Abstract
Awareness, diagnosis and treatment of COPD, compared to other major causes of death, remains far too low. This article describes the protocol objectives, design and the approaches taken in the Canadian Chronic Obstructive Lung Disease (CanCOLD) study, an epidemiological and integrated research. The CanCOLD study aims at better understanding heterogeneity of COPD presentation and disease progression. We hypothesize that individuals with unfavourable COPD “phenotypes” and subjects at-risk (ever smokers) with unhealthy lifestyle habits, environmental/work exposure, or co-morbidities will have increased risk of lung function decline independent of their cumulative exposure to cigarette smoke. The study is a prospective multi-center cohort study (9 sites in 6 provinces) built on the Canadian COPD prevalence study “COLD.” The study plan is to include 1800 subjects at least 40 years old who were sampled from the general population and who were found to fall within 4 groups: 1) COPD moderate–severe (GOLD 2–4); 2) COPD mild (GOLD 1); 3) subjects at-risk (ever smoker); and, 4) subjects never-smoker free of airflow obstruction. Data collection is based on using strictly standardized methods involving questionnaires, pulmonary function and cardiorespiratory exercise tests, CT scans, and blood sampling. CanCOLD is a unique study that will address challenging and important research questions on COPD disease evolution and disease management and will help to define the natural history of COPD disease evolution in individuals at-risk for COPD and in those with COPD who have mild disease.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is the 4th leading cause of mortality and it is projected to move up to the 3rd rank by 2020 (1,2). Mortality has increased (3) and COPD results in higher rates of hospitalization than heart failure, angina and other chronic diseases, and often has worse outcomes post-hospitalization than after myocardial infarction (4,5). Despite this, COPD is under diagnosed and undertreated, and it receives scant attention from health policymakers.
The Canadian COPD prevalence study (“COLD”) study has shown that in Canada the prevalence of COPD is 4-fold higher (6) than previous estimates suggested. Earlier estimates were based on self-reports of doctor diagnosis from the Canadian Community Health Survey (CCHS 2008). This study highlighted a major issue related to underdiagnosis; among subjects with mild and moderate COPD, it has been found that 70% had not been diagnosed despite many being symptomatic. Compared with other chronic diseases such as cardiovascular diseases and diabetes, the limited understanding of COPD pathogenesis and risk factors (beyond smoking), early disease processes, sex differences, disease heterogeneity and related prognosis may contribute to poor outcomes for many patients. Major obstacles that impede progress addressing important care gaps include: 1) the limited number of prospective studies; 2) the underrepresentation of early disease and female with COPD; and, 3) the lack of well-coordinated, cohort studies that document and integrate the “biological march” in well characterized and representative population, from unaffected to those with severe respiratory impairment. This has been the justification to develop the Canadian Cohort of Obstructive Lung Disease (CanCOLD). It is a novel research project; the first prospective longitudinal study for COPD with population sampling. This study will offer data complementary to other cohort studies of COPD phenotype by including women, mild, and at-risk subjects, and more extended data testing.
Epidemiological research is needed to develop a framework to combat COPD. This framework needs to incorporate better characterization of men and women at-risk for COPD and characterization of those with early disease. This framework also must include a better understanding of which risk factors may be modifiable through interventions and how these risk factors are related to health perception and disease evolution. The primary objective of CanCOLD is to establish which potentially modifiable factors other than smoking such as environmental/work exposure, physical activity, dietary factors (dietary patterns, individual nutrients), and co-morbidities will impact on disease progression (especially subjects with mild “early disease”) and on development of COPD (subjects at-risk, i.e., ever smoker with normal lung function) in men and women.
