Abstract
Background: This Phase III study evaluated the efficacy and safety of twice-daily aclidinium 200 μg and 400 μg versus placebo in the treatment of moderate-to-severe COPD. Methods: In this 12-week, double-blind, multicenter trial, patients were randomized (1:1:1) to inhaled twice-daily aclidinium 200 μg, aclidinium 400 μg, or placebo. Primary and secondary endpoints were changes from baseline in trough FEV1 and peak FEV1 at Week 12, respectively. Health status (St. George's Respiratory Questionnaire [SGRQ]), COPD symptoms (Transitional Dyspnea Index [TDI], night and early morning symptoms), and safety were also assessed. Results: A total of 561 patients (mean age, 64 ± 9 years) with a mean baseline FEV1 of 1.36 ± 0.54 L (47.2% of predicted value) were randomized. At Week 12, aclidinium 200 μg and 400 μg showed significant improvements from baseline in mean (95% CI) trough FEV1 compared with placebo by 86 (45, 127) mL and 124 (83,164) mL, respectively, and in peak FEV1 by 146 (101, 190) mL and 192 (148, 236) mL, respectively (p ≤ 0.0001 for all). Both aclidinium doses also provided significant improvements in SGRQ, TDI and almost all COPD symptom scores compared with placebo (p < 0.05 for all). Incidences of adverse events (AEs) were similar across treatment groups. The incidence of anticholinergic AEs was low and similar across groups (dry mouth: 0.5%–1.6%; constipation: 0%-1.1%). Conclusions: Treatment of moderate-to-severe COPD patients with twice-daily aclidinium 200 μg and 400 μg was associated with significant improvements in bronchodilation, health status, and COPD symptoms. Both doses were well tolerated and had safety profiles similar to placebo.
Trial Registration: This ACCORD I study (AClidinium in Chronic Obstructive Respiratory Disease I) was registered on clinicaltrials.gov (NCT00891462) as “Efficacy and Safety of Aclidinium Bromide for Treatment of Moderate to Severe Chronic Obstructive Pulmonary Disease (COPD)”.
Keywords: :
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease characterized by airway obstruction that is not fully reversible (Citation1). COPD is a leading cause of morbidity and mortality with significant contributions to healthcare costs (Citation2–4). Bronchodilators are central to symptomatic COPD management, with long-acting agents such as muscarinic antagonists and β-agonists (LAMAs and LABAs, respectively) considered more effective than short-acting alternatives (Citation1). Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend that effective COPD treatment should be achieved with minimal side effects (Citation1).
Aclidinium bromide is a novel, inhaled LAMA with low systemic activity, developed for maintenance treatment of COPD. It is rapidly hydrolyzed into inactive metabolites (Citation5), resulting in low circulating concentration following inhalation (Citation6, 7) suggesting a reduced potential for systemic side effects.
Although previous Phase III studies with once-daily aclidinium 200 μg demonstrated significant trough FEV1 improvements in COPD patients (Citation8), these increases were below the suggested minimal clinically important difference (MCID) of 100 mL (Citation9), indicating that a higher total daily dose may be more effective. Safety of a higher total daily dose and more frequent aclidinium dosing regimen is supported by data which demonstrate that single aclidinium doses up to 6000 μg and twice-daily aclidinium up to 800 μg were well tolerated in healthy subjects (Citation7, Citation10).
In a 2-week crossover study in COPD patients, bronchodilation over a 24-hour period with twice-daily aclidinium 400 μg was comparable to that of once-daily tiotropium, the only LAMA currently available (Citation11). Aclidinium also significantly improved the average bronchodilation at night versus tiotropium, suggesting added benefits during the second half of day (Citation11).
Additionally, a dose-finding aclidinium study demonstrated dose-dependent bronchodilation; twice-daily aclidinium 400 μg provided statistically significant bronchodilation when compared to the lowest aclidinium dose tested (100 μg) (Citation12). Therefore, aclidinium 200 ug and 400 ug doses were considered appropriate to be further assessed in Phase III studies. The objective of this Phase III study was to evaluate the efficacy and safety of 12-week twice-daily aclidinium 200 μg and 400 μg in COPD patients.
