-year-olds who were hospitalized for asthma in 2002–2006. Then, we estimated three proportions: the prevalent COPD cases who had an asthma diagnosis in any hospitalization during 2002-2006, the COPD cases identified from pharmaceutical data only who had a hospital diagnosis of asthma and the asthmatics who never received a COPD diagnosis but could have been identified as prevalent COPD cases according to their medicine intake.
Results
All 76,701 35-plus-year-old subjects who were hospitalized in the 5-year period with a COPD diagnosis were included as COPD prevalent cases in 2006. COPD was registered as the principal diagnosis for 26% of these cases; this proportion grew to 34% adding, in turn, cases with respiratory failure or heart failure as the principal diagnosis and COPD as the secondary. Subjects who died from COPD in 2006 and had not been hospitalized, contributed 397 more prevalent cases. Pharmaceutical data identified 95,259 additional cases. There were a total of 77, 098 prevalent cases of COPD in 2006 according to the hdr-cmr algorithm and 172,357 using the hdr-cmr-drug algorithm ().
Table 1. Study population, according to different algorithms, four areas, 2006.
The two estimates of COPD prevalence had similarities, in all areas; age-standardized rates increased adding the pharmaceutical data; the values obtained using the hdr-cmr-drug algorithm ranged between 4.03% of the residents in the FVG region and 6.71% of the residents in Rome, with estimates that are between 2.5 and 2.2 times higher than thehdr-cmr algorithm, respectively (). In each area, COPD prevalence increased proportionally with age and males showed higher values than females at any age ().
Figure 1. Age specific prevalence rates, for men and females, using the algorithm that includes pharmaceutical data - four Italian areas, 2006.
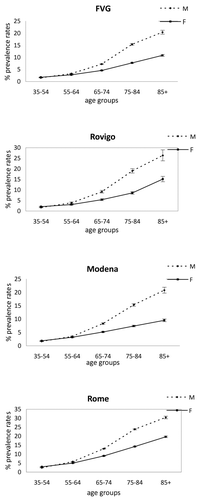
Table 2 Raw and standardized prevalence rates of COPD in 35-plus-old subjects.
According to the hdr-cmr-drug algorithm, the COPD cases were 33% in 35–64 year-old, 55% in 65–84 and 11% in 85-plus-year-olds (). Deaths increased from 0.02% in 35–64 year-olds to 0.2% and 1.1% in the older groups, respectively; hospitalizations increased from 23% to 53% and 69%, respectively, whereas pharmaceutical-identified cases decreased from 77% to 47% and 30%, respectively, in the older groups.
The proportion of pharmaceutical COPD cases who were hospitalized or died from COPD, was low (22.7%; 95% CI = 19.9% –25.9%) (). Even the proportion of COPD cases who took medicines after hospital discharge or before death was low (37.2%; 95% CI = 29.4%-47.1%) (). The 65-84 year-olds showed the highest values of predictive value, whichever COPD hospital case definition had been used (). Sensitivity decreased but predictive value increased when hospital cases were selected by principal diagnoses, in all age groups (Tables and ). Excluding COPD cases who had received an asthma diagnosis slightly reduced both sensitivity and predictive value. A high heterogeneity of sensitivity was observed across cities, for any COPD case definition in subjects over 85.
Table 3. Pooled results: % of pharmaceutical COPD cases who were dicharged with a COPD or died from COPD, by hospital case definition and age, 2006.
Table 4. Pooled results: % hospitalised or dead COPD cases who consumed drugs after discharged or before death, by hospital case definition and age, 2006.
When the analyses were restricted to those who took medicines to compare probable and possible COPD cases, the proportion of the pharmaceutical COPD cases who were hospitalized or died from COPD was still low: 24.2% (95% CI = 21.4% –27.0%) (). In contrast, the proportion of COPD hospitalized or dead cases who took medicines coherently with a reliable definition of pharmaceutical case (probable cases), reached 96.5% (95% CI = 95.7% - 97.2 0%) (); likewise, the proportion of those not hospitalized or dead subjects who took medicines coherently with a less-definite definition of COPD case (possible cases), reached 91.3% (95% CI = 89.4% –93.3%) (). Sensitivity increased with age, PPV was similar in all age groups, but specificity decreased at older ages.