Secondary objectives are: 1) to determine the combinations of disease and patient attributes that differentiate individuals (men/women) with COPD as they relate to relevant outcomes (symptoms, disease progression or death); 2) to determine if early detection of COPD with spirometry is meaningful according to sex and ageing. CanCOLD will also create a research platform that will give access to a large data set and blood samples (tissue bank). This will allow implementation of “substudies” to answer other important questions; it will build transdisciplinary research and sustainable partnerships within the global COPD community. This article describes the protocol design and the approaches taken in the CanCOLD study.
Methods
Study Design and Participants
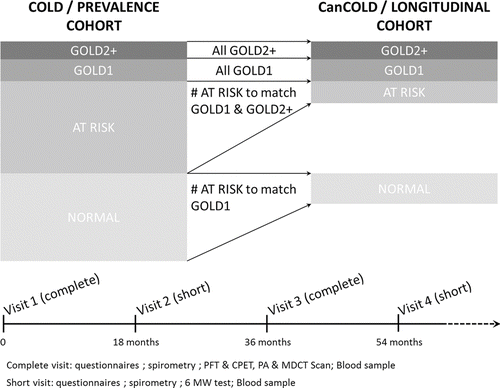
CanCOLD is a prospective longitudinal cohort study, tracking 1800 subjects with assessment at baseline, 18 months, 3 years and beyond following the same scheme (). The cohort comprises 2 COPD balanced subsets (GOLD ≥2 and GOLD 1) and 2 subsets of non-COPD peers, i.e., normal post bronchodilator spirometry (ever smoker for those at-risk and never-smoker for the healthy controls), matched for sex and age. Subjects are recruited from 9 study site participants in Canada: Calgary, Halifax, Kingston, Montreal, Ottawa, Quebec, Saskatoon, Toronto and Vancouver. Subjects from the prevalence study COLD(1) (random sample of >5,000 non-institutionalized adults aged 40 years and over, split evenly between men and women) are invited to participate in CanCOLD. Each of the COLD sites randomly sampled the population living in a well-defined area that had a total population of at least 250,000 people. CanCOLD sampling is based on the selection and contact of COPD subjects. Then matched non-COPD peers are selected and are contacted.
Study procedures- Study Evaluation Forms and Study Flow Chart
The study protocol and the written consent form have been approved by each Institutional Research Ethical Board. The flow chart and procedures are described in . Measurements are distributed in five categories: questionnaires; pulmonary function and assessment of exercise condition (field and laboratory tests); CT scan of the chest; blood tests; and health administrative databases.
Table 1. Study evaluation forms and study flow chart
Questionnaires. Questionnaires are covering sociodemographic information, smoking history and work exposure, consequences of health problems for employment (Short Form of the Health and Labour Questionnaire (7)), symptoms and disability (MRC (8); Global Chest Symptoms Questionnaire and Capacity of Daily Living Questionnaire (9,10) and medication, co-morbid conditions, lifestyle such as dietary habits (Food Frequency Questionnaire (11)) and physical activities (CHAMPS (12)) and health status (general (Short Form-36 version 2 (13)), disease specific (COPD Assessment Test (14); McGill COPD questionnaire (15); St. George's Respiratory Questionnaire (16)) and psychosocial (Hospital Anxiety and Depression Scale (17–19)).
Questionnaires are administered by a research coordinator during a face to face visit. For exacerbation, subjects will fill out an online questionnaire once every 3 months and for those who do not have access to the internet, a telephone interview will be conducted. An exacerbation occurs when a patient experiences either two or more of three major symptoms (increase in dyspnea, sputum purulence, and increased sputum volume), or any major symptom with a minor symptom. Based on the level of healthcare resources required, exacerbation severity is classified as mild (the need for only increased use of inhaled bronchodilators), moderate (the need for systemic corticosteroids and/or antibiotics), or severe (the need for hospital admission).