Methods
Study design
This randomized, double-blind, placebo-controlled, parallel-group study in moderate-to-severe COPD patients consisted of a 2-week run-in, a 12-week treatment period, and a 2-week follow-up phone contact/study visit (NCT00891462). Patients were evaluated for eligibility at screening and at baseline before being randomized (1:1:1) to twice-daily aclidinium 200 μg, aclidinium 400 μg, or placebo. Patients were instructed to administer study treatments at the same time in the morning (between 8:00 and 10:00 AM) and in the evening (between 8:00 and 10:00 PM) via a multiple-dose dry powder inhaler (Genuair®)*. Efficacy and safety of the patients were evaluated during study visits at Week 1, 4, 8 and 12. This study was conducted according to ICH/GCP guidelines and the Declaration of Helsinki in centers in United States and Canada and approved by the Western Institutional Review Board and Biomedical Research Alliance of New York. All patients gave written informed consent before any study procedure.
Study population
Male and female patients ≥40 years of age who were current or former cigarette smokers with a smoking history ≥10 pack-years and diagnosed with moderate-to-severe COPD (postbronchodilator FEV1/FVC <70% and FEV1 ≥30% but <80% of predicted) (Citation1) were eligible for study participation. Exclusion criteria included other significant respiratory conditions (including asthma), respiratory infection or COPD exacerbation ≤6 weeks prescreening (≤3 months if it resulted in hospitalization), clinically significant cardiovascular conditions including myocardial infarction during the previous 6 months, unstable arrhythmia, Bazett-corrected QTc >470 msec, and medical conditions wherein anticholinergic drugs are contraindicated.
Permitted concomitant medications included albuterol (rescue medication), inhaled corticosteroids (ICS), systemic corticosteroids equivalent to ≤10 mg/day of prednisone or 20 mg every other day, and theophylline if treatment was stable for ≥4 weeks prior to screening. Inhaled anticholinergics and LABAs were prohibited during the study. Rescue medication was discontinued ≥6 hours before each study visit, while theophylline and ICS were discontinued the morning before each study visit.
Assessments
Standardized spirometry (Citation13) was performed predose (-1 hour and at -10 minutes) at each visit and at 0.5, 1, 2, and 3 hours following the morning dose at each visit after randomization. St. George's Respiratory Questionnaire (SGRQ) and Baseline Dyspnea Index (BDI)/Transition Dypsnea Index (TDI) were completed at baseline and at every month.
COPD symptoms (early morning and at night) and rescue medication use were assessed using a Nighttime Symptoms Questionnaire; sleep quality was assessed using a non-disease-specific Daily Sleep Diary (Citation14). These questionnaires developed by the sponsor were self-administered by the patient each morning using an electronic diary (eDiary), beginning at screening through Week 12 of treatment. The COPD nighttime symptoms questionnaire, designed with a ≤24-hour recall period, assessed the frequency of COPD symptoms during the previous night, the severity and impact of nighttime symptoms (on activity and on sleep) and of early morning symptoms, sputum production, and rescue medication use.
The Sleep Diary questionnaire (Citation14) assessed the time the patient first went to sleep the previous night, the frequency of waking up and having difficulty falling back to sleep, the total number of hours slept, the overall sleep quality the previous night, how rested the patient felt that morning, and how the patient's sleep the prior night compared to their normal sleep.
COPD exacerbations (an increase in COPD symptoms ≥2 consecutive days resulting in medical intervention) were evaluated at each visit and categorized as mild (increased use of rescue medication), moderate (treatment with antibiotics and/or systemic corticosteroids), or severe (hospitalization). Safety was assessed using adverse events (AEs), laboratory tests, vital signs, and ECGs.
Endpoints
The primary efficacy endpoint was change from baseline to Week 12 in morning predose (trough) FEV1, the average of 2 predose FEV1 values. The secondary efficacy endpoint was change from baseline to Week 12 in peak FEV1, the highest value observed within 3 hours postmorning dose. Additional pulmonary function endpoints included changes from baseline on Day 1 (peak FEV1 only), Weeks 1, 4, and 8 (trough and peak FEV1) and Week 12 (AUC0-3/3h FEV1, trough, peak, and AUC0-3/3h FVC, and trough IC).
