Abstract
GOLD stage II COPD encompasses patients with FEV1 50–80% predicted. A published trials review suggested that benefits of maintenance therapy are limited to patients with FEV1 <60% predicted. We previously reported data demonstrating the efficacy of tiotropium in GOLD stage II disease in the 4-year UPLIFT® trial, and present here a further analysis of a sub-category of GOLD stage II patients with post-bronchodilator FEV1 ≥60% predicted from UPLIFT®. Outcomes included pre- and post-bronchodilator spirometry, exacerbations, SGRQ and mortality. Of the 5,992 UPLIFT® cohort, 1,210 (632 tiotropium, 578 control) had baseline post-bronchodilator FEV1 ≥60% predicted (range 60–78%), mean age was 64 years, 70% were men, and mean SGRQ total score was 39.9 units. Mean annual rate of post-bronchodilator FEV1 decline was 41 (tiotropium) and 49 (control) mL/year (P = 0.07); corresponding pre-bronchodilator values were 32 and 37 mL/year (P = 0.24). Morning pre-drug FEV1 and FVC improvements for tiotropium versus control were 87–127 mL and 139–186 ml, respectively (P < 0.001, all time-points). SGRQ total score improvements (tiotropium–control) were 2.0–3.4 units (P < 0.05 for all); a higher percentage of patients had an improvement of ≥4 units with tiotropium (P <0.05). Tiotropium reduced risk for an exacerbation (HR [95% CI] = 0.83 [0.71, 0.96]) and mortality for the 4-year protocol-defined treatment period (HR [95% CI] = 0.66 [0.45, 0.96]). Tiotropium treatment provides clinical efficacy in patients with GOLD stage II disease with an FEV1 ≥60% predicted, supporting current GOLD guidelines for COPD treatment. (ClinicalTrials.gov number NCT00144339).
Introduction
International guidelines for the management of chronic obstructive pulmonary disease (COPD) utilize a categorization system of disease severity based on post-bronchodilator forced expiratory volume in 1 second (FEV1) values (Citation1). Initiation of maintenance bronchodilator therapy is recommended in patients with Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage II disease or worse; GOLD stage II is defined by a post-bronchodilator FEV1/forced vital capacity (FVC) ratio of < 0.70 and a FEV1 between 50–80% predicted normal (Citation1). Guidelines have indicated a preference for inhaled long-acting bronchodilators (Citation1).
Clinical trial data have demonstrated improvements in both lung function and health outcomes with inhaled bronchodilator treatment in patients with GOLD stage II disease, which support current treatment recommendations (Citation2,3). However, many clinical trials have inclusion criteria that do not encompass the full spectrum of GOLD stage II patients because they exclude the upper end of the FEV1 range within this category. A previously published review of trials suggested that benefits of maintenance therapy are limited to patients with FEV1 < 60% predicted, based on the limited data in the upper end of the spectrum of GOLD stage II patients in clinical trials (Citation4). Moreover, the efficacy of tiotropium specifically in patients with an FEV1 above 60% predicted has also been questioned (Citation5).
The Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT®) trial is a 4-year, randomized, double-blind, placebo-controlled trial of tiotropium in COPD patients with post-bronchodilator FEV1 of ≤70% of predicted (Citation6). Although tiotropium did not alter the rate of decline of FEV1 in the full cohort, there were sustained improvements in FEV1, FVC, slow vital capacity (SVC), health-related quality of life (HRQoL) and exacerbations relative to the control group (primary endpoint). We previously reported data demonstrating the clinical efficacy of tiotropium in a prespecified subgroup analysis of GOLD stage II patients in the UPLIFT® trial (Citation2); however, data in that analysis were not specifically broken down into further severity groups within GOLD stage II. Therefore, we conducted a further post-hoc subgroup analysis of COPD patients with a baseline post-bronchodilator FEV1 ≥ 60% predicted in the UPLIFT® trial, in order to add to the limited available data demonstrating the benefits of treating patients within this GOLD stage II subcategory.
Methods
Study design
UPLIFT® was a 4-year, randomized, placebo-controlled, clinical trial of tiotropium in 5,992 treated patients with COPD (Citation6). Details of the methodology have been described elsewhere (Citation6). In brief, patients were randomized to 18 μg tiotropium or placebo (control) once daily. All respiratory medications with the exception of other inhaled anti-cholinergics were permitted throughout the trial. The co-primary endpoints were the yearly rate of decline in pre- and post-bronchodilator FEV1. Secondary endpoints were: time to first COPD exacerbation and hospitalization for exacerbation; numbers of exacerbations and hospitalizations for exacerbations; FEV1, FVC and SVC at all time points; HRQoL measured using the St George's Respiratory Questionnaire (SGRQ); and mortality (on-treatment and on-treatment with vital status follow-up). All patients gave written informed consent for participation in the study. The study was approved by local ethical review boards and conducted in accordance with the Declaration of Helsinki.