Table 5. Pooled results: % pharmaceutical probable COPD cases who died or were hospitalised; % of hospitalised/dead COPD cases who took medicines (probable case); percentage of not hospitalised/dead COPD cases who took medicines (possible case), by age, 2006.
Among prevalent COPD cases identified by algorithm including pharmaceutical data, 3.1% received a hospital diagnosis of asthma. The COPD cases who had also an asthma diagnosis were slightly younger (average age 66.2 years) than those without asthma (average 68.7 years). Among prevalent COPD from pharmaceutical data only, 2.6% had an asthma diagnosis in the period 2002-2006. In contrast, among 4981 subjects who received a hospital diagnosis of asthma but no COPD diagnosis, 47.6% could have been classified as COPD according to their medicine intake.
Up to 98% of subjects consumed respiratory medicines from the ATC groups, which were recommended for COPD. All probable COPD cases used beta-2-agonists, 36.4% took anticholinergics, 18.2% xantines and at least 61.5% glucocorticoids (). It is noteworthy that 12% of probable COPD cases took leukotriene receptor antagonists, though they are recommended to asthmatics ().
Table 6. Subjects classified as COPD cases, according to pharmaceutical COPD definitiona by disease reliability and ATC groups.
Discussion
We found that adding pharmaceutical data to hospital and mortality data, contributed 55% additional COPD prevalent cases. Few cases among those treated required hospitalization in the previous years, the highest values were observed in 85-plus-year-olds; few cases among those discharged or who died from COPD, took respiratory medicines, the highest values were observed in 65–84 year-olds when principal diagnoses were used to define cases. The COPD prevalent cases who had an asthma diagnosis were 3.1%.
Using both a longitudinal approach and adding pharmaceutical data contributed to higher estimates of COPD prevalence. The former leads to estimates that are three times higher than the cross-sectional approach and the latter doubled the prevalence as estimated using hdr-cmr algorithm. As there is a lack of external validation for the algorithms that included all the available health data (hospitalizations, deaths and medicines), the previous Italian estimates of COPD prevalence and the relationship observed between gender and age groups make us confident in our results. It is possible however that true COPD cases were underestimated due to under-diagnosis and therefore under-treatment (Citation22). An average COPD prevalence of about 13% has been estimated in seven Italian surveys (Citation2,Citation4) carried out between 1999 and 2003. These studies included all severity GOLD stages, and a large meta-analysis (Citation4) showed that including GOLD first stage cases doubled the prevalence based on GOLD second stage cases. Moreover, one of the aforementioned surveys estimated a 3.6% COPD prevalence for moderate/severe cases (Citation23). In spite of large variations in estimates, many countries have data coherent with characteristics we found for COPD, like increases with age and more frequent occurrence in males (Citation1,Citation24). Results from a recent 10-European-country cohort (Citation25) also confirm higher incidence of COPD in males than in females and a clear age-trend for COPD cases according to the GOLD definition. An increasing trend in females has been reported in some countries in more recent years (Citation26). Nevertheless, the caution in using pharmaceutical data to estimate COPD prevalence in previous studies is the result of significant challenges, such as the difficulties in differentiating COPD treatment from asthma treatment, finding a temporal definition that corresponds to a chronic treatment and having an exhaustive validation of the pharmaceutical data.
Sensitivity and predictive values of hdr-cmr algorithm were very low. The sensitivity increased with age, confirming that exacerbations and complications of COPD are likely to be more frequent in older subjects (Citation27). Nevertheless, sensitivity never exceeded 40%; thus indicating that data from hospital and mortality registers underestimate prevalent COPD cases.
Comparing hospital case definitions showed that principal diagnoses of COPD, respiratory failure or congestive heart failure detected fewer COPD cases but they were more likely to be true cases, as a higher percentage of these cases also took respiratory medicines. In other words, a COPD case definition based on the principal diagnoses further underestimated prevalence but improved the reliability of hospital COPD cases making them suitable for enrolment in a COPD cohort, as has been suggested for other chronic diseases (Citation28).
Our conclusions are conditioned by the validation criterion we used; the Bayesian latent class analysis could represent a possible alternative to validate COPD cases using pharmaceutical data, since it would be able to assess the validity of multiple sources and adjust for the imperfect sensitivity and specificity of each source, in the absence of a real gold standard (Citation29–31).