Additional efficacy endpoints were changes from baseline at Weeks 4, 8 and 12 in SGRQ and TDI (including percentage of subjects with a clinically meaningful improvement [decrease of ≥4 points for SGRQ (Citation15) or increase of ≥1 unit for TDI (Citation16)]), changes from baseline at Week 12 in COPD Nighttime Symptoms Questionnaire and Daily Sleep Diary scores, rescue medication use over 12 weeks, and COPD exacerbation rate.
Statistical analysis
Demographic, baseline, and safety data were summarized by treatment group for the safety population, defined as subjects who took ≥1 dose of study treatment.
All efficacy analyses were based on the intent-to-treat (ITT) population, defined as subjects in the safety population who had baseline and at least 1 postbaseline FEV1 assessment. Efficacy outcomes were analyzed using ANCOVA with treatment group and gender as factors and baseline value and age as covariates. Results are presented as estimated adjusted treatment effects (least square means [LSM] and LSM differences) with 95% confidence intervals (CIs) and two-sided p-values. Missing values were imputed using the last-observation-carried-forward approach. Assuming a 240 mL standard deviation, 165 patients per treatment arm would give >90% statistical power to detect a 100-mL treatment difference in trough FEV1, adjusting for multiple comparisons.
The percentage of patients who achieved clinically meaningful improvements from baseline in SGRQ total score (≥4 points) or TDI focal score (≥1 unit) was analyzed using a logistic regression model with treatment group, sex, age, and baseline value as explanatory variables. Statistical significance was based on the Wald test. The effect of aclidinium treatment compared with placebo was estimated by odds ratio and its 95% CI.
Mean (SD) values for the daily COPD symptoms and sleep scores were calculated using weekly averages from the sum of daily averages starting from the week prior to randomization (baseline) until Week 12. Overall daily rescue medication use was calculated using the total number of puffs of rescue medication used divided by the number of nonmissing days during the period from first dose of study drug to last available rescue medication use recorded in the eDiary. COPD symptoms, sleep, and rescue medication use were analyzed using an ANCOVA model with treatment group as a factor and the baseline value as covariate. The number of COPD exacerbations per patient/year was analyzed using Poisson regression with overdispersion for rates and with treatment, sex, and baseline COPD severity as factors and age as covariate.
Results
Patient demographics and baseline characteristics
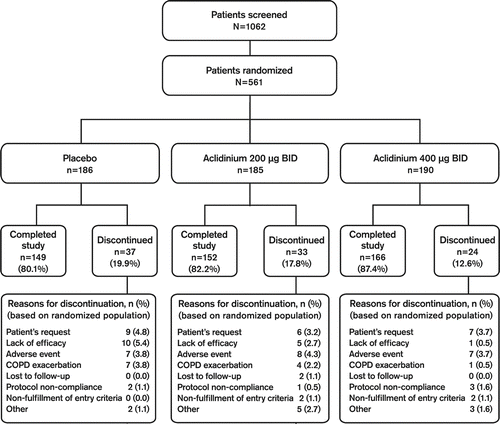
Of the 561 randomized subjects, 467 completed the study (). Although the percentage of patients who discontinued due to consent withdrawal and AEs was generally similar across treatment groups, there was a dose-related trend toward fewer discontinuations due to COPD exacerbation or lack of efficacy with aclidinium versus placebo. Demographic and baseline characteristics were similar across treatment groups (). Patients had a mean (SD) age of 64 (Citation9) years and a baseline FEV1 of 1.36 (0.54) L (47.2 [14.1]% of predicted value). A majority of the patients in all treatment groups used COPD medications before screening and these medications were taken by similar proportions of patients in each group ().
Table 1. Demographic data and baseline characteristics (safety population)
Pulmonary function
After 12 weeks of treatment, twice-daily aclidinium 200 μg and 400 μg significantly improved the change from baseline in trough FEV1 over placebo by 86 mL and 124 mL, respectively (p < 0.0001; ). Additionally, aclidinium 200 μg and 400 μg significantly improved the change from baseline in peak FEV1 over placebo by 146 mL and 192 mL, respectively (p < 0.0001; ).
Figure 2. Mean (SE) change from baseline in (A) trough FEV1 and (B) peak FEV1 at Day 1 (peak only) and at Weeks 1, 4, 8 and 12.