Study population
Patients were aged ≥ 40 years, with a smoking history of ≥10 pack-years, post-bronchodilator FEV1 of ≤ 70% of predicted and FEV1/FVC ratio of ≤ 0.70. Exclusion criteria included a history of asthma, COPD exacerbation or respiratory infection within 4 weeks of screening, prior pulmonary resection and use of supplemental oxygen for >12 hours per day. The current analysis was conducted in the subgroup of patients with a post-bronchodilator FEV1 ≥ 60% at baseline.
Statistical analysis
For this report, the analysis was confined to the subgroup of subjects with a post-bronchodilator FEV1 ≥ 60% at baseline. The decline of pulmonary function over time was analyzed with random coefficient regression in which the FEV1 changed linearly after 30 days for each patient, intercepts and slopes were random and the treatment effect was fixed. The mean FEV1 at each visit was estimated using a repeated measures ANCOVA model including treatment, treatment by visit and baseline by visit interactions. Similarly, the mean SGRQ was estimated using the repeated measures ANCOVA model. Kaplan-Meier curves for the probability of first exacerbation and death from any cause were constructed and Cox regression was used to estimate hazard ratios (HR). The number of events between the study groups and relative risks were compared using Poisson regression, with adjustment for over-dispersion. No explicit corrections for multiplicity were made in this presentation of post-hoc analyses.
Results
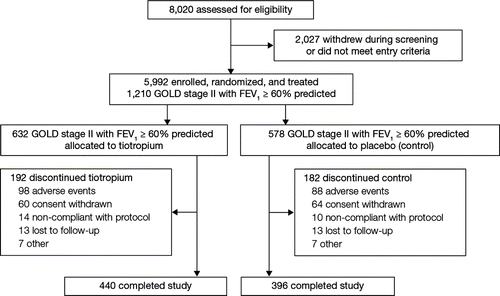
Among the 5,992 treated patients in the total UPLIFT® cohort, 2,739 had GOLD stage II disease. Of these, 1,210 patients (632 in the tiotropium group; 578 in the control group) had a baseline post-bronchodilator FEV1 ≥ 60% predicted (range, 60–78%) and were included in the current analysis (). This includes 23 GOLD stage II patients randomized with post-bronchodilator FEV1 > 70% which, although representing a protocol violation, are included in this analysis. There were a comparable percentage of randomized patients with a baseline post-bronchodilator FEV1 ≥ 60% predicted who discontinued tiotropium versus control treatment (30.4 versus 31.5%).
Patient baseline characteristics
Baseline characteristics of patients with a baseline post-bronchodilator FEV1 ≥ 60% predicted are summarized in . Although a higher proportion of the control patients were current smokers (36 versus 29%; P = 0.011), none of the other listed baseline characteristics differed significantly between the two treatment groups. It is noteworthy that over 50% of these patients with a post-bronchodilator FEV1 ≥ 60% predicted were already receiving maintenance therapy with a long-acting β2-agonist (LABA) and/or inhaled corticosteroid (ICS) at baseline and over a third had been receiving anti-cholinergic therapy.
Table 1. Baseline characteristics of patients with post-bronchodilator FEV1 ≥ 60% predicted in the UPLIFT® trial
Lung function outcomes
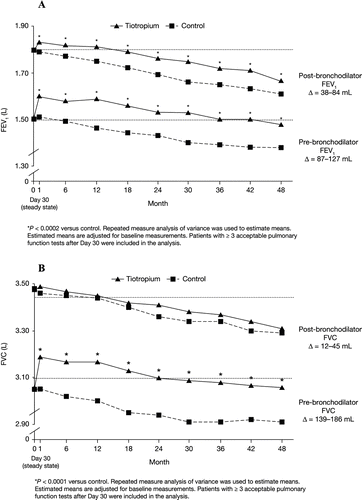
The mean ± standard error (SE) annual rate of decline in post-bronchodilator FEV1 was 41 ± 3 mL/year in the tiotropium group and 49 ± 3 ml/year in the control group (P = 0.07). The corresponding pre-bronchodilator values were 32 ± 3 ml/year and 37 ± 3 mL/year, respectively (P = 0.24). Improvements in pre- and post-bronchodilator FEV1 for tiotropium versus control ranged from 87–127 mL and 38–84 mL, respectively (P ≤ 0.002 for all time-point comparisons) (). Tiotropium also improved pre-bronchodilator FVC values versus control by 139–186 mL (P < 0.0001 for all time-point comparisons) ().