Hospitalized COPD cases showed a low reliability. Now, if it is reasonable to expect that only 22.7% of COPD cases among those treated had required hospitalization in a 5-year period, it is surprising that only 37.2% of discharged or dead COPD cases took respiratory medicines. Although this proportion increased using principal diagnoses, it never exceeded 65%. Similar results have been reported previously in Canada (Citation13), where up to 47% of clinically diagnosed COPD patients were not prescribed beta-2 agonist inhalants. These results are difficult to interpret as they clearly reject the hypothesis that hospital-diagnosed COPD cases are the most reliable; rather, they suggest a possible negative misclassification of hospital cases.
When we assessed the severity of COPD cases among those who took medicines, the hypothesis that hospitalized COPD cases were the most severe (Citation32) was confirmed, since they were prescribed as probable cases much more frequently (96.5%) than as possible cases, and up to 92.6% of possible cases were not hospitalized or died. It is worthy to note that a very recent study (Citation33) which validated hospital and pharmacy COPD cases with spirometry results, reported validity estimates (36% the sensitivity, 92% the specificity and 81% the PPV) very similar to those observed here.
On the other hand, if we wanted a more reliable definition of COPD, to be enrolled into a cohort we should add to the previous criteria of a principal diagnosis and advanced age (65–84), meeting the criteria for a probable pharmaceutical case.
There are other implications from these results about the use of current health datasets. Younger cases were less frequently detected by the hospital/mortality dataset than the older ones, but they were similarly related to medicine consumption; one possible interpretation is that COPD exacerbations are less likely in young people, another is more adequate home treatment in younger than in older people. Younger cases had the highest specificity for treatments, thus the few cases hospitalized are likely to be reliable and severe. This pattern is more evident when principal diagnoses of COPD or its complications were analyzed, making the reliability of younger COPD patients identified by a secondary diagnosis very low. Moreover, the youngest pharmaceutical cases may suffer from lower reliability, since they increased four-fold (instead of two-fold in the older groups). In contrast, the 85-plus-year-old COPD cases who take respiratory medicines were more likely to be hospitalized or die from COPD in the study period, but those who were hospitalized or died were the least likely to take medicines after discharge or before death, and there were fewer possible cases who were never hospitalized or died; these results did not change with the principal or secondary diagnosis. Thus, the low specificity and predictive value of the oldest cases are more likely to be due to the validation criteria (based on medicines consumption) than to the poor reliability of hospital diagnoses. Good treatment compliance as well as appropriate prescriptions are more difficult in oldest patients (Citation34) but in turn, this may contribute to increasing hospitalizations. In conclusion, pharmacological COPD case definitions are less adequate to validate older COPD cases.
Only 3.1% of prevalent COPD and only 2.6% of those identified by medicines only had a diagnosis of asthma, suggesting a low misclassification between COPD and asthma in 35-plus-old subjects. These estimates cannot be conclusive since they were obtained from hospital cases of asthma, who account for almost 3% of the prevalent asthma in young adults in Italy (Citation35). Estimating COPD-asthma misclassification would have to take into account asthmatics in the general population. Previous studies selected older COPD patients (45-plus-year-olds or 50-plus-year-olds) (Citation11,12) to reduce the COPD-asthma misclassification; however not decreasing the asthmatic proportions in 65-year-old-or-older COPD cases in this study and observing an important consumption of leukotriene receptor antagonists among the definite pharmaceutical cases suggest that the hospital cases included different phenotypes of COPD, particularly patients with overlap syndrome asthma-COPD (Citation36) or those for whom long-standing asthma concurred to an irreversible airways obstruction (Citation27). We cannot exclude a misclassification due to frank diagnostic errors between COPD and adult asthma, since we had no data on reversible airways obstruction, bronchial responsiveness, smoking history or airway inflammation (Citation36, 37).
Although we recognize some limits of our analysis, the consistency of the reliability of the estimates among areas that differ so much in population size and health care management, makes us confident in the generalizability of our findings.
Conclusions
The use of hospitalization or mortality data in detecting COPD cases is perhaps too conservative a method. The use of pharmaceutical data in identifying COPD cases should be optimized in order to obtain a more reliable COPD prevalence estimate.
Declaration of Interest
All the authors declare that they have no competing interests.