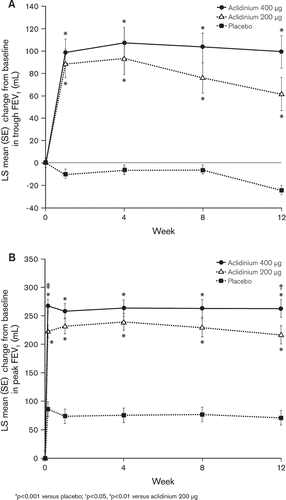
Changes from baseline in trough and peak FEV1 were significantly higher with aclidinium than placebo at all study visits (p < 0.0001 for all). Maximum bronchodilation provided by aclidinium was reached on the first timepoint assessed (first day for peak FEV1 and first week for trough FEV1) and maintained throughout the 12-week study period (). At 30 minutes after treatment administration on Day 1, the first postdose FEV1 time point assessed in the study, significant improvements in the change from baseline in FEV1 were already observed with aclidinium 200 μg and 400 μg over placebo (89 mL and 125 mL, respectively, p < 0.0001 for both).
Similar results were observed for AUC0-3/3h FEV1, with mean improvements over placebo at Week 12 of 144 mL and 192 mL for aclidinium 200 μg and 400 μg, respectively (p < 0.0001 for both). Both aclidinium doses also showed significant improvements in FVC (trough, peak, and AUC0-3/3h) and trough IC compared with placebo ().
Table 2. Mean (SE) change from baseline in pulmonary function parameters (FVC and IC) after 12 weeks of treatment (ITT population)
Aclidinium 400 μg provided greater placebo-adjusted improvements in bronchodilation than aclidinium 200 μg throughout the study, with statistically significant differences in peak FEV1 at Day 1 and Week 12, in favor of the higher dose (p = 0.004 and 0.041, respectively).
Clinical outcomes
Significant improvements in SGRQ total scores were observed at all study visits with aclidinium (). The largest improvement was at Week 4, with differences over placebo of –3.2 and –3.6 for aclidinium 200 μg and 400 μg, respectively (p < 0.001 for both). At study end, improvements in SGRQ total score over placebo were –2.7 (aclidinium 200 μg, p = 0.013) and –2.5 (aclidinium 400 μg, p = 0.019). At all timepoints, a higher percentage of patients in each aclidinium group (ranging from 41% [Week 4, 400 μg] to 49% [Week 12, 200 μg]) achieved a clinically meaningful improvement in SGRQ total score (≥4-point decrease from baseline) (Citation15) compared with placebo (ranging from 27% to 36%; p < 0.05 for all versus placebo based on odds ratios, except at Week 12 for the aclidinium 400 μg group, p = 0.139; ).
Figure 3. Mean (SE) change from baseline in A) SGRQ total score and B) TDI focal score at Weeks 4, 8, and 12.
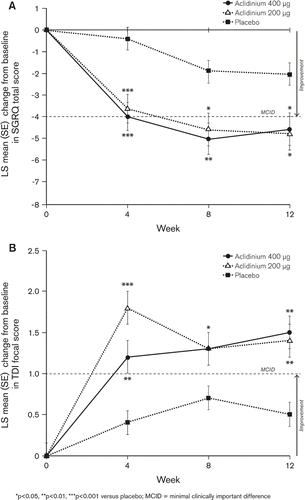
Figure 4. Percentage of patients who achieved a clinically meaningful difference in (A) SGRQ total score (≥4 units) and (B) TDI focal score at Weeks 4, 8 and 12.
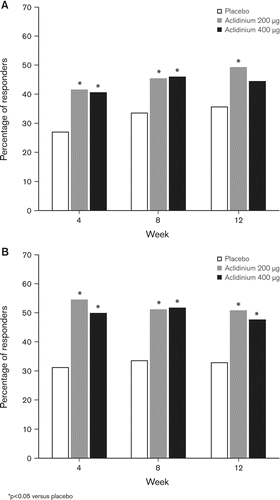
Similarly, both aclidinium doses significantly improved TDI focal scores compared with placebo at each study visit (p < 0.05 for all except at Week 8 for aclidinium 200 μg, p = 0.060; ), with maximum differences from placebo for aclidinium 200 ug and 400 μg observed at Week 4 (1.4) and Week 12 (1.0), respectively (p < 0.005 for both). Aclidinium 200 μg resulted in a 0.9 difference in TDI focal score from placebo at Week 12 (p = 0.005). A higher percentage of patients in each aclidinium group (ranging from 48% [Week 12, 400 μg] to 55% [Week 4, 200 μg]) achieved a clinically meaningful improvement in TDI (≥1 unit) (Citation16) compared with placebo (ranging from 31% to 34%) at all timepoints (p < 0.05 for all versus placebo based on odds ratios, ).