Other outcomes
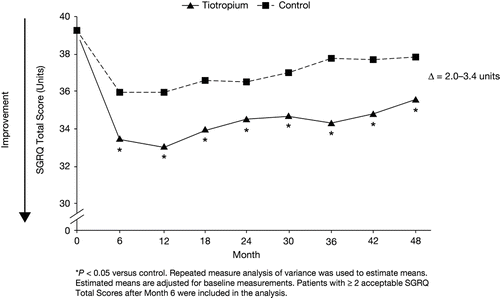
SGRQ total scores were improved with tiotropium versus control (treatment difference of 2.0–3.4 units; P < 0.05 for all comparisons) (). A significantly higher proportion of patients in the tiotropium group compared with the control group had an improvement of SGRQ total score ≥ 4 units after 4 years (52% versus 44%; P < 0.05).
Figure 3. St George's Respiratory Questionnaire total score over 4 years in the tiotropium and control groups with FEV1 ≥ 60% predicted normal.
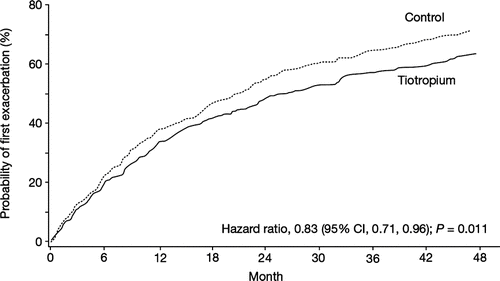
A total of 56% of patients in the tiotropium group experienced an exacerbation during the trial period compared with 62% of patients in the control group, corresponding to a 17% reduction in the risk for an exacerbation with tiotropium (HR 0.83; 95% confidence interval [CI] 0.71, 0.96; P = 0.011) (). Exacerbations requiring hospitalization (severe exacerbations) were experienced by 13% and 15% of tiotropium and control patients, respectively; the risk of experiencing a hospitalized exacerbation was not significantly different between groups (HR 0.86; 95% CI 0.64, 1.16; P = 0.334).
Figure 4. Probability of first exacerbation in the tiotropium and control groups with FEV1 ≥ 60% predicted normal.
Overall survival was improved with tiotropium for the protocol-defined treatment period (censored at Day 1,440): 47 patients died from any cause in the tiotropium group compared with 64 patients in the control group (HR 0.66; 95% CI: 0.45, 0.96; P = 0.031) (). A Mortality Adjudication Committee (MAC) classified the primary causes of death in all UPLIFT® participants who were reported to have died and for whom sufficient records were available (7). The adjudicated primary causes of death in the 1210 subjects in the GOLD Stage II subcategory of post-bronchodilator FEV1 ≥ 60% predicted by MedDRA system organ class and preferred term were as follows: other than causes classified under general disorders and administration site conditions, the most common cause was malignancy (13.5% non-respiratory, 21.6% lung cancer and 3.6% other respiratory system neoplasms), followed by cardiac disorders (10.8%), non-malignant lower respiratory disorders (10.8%, including 8.1% COPD exacerbations and 1.8% pneumonias).
Figure 5. Probability of death (any cause) in the tiotropium and control groups with FEV1 ≥ 60% predicted normal.
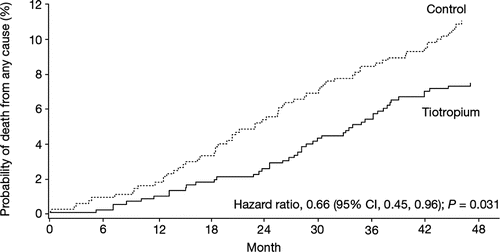
Among general disorders 6.3% were adjudicated as sudden cardiac death and 0.9% as sudden death. Numerically more cardiac deaths and deaths due to COPD exacerbation occurred in the placebo group and numerically more deaths due to cancer occurred in the tiotropium group. These findings are similar to those that have been reported for all the GOLD Stage II participants in the UPLIFT® trial, as reported by McGarvey et al. (Citation7).