Pulmonary function and exercise assessment. Spirometry, lung volumes and DLCO are obtained according to standard techniques (20). Disease severity is categorized according to the GOLD classification (21). Spirometry (pre- and post-BD) is done according to stringent standards (22). In each site, technicians are initially trained and certified with respect to study procedures. Then, there is a continuous web-based grading of all spirometry (weekly reports) ≥3/4 and grading of technician performance (updated) ≥3/4. Exercise condition is assessed with three tests: 1) 6-Minute walk test (6MWT) performed accordingly to the ATS guideline (23,24); 2) Cardiopulmonary exercise test (CPET)- Symptom-limited incremental exercise is conducted on an electronically braked cycle ergometer according to recommended guidelines (25). Breath-by-breath data are collected at baseline and throughout exercise while subjects breath through a mouthpiece, nose occluded. Exercise variables (V˙E, V˙O2, V˙CO2, PETCO2, f, TI and TE, TI/Ttot, VT/TI and VT/TE) are measured and averaged over the last 30 seconds of each minute and at peak exercise.
Changes in EELV, estimated from IC measurements, and symptoms, assessed using modified Borg scale for perceived exertion, are assessed at rest, at the end of each 2-minute increment of exercise, and at peak exercise (26), reference for Borg; 3) Cardiac bioelectrical impedance (Physioflow®) measured during CPET- It has the advantage of being simple, non-invasive and it is independent of subject's collaboration. The reproducibility/accuracy of the PhysioFlow® derived cardiac output measures have been assessed at rest and during exercise (27,28).
CT scans of the chest. CT scan is acquired using a multi-slice CT scanner (≥16 detectors). The images are acquired with the subject supine at suspended full inspiration from the base to the apex of the lung to minimize motion artefact. The technical parameters for the CT acquisition are as follows: 120 kVp, 40 mAs, 0.5 second gantry rotation, pitch of 1.25 and 1 mm slice thickness. The images are reconstructed using both a low (“b35f”) and a high spatial frequency (edge enhancing) reconstruction algorithms and the smallest field of view that contains both lungs. The images are transferred the Department of Radiology of the University of British Columbia in Vancouver using the DICOM 3.0 format. The CT scans are graded for emphysema severity and distribution in accordance with the Fleischner Glossary of terms (29) and further classified into centrilobular, panlobular, and paraseptal. A simplified bronchiolitis score is also included and other incidental findings reported.
The extent and distribution of the emphysema as well as the airway wall dimensions (airway lumen area, wall area) is also quantified using the Apollo Image analysis software (VIDA Diagnostics Cedar Rapids IA). For each patient, the square root of wall area of all these bronchi against the internal perimeter is plotted, and the intercept corresponding to a bronchus of 10 mm internal perimeter is identified. The resultant value for square root of wall area is the primary measure of airway wall thickening. This parameter was chosen because it has been shown to predict the mean dimensions of histological small airways in COPD(30). The% wall area for 3rd, 4th, 5th and 6th generation bronchi will be measure as secondary measures.
Blood tests. Venous blood is collected for 4 types of samples. From Vacutainer Serum tubes, blood is processed to separate the serum and the buffy coat white blood cells, to obtain 10 aliquots of 0.5 ml of serum. From EDTA tubes, blood is processed to obtain 10 aliquots of 0.5 ml of plasma. Blood is also collected for genomic extraction (using commercial kits): 5 aliquots of 2.5 ml of whole Blood in PAXgene RNA tubes and 7 aliquots of 0.5 ml of whole Blood for DNA preparation. Samples are frozen at –80 ºC and sent to one of the two tissue bank sites for long-term storage and later assays. From the serum/plasma samples, among the measurements we will perform are the following: 1) SP-D; 2) CRP; 3) IL-6; 4) fibrinogen and 5) CC-16. At the tissue banks, in addition to basic clinical data access, functions for managing samples and inventory based on barcode technology, are available.
Health administrative databases. Subjects, recruited from CanCOLD, have been asked to provide permission to follow up their health care utilisation (including use of medications), health outcomes and mortality using provincial health care administrative and vital statistic databases. Canada has universal, government administered healthcare system, so that data for all physician visits and hospital and emergency department visits can be captured in this manner. The request has been to obtain follow-up for a period of up to 15 years. Such permission has been requested of subjects in all GOLD stages as well as those from the peer non COPD groups, an unaffected control group.