Nighttime Symptoms, Sleep, and Rescue Medication Use
Compared with placebo at Week 12, both aclidinium doses significantly reduced frequency of nighttime symptoms (breathlessness, cough, sputum production, and wheezing), severity and impact of breathlessness and cough on nighttime activity, severity and impact of breathlessness on early morning activity, and 24-hour sputum production (p < 0.05 for all versus placebo; ). Sputum produced during sleeping hours at Week 12 was not significantly reduced with aclidinium compared with placebo. Almost all nighttime symptom improvements at Week 12 with aclidinium 400 μg were numerically higher than those with aclidinium 200 μg ().
Table 3. Mean (SD) change from baseline in daily average of COPD nighttime and early morning symptom scores at Week 12 (ITT population)
Generally, sleep diary results were not significantly different between treatment groups; however, a significant difference in the frequency of nighttime awakenings was observed with aclidinium 400 μg versus placebo at Week 12 (p < 0.05).
Aclidinium 200 μg and 400 μg significantly reduced total daily rescue medication use from placebo over the 12-week period by 0.7 puffs/day (p = 0.001) and 0.9 puffs/day (p < 0.0001), respectively.
COPD exacerbations
A trend towards a reduction in the rate of moderate-to-severe COPD exacerbations per patient/year were observed with aclidinium 200 μg and 400 μg (i.e., 33% and 34%, respectively, compared with placebo), although these changes were not significant (p = 0.103 and p = 0.091, respectively). Rates of exacerbation of any severity per patient/year were low (i.e., 0.79, 0.55, and 0.41 for placebo, aclidinium 200 μg, and 400 μg, respectively), with a significant reduction with aclidinium 400 μg versus placebo (rate ratio = 0.52, p = 0.009).
Safety
Twice-daily aclidinium 200 μg and 400 μg were well tolerated; most AEs were mild to moderate in severity. A numerically smaller percentage of patients treated with aclidinium 400 μg (44.7%) reported a treatment-emergent AE (TEAE) versus aclidinium 200 μg (50.5%) or placebo (52.2%). COPD exacerbation was the only AE reported by >5% of patients in all groups, with a lower incidence with aclidinium 400 ug versus aclidinium 200 μg and placebo (). Incidences of anticholinergic-related AEs (dry mouth, constipation) and cardiac AEs were low and similar across treatment groups (<2% for any event in any group).
Table 4. Most frequently reported (≥2% of subjects in any group) adverse events by treatment group (n [%]; safety population; N = 560)
The percentage of subjects experiencing a serious AE (SAE) was low and similar among the treatment groups (2.2% placebo, 4.3% aclidinium 200 μg, and 3.2% aclidinium 400 μg). The most frequently reported SAE was COPD exacerbation (n = 1 each for placebo and aclidinium 200 μg, n = 3 for aclidinium 400 μg). One subject in the aclidinium 400 μg group died due to metastatic lung cancer 23 days after first drug intake; this was not considered related to study treatment. No clinically significant differences in clinical laboratory values, vital signs, or ECG parameters were observed.
Discussion
In this study, twice-daily aclidinium 200 μg and 400 μg resulted in significant bronchodilation compared with placebo in moderate-to-severe COPD patients, as assessed by morning predose (trough) FEV1 and peak FEV1 after 12 weeks of treatment. These improvements were evident by the first day of treatment (for peak FEV1) and maintained throughout the 12-week study period.