Discussion
The authors of a comprehensive review of 42 randomized controlled trials (RCTs) and 8 previous meta-analyses of pharmacotherapy for COPD concluded that evidence of treatment effectiveness was limited to symptomatic patients (especially those with dyspnoea and frequent exacerbations) with a FEV1 < 60% predicted (Citation4). The threshold of 60% predicted FEV1 suggested by these authors for evidence of therapeutic efficacy was based largely on the scarcity of reports of treatment trials that either enrolled patients with a FEV1 above this threshold or included sub-analyses of treatment results in such patients. Nearly all of the treatment trials included in their review enrolled symptomatic COPD patients with a mean FEV1 less than 50% predicted. On the other hand, it is possible that post-hoc analyses of subgroups of patients with less severe disease who were included in these trials, including those with a FEV1 ≥ 60% predicted, would demonstrate significant treatment benefits.
We have previously demonstrated that tiotropium improved outcomes relative to control in the GOLD II subgroup of patients in the UPLIFT® trial (i.e., those with a post-bronchodilator FEV1 of > 50% predicted and < 80% predicted, in a prespecified analysis (Citation2). To address the possibility that the portion of UPLIFT® patients in the GOLD stage II subcategory with FEV1 ≥ 60% would show similar efficacy with tiotropium, we performed the present post-hoc sub-analysis. We found that, in the subgroup of patients with a FEV1 ≥ 60% predicted normal, treatment with tiotropium (with or without concomitant therapy with a LABA and/or ICS as prescribed by the treating physician) provided clinical efficacy in terms of significant improvements in lung function and HRQoL and reduced risk of exacerbations and all-cause mortality compared to placebo (with or without concomitant prescribed therapy with a LABA and/or ICS).
A previously reported subgroup analysis of the results in the entire UPLIFT® population by smoking status indicated that the benefits of tiotropium were similar across different smoking categories (sustained ex-smokers, continuing smokers and intermittent smokers), except for a significant reduction in all-cause mortality in the sustained ex-smokers but not the continuing smokers (Citation8). Because a significantly higher proportion of control patients than those assigned to tiotropium among the subset with a post-bronchodilator FEV1 ≥ 60% predicted were current smokers at baseline (), we conducted a sensitivity analysis to adjust for the potential influence of the baseline imbalance.
The results were very similar to those without adjusting for smoking status. For example, the HR for all-cause mortality changed only slightly from 0.66 (without the adjustment) to 0.68 (with the adjustment). Therefore, it appears that the mortality benefit in tiotropium group was not driven by fewer current smokers compared with the placebo group. The totality of the beneficial results with tiotropium in the present subgroup of moderate COPD patients supports the current GOLD guidelines for the treatment of COPD, which recommend initiating inhaled long-acting bronchodilator maintenance therapy in persistently symptomatic patients with GOLD stage II disease, without distinction as to the degree of impairment in FEV1% predicted within the moderate severity category.
The current results extend the findings from subgroup analyses of data from two long-term global randomized placebo-controlled trials in patients with GOLD stage II COPD. In the Towards a Revolution in COPD Health (TORCH) trial (Citation9), twice daily salmeterol 50 μg, fluticasone 500 μg and their fixed combination were compared with each other and with placebo over 3 years in 6,112 COPD patients with a pre-bronchodilator FEV1 < 60% predicted. A subsequent post-hoc sub-analysis included 2,156 patients with GOLD stage II to IV (Citation3).
The salmeterol/fluticasone combination demonstrated improvements over placebo in adjusted mean FEV1 in GOLD stage II patients that were at least comparable to those observed in GOLD stages III and IV patients. Significant improvements in health-related quality of life and reductions in the annual rate of moderate/severe exacerbations and in all-cause mortality were also noted. Close examination of the data suggest at least numeric benefits of salmeterol and fluticasone monoproducts versus placebo with respect to improving these outcomes among the GOLD stage II patients. Data relating to the 796 (37%) of patients within the GOLD stage II subgroup who had milder COPD (post-bronchodilator FEV1 ≥ 60% predicted) were not reported separately.
In a subgroup analysis of 2,739 GOLD stage II participants in the other large-scale, long-term trial of pharmacotherapy in COPD, the UPLIFT® trial, improvements in pre- and post-bronchodilator FEV1 with tiotropium compared with control over the 4-year course of the study were at least comparable to those noted in the subjects with severe and very severe disease, as were improvements in health status and reductions in exacerbations and all-cause mortality (Citation2).