Acknowledgments
We thank Margaret Becker for revising the English.
References
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, Menezes AM, Sullivan SD, Lee TA, Weiss KB, Jensen RL, Marks GB, Gulsvik A, Nizankowska-Mogilnicka E; BOLD Collaborative Research Group. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007; 370:741–750.
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370:765–773.
- Pawels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004; 364:613–620.
- Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J 2006; 28:523–532.
- Sunyer J, Schwartz J, Toblas A, Macfarlane D, Garcia J, Antò JM. Patients with chronic obstructive pulmonary disease are at increased risk of death associated with urban particle air pollution: a case-crossover analysis. Am J Epidemiol 2000; 151:50–56.
- Ackermann-Liebrich U, Kuna-Dibbert B, Probst-Hensch NM, Schindler C, Felber Dietrich D, Stutz EZ, Bayer-Oglesby L, Baum F, Brändli O, Brutsche M, Downs SH, Keidel D, Gerbase MW, Imboden M, Keller R, Knöpfli B, Künzli N, Nicod L, Pons M, Staedele P, Tschopp JM, Zellweger JP, Leuenberger P; SAPALDIA Team. Follow-up of the Swiss Cohort Study on Air Pollution and Lung Diseases in Adults (SALPADIA 2) 1991–2003: methods and characterization of participants. Soz Präventivmed 2005; 50:245–263.
- Pope CA 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc 2006; 56:709-742.
- Analitis A, Katsouyanni K, Dimakopoulou K, Samoli E, Nikoloulopoulos AK, Petasakis Y, Touloumi G, Schwartz J, Anderson HR, Cambra K, Forastiere F, Zmirou D, Vonk JM, Clancy L, Kriz B, Bobvos J, Pekkanen J. Short-term effects of ambient particles on cardiovascular and respiratory mortality. Epidemiology 2006; 17:230–233.
- Zanobetti A, Schwartz J. Air pollution and emergency admissions in Boston, MA. J Epidemiol Commun Health 2006; 60:890–895.
- Peel JL, Paige E, Tolbert PE, Klein M, Metzger KB,Dana Flanders W, Todd K, Mulholland JA, Ryan PB, Frumkin H. Ambient air pollution and respiratory emergency department visits. Epidemiology 2005; 16:164–174.
- Anechino C, Rossi E, Fanizza C, De Rosa M, Tognoni G, Romero M for working group “ARNO project”. Prevalence of chronic obstructive pulmonary disease and pattern of comorbidities in a general population. Inter J COPD 2007; 2:567–574.
- Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest 2005; 128:2640–2646.
- Lacasse Y, Montori VM, Lanthier C, Maltis F. The validity of diagnosing chronic obstructive pulmonary disease from a large administrative database. Can Respir J 2005; 12:251–256.
- Wigertz A, Westerling R. Measures of prevalence: which healthcare registers are applicable? Scand J Publ Health 2001; 29:55–62.
- Motheral B, Brooks J, Clark MA, Crown WH, Davey P, Hutchins D, Martin BC, Stang P. A checklist for retrospective database studies–report of the ISPOR Task Force on Retrospective Databases. Value Health 2003; 6:90–97.
- Wiréhn AB, Karlsson HM, Carstensen JM. Estimating disease prevalence using a population-based administrative healthcare database. Scand J Public Health 2007; 35:424–431.
- Faustini A, Cascini S, Arcà M, Balzi D, Barchielli A, Canova C, Galassi C, Migliore E, Minerba S, Protti MA, Romanelli A, Tessari R, Vigotti MA, Simonato L. Chronic obstructive pulmonary disease prevalence estimated using a standard algorithm based on electronic health data in various areas of Italy. Epidemiol Prev 2008; 32 (Suppl):46–55. (Italian)
- Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic Obstructive Pulmonary Disease surveillance—United States, 1971–2000. MMWR — Surveillance Summary 2002; 51:1–16.
- Soriano JB, Maier WC, Visick G, Pride NB. Validation of general practitioner-diagnosed COPD in the UK general practise research database. Eur J Epidemiol 2001; 17:1075–1080.