Although an MCID in FEV1 has not yet been clearly defined, the improvement over placebo in trough FEV1 with aclidinium 400 μg in this study (124 mL at Week 12) is within the suggested MCID of 100–140 mL (Citation9, Citation17), similar to what was previously reported in an earlier Phase II study with twice-daily aclidinium (Citation11). The improvements in trough FEV1 with aclidinium reported here are comparable to those observed in tiotropium registration studies (120–150 mL) (Citation18–20).
The comparable improvements in peak FEV1 observed at Day 1 and Week 12 with aclidinium 400 μg twice daily in this study suggest that aclidinium reaches its maximum effect with the first dose. As delayed onset of effect is considered a potential barrier to adherence to prescribed therapies in COPD patients (Citation21), the rapid onset of action seen with aclidinium treatment may positively affect patient compliance.
The numerically greatest number of patient discontinuations was found in the placebo group while the least number of discontinuations was observed in the aclidinium 400 μg group, similar to the pattern of differential withdrawal frequently observed in COPD trials (Citation18, 19, Citation22, 23). In particular, the aclidinium 400 μg group had the numerically least number of patient discontinuations due to COPD exacerbation or lack of efficacy, suggesting that the higher aclidinium dose provides greater efficacy in COPD patients compared with the lower dose.
The GOLD guidelines emphasize that treatment of stable COPD should include managing symptoms and improving health status (Citation1). In this study, both aclidinium doses significantly improved dyspnea, with a clinically meaningful improvement in TDI focal score (≥1 unit) (Citation16) at Week 12 with aclidinium 400 μg. Although significant improvements from baseline in SGRQ were observed with aclidinium over placebo throughout the study, the MCID (≥4 units) (Citation15) was not met, most probably due to the short study duration. These results suggest that aclidinium may improve health status and that a longer treatment period (i.e., 6 months) may be needed to better evaluate such changes. A similar phenomenon has been observed in recent studies wherein the magnitude of improvement in SGRQ total scores with tiotropium versus placebo increased with longer study duration (Citation24, 25).
Nighttime COPD symptom incidence has not been extensively studied but it has been reported that 89% of COPD patients experience ≥1 nighttime symptom (Citation26) and that COPD symptoms are at their worst at night or early morning (Citation27, 28). These could result in nocturnal awakenings and difficulties with morning activities, negatively impacting patient quality of life. Thus, it is essential to evaluate the effect of COPD treatment on these parameters. Since no validated instrument currently exists to assess COPD nighttime symptoms and their influence on morning activities, a questionnaire was developed for this study to evaluate these aspects. Similar to the reduction in nighttime symptoms observed in a 2-week study with twice-daily aclidinium 400 μg (Citation11), twice-daily aclidinium 200 μg and 400 μg reduced the frequency, severity, and impact of nighttime symptoms compared with placebo in COPD patients after 12 weeks of treatment.
The amount of sputum produced during sleeping hours at Week 12 was the only symptom parameter that did not show a significant reduction with aclidinium treatment compared with placebo at study end; however, this may have been due to a reduction in sputum production in the placebo group at this time point. Results from this study suggest that the evening dose of aclidinium provides sustained bronchodilation and improvement of nighttime and early morning symptoms, although no direct correlation was investigated between these parameters. Other twice-daily COPD medications have been reported to improve nighttime awakenings (Citation29–32), morning activity (Citation33), and daytime symptoms (Citation34).
However, the study reported here provides a more detailed investigation and is the first to evaluate the effect of treatment on particular COPD symptoms (ie, breathlessness, cough, sputum production) and their severity and impact specifically at night and at early morning. The positive impact of twice-daily aclidinium on COPD nighttime and early morning symptoms will need to be confirmed in future studies.
Although a trend towards a reduction in moderate-to-severe exacerbation rates with aclidinium was observed in this study, this trial was not designed to assess exacerbation frequency. Studies with an enriched population for patients at risk for COPD exacerbations, longer treatment duration, and adequate power to determine between-group differences are necessary to establish the treatment benefit of aclidinium on COPD exacerbations.
In this 12-week study, both aclidinium doses had safety profiles similar to placebo. Incidences of anticholinergic and cardiac AEs with aclidinium were low and also similar to placebo. This is most likely due to the low and transient systemic exposure of aclidinium, a result of its rapid hydrolysis in plasma (Citation5-7). Studies with a longer treatment duration would enable a more comprehensive evaluation of the safety profile of aclidinium.