A further breakdown of these subjects with moderate COPD to include only the 1,210 patients with a post-bronchodilator FEV1 ≥ 60%, as reported herein, revealed similar benefits of tiotropium over control. The latter findings fill the gaps in either the design of, or analyses of findings from, previous reports that led to the conclusion that data on the impact of pharmacotherapy in patients within the milder spectrum of GOLD stage II COPD were too sparse to support the benefits of inhaled therapies in patients with a post-bronchodilator FEV1 > 60% predicted (Citation4).
A small (N = 224), relatively short-term (3-month) RCT was designed specifically to compare the efficacy of tiotropium versus placebo in symptomatic patients (Medical Research Council dyspnoea score ≥ 2) with mild to moderate COPD, namely those with a post-bronchodilator FEV1 ≥ 60% predicted (Citation10). Findings from this RCT, in which the average baseline pre-bronchodilator FEV1 was 73.4% predicted, showed significant improvements with tiotropium over placebo in trough FEV1 at 3 months that were comparable to those observed over a 4-year period in the current subgroup analysis of UPLIFT® subjects with a post-bronchodilator FEV1 ≥ 60% predicted.
The categorization of COPD severity by FEV1% predicted by the GOLD guidelines (Citation1) is somewhat arbitrary and needs to be qualified since the correlation between FEV1% predicted and both dyspnoea (the cardinal symptom of COPD) and disease-specific HRQoL is relatively weak (Citation11–13). Indeed, it has been shown that health status is significantly impaired in patients with all severities of COPD, including those with mild airflow obstruction (Citation14). One of the reasons for the poor correlation between symptoms of COPD and FEV1% predicted is the important influence of dynamic hyperinflation on dyspnoea during exercise (Citation15,16), which accounts for exercise limitation even in patients with only relatively modest reductions in FEV1% predicted (Citation17).
Therefore, it is not surprising that bronchodilator therapy, which reduces hyperinflation and improves exercise performance (Citation18–20), is often effective in COPD patients with only modest degrees of airflow obstruction. Furthermore, 22% of patients with only moderate COPD have been found to have frequent exacerbations (≥ 2 per year) (Citation21), suggesting the potential advantages of pharmacotherapy, which has been shown to be effective in reducing exacerbations of COPD (Citation22–24), in this particular subgroup of patients with moderate disease.
There are some limitations to the present analysis. Due to its post-hoc nature, results can be considered as supportive of other study results rather than conclusive. In addition, GOLD stage II disease is classified as FEV1 50–80% normal; however, the inclusion criteria for the UPLIFT® trial stipulated FEV1 ≤ 70% predicted. Therefore patients with FEV1 between 70–80% were generally not available for this analysis, although 23 GOLD stage II patients were inappropriately randomized in violation of the protocol but were included in the analysis in accordance with the intention-to-treat principle.
Conclusions
The present results demonstrate the benefits of tiotropium in a sub-population of patients with relatively mild COPD studied over a 4-year period with respect to improvements not only in lung function but also in patient-reported outcomes. When considered along with previous reports in patients with moderate COPD (Citation2,3,Citation10), these results are consistent with the view that the benefits of pharmacotherapy extend to patients within the milder spectrum of COPD severity and support current guideline recommendations for the introduction of maintenance long-acting inhaled bronchodilator therapy in persistently symptomatic patients with moderate COPD.
Declarations of Interest
DT has received: honoraria from Boehringer Ingelheim, Pfizer, Dey Labs, AstraZeneca, Teva Pharmaceuticals, GlaxoSmithKline; consultancy fees from Boehringer Ingelheim, Novartis, Dey Labs, Schering-Plough, AstraZeneca; grants from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Dey, GlaxoSmithKline, Novartis, Pfizer, Schering-Plough, Sepracor; and speaker bureau fees from Boehringer Ingelheim, Novartis, Dey Labs, Schering-Plough, and AstraZeneca. BC has received: honoraria and consultancy fees from Almirall, AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline; and grants from Boehringer Ingelheim, Forest, and GlaxoSmithKline. MD has received: honoraria from Boehringer Ingelheim, Pfizer, and AstraZeneca; consultancy fees from Boehringer Ingelheim and GlaxoSmithKline; grants from AstraZeneca and GlaxoSmithKline; and speaker bureau fees from Boehringer Ingelheim, GlaxoSmithKline, Nycomed, and Dompé. TL and SK are previous employees of Boehringer Ingelheim. SK is a current employee of, and holds shares in, Uptake Medical Corp. TL is a current employee of Medtronic, and holds shares in Affymetrix, Amgen, AstraZeneca, Medco Health Solutions, Medtronic, Merck, and Oncogenex. Editorial assistance was provided by Natalie Dennis from PAREXEL, work which was funded jointly by Boehringer Ingelheim and Pfizer.