- Hansel A, Hollowell J, McNiece R, Nichols T, Strachan D. Validity and interpretation of mortality, health service and survey data on COPD and asthma in England. Eur Respir J 2003; 21:279–286.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease, GOLD 2006 pp 1–100 (available at the site http://www.goldcopd.com/; last access 1/22/07).
- Price D, Duerden M. Chronic obstructive pulmonary disease. The lack of a national service framework should not allow us to ignore it. BMJ 2003; 326:1046–1047.
- Viegi G, Pedreschi M, Pistelli F,Di Pede F, Baldacci S, Carrozzi L, Giuntini C. Prevalence of Airways Obstruction in a General Population: European Respiratory Society vs American Thoracic Society Definition. Chest 2000; 117:339S–345S.
- Halbert RJ, Isonaka S, George D, Iqbal A. Interpreting COPD prevalence estimates. Chest 2003; 123:1684–1692.
- de Marco R, Accordini S, Marcon A, Cerveri I, Antó JM, Gislason T, Heinrich J, Janson C, Jarvis D, Kuenzli N, Leynaert B, Sunyer J, Svanes C, Wjst M, Burney P; for the European Community Respiratory Health Survey (ECRHS). Risk factors for chronic obstructive pulmonary disease in a European cohort of young adults. Am J Respir Crit Care Med 2011; 183:891–897. (Epub ahead of print).
- Han ML, Postma D, Mannino D, Giardino ND, Buist S, Curtis JL, Martines FJ. Gender and COPD: why it matters. Am J Respir Crit Care Med 2007; 176:1179–1184.
- Eisner MD, Anthonisen N, Coultas D, Kuentzli N, Peres-Padilla R, Postma Det, Romieu I, Silverman EK, Balmes JR; Committee on Nonsmoking COPD, Environmental and Occupational Health Assembly. An official American Thoracic Society Public Policy Statement: novel risk factors and the global burden of chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2010; 182:693–718.
- Saydah SH, Geiss L, Tierney ED, Benjamin SM, Engelgau M, Brancati F. Review of the performance of methods to identify diabetes cases among vital statistics, administrative, and survey data. Ann Epidemiol 2004; 14:507–516.
- Bernatsky S, Joseph L, Belisle P, Boivin JF, Rajan R, Moore A, Clarke A. Bayesian modelling of imperfect ascertainment methods in cancer studies. Stat Med 2005; 24:2365–2379.
- Nascimento MC, de Souza VA, Sumita LM, Freire W, Munoz F, Kim J, Pannuti CS, Mayaud P. Comparative study of Kaposi's sarcoma-associated herpes virus serological assays using clinically and serologically defined reference standards and latent class analysis. J Clin Microbiol 2007; 45:715–720.
- Bernatsky S, Joseph L, Pineau CA, Belisle P, Lix L, Banerjee D, Clarke AE. Polymyalgia rheumatica prevalence in a population-based sample. Arthritis Rheum 2009; 61:1264–1267.
- O'Brien JA, Ward AJ, Jones MK, McMillan C, Lordan N. Utilization of health care services by patients with chronic obstructive pulmonary disease. Respir Med 2003; 97 Suppl A:S53–58.
- Cooke CR, Joo MJ, Anderson SM, Lee TA, Udris EM, Johnson E, Au DH. The validity of using ICD-9 Codes and pharmacy records to identify patients with chronic obstructive pulmonary disease. BMC Health Serv Res 2011; 11:37
- Gallagher PF, Barry PJ, Ryan C, Hartigan I, O'Mahony D. Inappropriate prescribing in an acutely ill population of elderly patients as determined by Beers’ Criteria. Age Aging 2008; 37: 96–101.
- Tessari R, Migliore E, Balzi D, Barchielli A, Canova C, Faustini A, Galassi C, Simonato L. Asthma prevalence estimated using a standard algorithm based on electronic health data in various areas of Italy. Epidemiol Prev 2008; 32 suppl 1:56–65.
- Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it ? Thorax 2009; 64:728–735.
- Contoli M, Baraldo S, Marku B, Casolari P, Marwick JA, Turato G, Romagnoli M, Caramori G, Saetta M, Fabbri LM, Papi A. Fixed air flow obstruction due to asthma or chronic obstructive pulmonary disease: 5-year follow-up. J Allergy Clin Immunol 2010; 125:830–837.
Appendix 1
Pharmaceutical definition of COPD case