Conclusions
Overall, twice-daily aclidinium 200 μg and 400 μg significantly improved lung function, health status, and reduced COPD symptoms, with aclidinium 400 μg providing numerically greater benefits than aclidinium 200 μg throughout the study. Both doses were well tolerated and had similar safety profiles. Twice-daily aclidinium may thus be an effective new treatment option for COPD patients.
Declaration of Interest
This work was funded by Forest Research Institute, Inc. (FRI), a wholly owned subsidiary of Forest Laboratories, Inc., and Almirall, S.A. Esther Garcia Gil is an employee of Almirall, S.A. Hassan Lakkis and Cynthia Caracta are employees of FRI. Dr. Kerwin has done consulting or lectures for Alkermes, AstraZeneca, Dey Laboratories, GlaxoSmithKline, Ironwood, Merck, SanofiAventis, and Sunovion. He has performed multicenter clinical trials for fifty pharmaceutical companies including Forest and Almirall. Dr. D’ Urzo has received research, consulting, and lecturing fees from GlaxoSmithKline, Sepracor, ScheringPlough, Altana, Methapharma, AstraZeneca, ONO pharma, Novartis Canada/USA, Hoffmann-La Roche Limited, and KOS Pharmaceuticals. Dr. Gelb has been a speaker for Boehringer-Ingelheim, Pfizer, and Astra Zeneca and conducted clinical trials with Forest and Novartis. Editorial assistance by Joy Ramos, PhD, of Prescott Medical Communications Group was funded by FRI. There are no other financial disclosures/conflicts of interest.
Acknowledgments
All the authors were involved in the creation and review of the manuscript, had full access to study data, and take full responsibility for data integrity and accuracy of all data analysis. The authors would like to thank the investigators and the study team in each of the participating centers.
References
- Rabe KF, Hurd S, Anzueto A, Global Strategy for the Diagnosis, Management, and Prevention Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2007 Sept; 176(6):532–55.
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007 Sept; 370(9589):765–73.
- Hurd S. The impact of COPD on lung health worldwide: epidemiology and incidence. Chest 2000 Feb; 117(2 Suppl):1S–4S.
- Minino AM, Heron MP, Murphy SL, Kochanek KD. Deaths: final data for 2004. Natl Vital Stat Rep 2007 Aug; 55(19):1–119.
- Sentellas S, Ramos I, Alberti J, Aclidinium bromide, a new, long-acting, inhaled muscarinic antagonist: in vitro plasma inactivation and pharmacological activity of its main metabolites. Eur J Pharm Sci 2010 Mar; 39(5):283–90.
- Jansat JM, Lamarca R, de Miquel G, Schrodter A, Miletzki B, Gurniak M. Safety and pharmacokinetics of multiple doses of aclidinium bromide, a novel long-acting muscarinic antagonist for the treatment of chronic obstructive pulmonary disease, in healthy participants. J Clin Pharmacol 2009 Oct; 49(10):1239–46.
- Jansat JM, Lamarca R, Garcia Gil E, Ferrer P. Safety and pharmacokinetics of single doses of aclidinium bromide, a novel long-acting, inhaled antimuscarinic, in healthy subjects. Int J Clin Pharmacol Ther 2009 Jul; 47(7):460–8.
- Jones PW, Rennard SI, Agusti A, Efficacy and safety of once-daily aclidinium in chronic obstructive pulmonary disease. Respir Res [Internet] 2011 April 26 [cited 2011 April 27];12:55. Available from: http://respiratory-research.com/content/12/1/55.
- Cazzola M, MacNee W, Martinez FJ, Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J 2008 Feb; 31(2):416–69.
- Lasseter KC, Dilzer S, Jansat JM, Garcia Gil E, Caracta C, Ortiz S. Safety and pharmacokinetics of multiple doses of aclidinium bromide administered twice daily in healthy volunteers. In: American Thoracic Society International Conference Abstract Issue; 2011 May 13–18; Denver, CO. New York: Am J Respir Crit Care Med; c2011. p. A1615.