Retention Plan
Loss to follow-up of subjects is an issue in long-term cohorts. CanCOLD has developed a retention plan that includes, but is not limited to, the following strategies: 1) following each visit, subjects will receive a report with some salient results of tests and questionnaires, written in lay language; this report is independent of test results that can be provided to the subject if required, or if needed for follow-up (e.g., CT scan) as part of good clinical practice; 2) an annual report of the study progress; 3) enrolled subjects will receive a birthday card and a new year's card, thanking them for their participation to the study; 4) additional strategies (e.g., social gathering, raffles) are under evaluation. All these strategies are coordinated by the central office, and are implemented once approved by the local site Research Ethics Boards.
Quality Control Policy and Good Practice
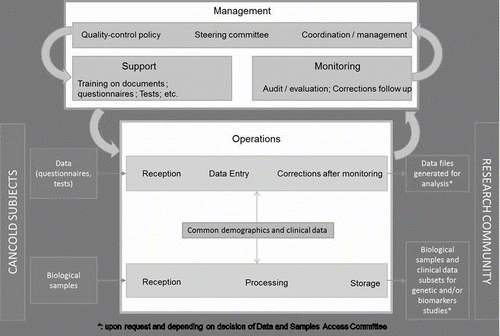
The quality control policy is of great importance as it emphasizes internally the responsibility and commitment every participating site needs to have, and the great value of the study externally. The quality control policy is aiming and making sure through national coordination that: 1) Each subject has complete and unbiased testing (questionnaires, lung/exercise and blood tests) according to the study protocol and the manual of procedures; 2) All data collected including the blood biological samples are traceable in a centralized platform and are properly stored for future analysis; a web-based informatic data management system has been developed by the Laboratoire de Télématique Biomédicale of the Respiratory Health Network of the FRSQ; 3) Feedback is provided on a continuous basis to each site ascertaining good practice and quality improvement.
Raw data from each test is checked for quality by an expert before being entered in the central database. Within one month of subject testing in a given site, data is entered, cleaned and ready to be accessed for specific analysis. describes the CanCOLD quality-control approach and implementation. The management includes: 1) definition of the process and following its application; 2) definition of role/responsibility of participating site, follow-up and communication; 3) support for training (initial and follow-up), equipment and site facilities; 4) system quality improvement as part of a continuous process: initiation visit and training/certification, monitoring visits, evaluation to assess conformity, actions for correcting problems and prevention.
The operational level implements and complies with the quality-control policy. A national expert in charge is identified for each test (questionnaires, PFT/exercise, blood samples, CTScan) and a very precise audit process for each stage is applied by the site-coordinator. Quality control process also covers access to data and biological samples. A specific policy has been developed to access data and samples, making sure that data generated by the study are available and are used for ancillary projects and publications.
Statistical Analysis and Sample Size
Baseline characteristics are tabulated for men and women, and for each of the cohort subsets. Results are mean/standard deviation, median/interquartile or percent where appropriate. Statistical analysis and sample size calculations are only presented for the main objective, not for secondary objectives.
Statistical analysis: What exposures other than smoking determine the progression of COPD? Using linear mixed models with FEV1 as the dependent variable we will estimate the progression of COPD over time. The main determinants will be lifestyle factors other than smoking, such as environment exposure, physical activity and diet. We will develop mixed linear regression models with adjustment for age, sex, smoking status and “lifetime” tobacco exposure, BMI, weight change, education, personal resources, self-efficacy, baseline FEV1, exercise capacity, quantitative emphysema and airway disease (CT Scan), COPD exacerbations, “scores” of diet and exercise behavior, biomarkers (systemic inflammation), symptoms, treatment and co-morbidities.