All the authors participated in the writing of this manuscript and are responsible for its content.
Acknowledgments
The study was sponsored by Boehringer Ingelheim and Pfizer. The authors would like to thank Natalie Dennis from PAREXEL for editorial assistance, which was funded jointly by Boehringer Ingelheim and Pfizer.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Available from: http://www.goldcopd.org/Guidelineitem.asp?l1 = 2&l2 = 1&intId = 2003. Updated 2009.
- Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP; on behalf of the UPLIFT® study investigators. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet 2009; 374:1171–1178.
- Jenkins CR, Jones PW, Calverley PM, Celli B, Anderson JA, Ferguson GT, Yates JC, Willits LR, Vestbo J. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease; analysis from the randomised, placebo-controlled TORCH study. Respir Res 2009; 10:59.
- Wilt TJ, Niewoehner D, MacDonald R, Kane RL. Management of stable chronic obstructive pulmonary disease: a systematic review for a clinical practice guideline. Ann Intern Med 2007; 147:639–653.
- Enright P. Don't prescribe tiotropium for smokers with an FEV1 above 60% predicted. Prim Care Respir J 2009; 18:119–120.
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M; UPLIFT Study Investigators. A four-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359:1543–1554.
- McGarvey LP, Magder S, Burkhart D, Kesten S, Liu D, Manuel RC, Niewoehner DE. Cause-specific mortality adjudication in the UPLFIT COPD trial: Findings and recommendations. Respir Med 2011 [Epub ahead of print].
- Tashkin DP, Celli B, Kesten S, Lystig T, Mehra S, Decramer M. Long-term efficacy of tiotropium in relation to smoking status in the UPLIFT trial. Eur Respir J 2009; 103:963–974.
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356:775–789.
- Johansson G, Lindberg A, Romber K, Nordström L, Gerken F, Roquet A. Bronchodilator effiacy of tiotropium in patients with mild to moderate COPD. Prim Care Respir J 2008; 17:169–175.
- Mahler DA, Harver A. A factor analysis of dyspnea ratings, respiratory muscle strength, and lung function in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1992; 145(2 Pt 1):467–470.
- Jones PW. Issues concerning health-related quality of life in COPD. Chest 1995(May Supplement); 107:187s–193s.
- Jones PW. Health status measurement in chronic obstructive pulmonary disease. Thorax 2001; 56:880–887.
- Jones PW, Brusselle GDal Negro RW, Ferrer M, Kardos P, Levy ML, Perez T, Soler-Cataluña JJ, van der Molen T, Adamek L, Banik N. Health-related quality of life in patients by COPD severity within primary care in Europe. Respir Med 2011; 105:57–66.
- O'Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164:770–777.
- Cooper CB. Airflow obstruction and exercise. Respir Med 2009; 103:325–334.
- Babb TG, Viggiano R, Hurley B, Staats B, Rodarte JR. Effect of mild-to-moderate airflow limitation on exercise capacity. J Appl Physiol 1991; 70:223–230.
- O'Donnell DE, Flüge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, Make B, Magnussen H. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004; 23:832–840.
- O'Donnell DE, Voduc N, Fitzpatrick M, Webb KA. Effect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary disease. Eur Respir J 2004; 24:86–94.
- Maltais F, Hamilton A, Marciniuk D, Hernandez P, Sciurba FC, Richter K, Kesten S, O'Donnell D. Improvements in symptom-limited exercise performance overy 8 h with once-daily tiotropium in patients with COPD. Chest 2005; 128:1168–78.
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, Calverley P, Rennard S, Wouters EF, Wedzicha JA; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363:1128–1138.
- Rodrigo GJ, Castro-Rodriguez JA, Plaza V. Safety and efficacy of combined long-acting β-agonists and inhaled corticosteroids vs long-acting β-agonists monotherapy for stable COPD. Chest 2009; 136:1029–1038.
- Rodrigo GJ, Nannini LJ. Tiotropium for the treatment of stable chronic obstructive pulmonary disease: a systematic review with meta-analysis. Pulm Pharmacol Ther 2007; 20:495–502.
- Baker WL, Baker EL, Coleman CI. Pharmacologic treatments for chronic obstructive pulmonary disease: A mixed-treatment comparisons meta-analysis. Pharmacotherapy 2009: 29:891–905.