- Fuhr R, Magnussen H, Sarem K, Efficacy of aclidinium bromide 400 μg BID compared with placebo and tiotropium in patients with moderate-to-severe COPD. Chest [Internet] 2011 September 8 [cited 2011 September 8]:[29 p.]. Available from: http://chestjournal.chestpubs.org/content/early/2011/09/07/chest.11-0406.
- Singh D, Magnussen H, Kirsten A-M, Aclidinium bromide: a phase IIb, dose-finding study. In: British Thoracic Society Winter Meeting Programme and Abstracts Issue; 2011 December 7–9; London, UK. London, UK: Thorax; c2011. p. A172.
- Miller MR, Hankinson J, Brusasco V, Standardisation of spirometry. Eur Respir J 2005 Aug; 26(2):319–38.
- Haythornthwaite JA, Hegel MT, Kerns RD. Development of a sleep diary for chronic pain patients. J Pain Symptom Manage 1991 Feb; 6(2):65–72.
- Jones PW. St. George's Respiratory Questionnaire: MCID. COPD 2005 Mar; 2(1):75–9.
- Mahler DA, Witek TJ, Jr. The MCID of the transition dyspnea index is a total score of one unit. COPD 2005 Mar; 2(1):99–103.
- Donohue JF. Minimal clinically important differences in COPD lung function. COPD 2005 Mar; 2(1):111–24.
- Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax 2003 May; 58(5):399–404.
- Casaburi R, Mahler DA, Jones PW, A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J 2002 Feb; 19(2):217–24.
- Vincken W, van Noord JA, Greefhorst AP, Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. Eur Respir J 2002 Feb; 19(2):209–16.
- Make BJ. Chronic obstructive pulmonary disease: developing comprehensive management. Respir Care 2003 Dec; 48(12):1225–34; discussion 34–7.
- Tashkin DP, Celli B, Senn S, A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008 Oct; 359(15):1543–54.
- Calverley PM, Spencer S, Willits L, Burge PS, Jones PW. Withdrawal from treatment as an outcome in the ISOLDE study of COPD. Chest 2003 Oct; 124(4):1350–6.
- Bateman ED, Tashkin D, Siafakas N, A one-year trial of tiotropium Respimat plus usual therapy in COPD patients. Respir Med 2010 Oct; 104(10):1460–72.
- Bateman E, Singh D, Smith D, Efficacy and safety of tiotropium Respimat SMI in COPD in two 1-year randomized studies. Int J Chron Obstruct Pulmon Dis 2010; 5:197–208.
- Mocarski M, Caracta C, Tourkodimitris S, Park G, Garcia Gil E, Setyawan J. Nighttime symptoms of COPD in a clinical trial population: prevalence and impact. In: American Thoracic Society International Conference Abstract Issue; 2011 May 13–18; Denver, CO. New York: Am J Respir Crit Care Med 2011; p. A1495.
- Partridge MR, Karlsson N, Small IR. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an internet survey. Curr Med Res Opin 2009 Aug; 25(8):2043–8.
- Kessler R, Partridge MR, Miravitlles M, Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J 2011 Feb; 37(2):264–72.
- Tashkin DP, Pearle J, Iezzoni D, Varghese ST. Formoterol and tiotropium compared with tiotropium alone for treatment of COPD. COPD 2009 Feb; 6(1):17–25.
- Rennard SI, Tashkin DP, McElhattan J, Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trial. Drugs 2009; 69(5):549–65.
- Anzueto A, Ferguson GT, Feldman G, Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD 2009 Oct; 6(5):320–9.
- Campbell M, Eliraz A, Johansson G, Formoterol for maintenance and as-needed treatment of chronic obstructive pulmonary disease. Respir Med 2005 Dec; 99(12):1511–20.
- Partridge MR, Schuermann W, Beckman O, Persson T, Polanowski T. Effect on lung function and morning activities of budesonide/formoterol versus salmeterol/fluticasone in patients with COPD. Ther Adv Respir Dis 2009 Aug; 3(4):1–11.
- Make B, Hanania NA, ZuWallack R, The efficacy and safety of inhaled fluticasone propionate/salmeterol and ipratropium/albuterol for the treatment of chronic obstructive pulmonary disease: an eight-week, multicenter, randomized, double-blind, double-dummy, parallel-group study. Clin Ther 2005 May; 27(5):531–42.