Sample size calculation. We have ensured an adequate sample size to assess the questions/hypothesis that relate to the evolution of COPD (annual decline in FEV1). Using the tables presented in Hedeker et al. (31), for the longitudinal data, with 4 measurement occasions, and assuming that the data are auto-correlated (i.e., correlation depends on time lag between observations) with autocorrelation of 0.5 between observations on the same subject we will have adequate sample size to detect a medium-effect size (0.5*SD) (for a linear between-groups trend) with about 133 subjects/group. This estimate included a 10% attrition, and alpha = 0.05, with 80% power. Assuming that the SD of yearly decline in FEV1 ranges from 30 to 44 ml (32), this would allow us to detect a change of 15 to 22 ml/year over the follow-up period. In the same paper, they found average yearly decreases of 25 to 44 ml depending on starting state (32), thus we will have power to conduct our longitudinal analyses.
Statistical analysis. What exposures other than smoking determine the development of COPD? Logistic regression will be used to compare those who develop COPD during the duration of the study (subset of subjects at-risk) and those that do not.
Sample size calculation. As this has never been done before, we do not have any estimates of the proportion of these subjects who will develop COPD. For this reason, we present the detectable odds ratio for a range of values. Thus, we are well powered to detect moderate to large odds ratios depending on the proportion of at-risk subjects who go on to develop COPD and the prevalence of exposure.
Discussion
CanCOLD is a novel COPD longitudinal cohort as it represents the establishment of a well characterised “phenotyped” population based cohort of COPD subjects and matched non-COPD peers with the framework needed to address this major health problem with respect to early disease, sex, aging and related co-morbidities. It is a resource with high potential for interdisciplinary research collaborations.
Prevention should be implemented for COPD as it is for cardiovascular diseases. Other than smoking cessation before or after establishment of COPD (primary and secondary prevention), very little is known about subsequent modifiable risk factor exposure (secondary prevention). No doubt that cigarette smoking is the most important causal factor for developing COPD and for disease progression. However, the view that cigarette smoking is the sole meaningful risk factor in the natural history of COPD is a misconception (33). Novel less traditional risks factors such as physical activity, dietary factors and co-morbidities can best be accessed through a population sample of subjects at-risk and COPD patients with early disease. CanCOLD has been designed to provide a population-based sample in lieu of convenience samples. CanCOLD also comprises a balanced proportion of male and female that will allow assessing differences in sex. These are important and distinctive features compared to other large COPD cohort such as COPDGene, ECLIPSE and SPIROMICS.
The data set collected in CanCOLD will also allow grouping of key patient attributes such as biological, clinical, physiological, imaging and co-morbidities and allow us to relate these variables to meaningful clinical (patient-related) outcomes. Ultimately, this may be useful in defining specific biological pathways or therapies. Other cohorts of larger size such as COPDGene, ECLIPSE and SPIROMICS have also being conducted on the heterogeneity of COPD, collecting as well large amount of data, biological measures with state-of-the-art technology and rich tissue banks. CanCOLD will be an excellent cohort to extend or validate data such as candidate biomarkers and discovery of genes from other cohorts. It will offer a unique opportunity to extend the observations to include subjects with mild disease. Furthermore, CanCOLD will take unique advantage of the administrative health databases available in Canada.
None of the existing COPD cohorts allows assessing the problem of underdiagnosing of COPD and the need for earlier intervention. The analysis of the CanCOLD data set will address whether undiagnosed COPD will be clinically important and a predictor of poor outcome, i.e., progression of lung hyperinflation, static (at rest) and dynamic (on exertion) and lung/airway structure abnormalities on CT Scan, systemic inflammation, absenteeism (days/hours missed from work) and presenteeism at work (illness/condition-related reductions in productivity while the person is at work) and health care utilization. This information will be mostly valuable in assisting public-health and health care system decision makers in developing policies to improve the diagnosis and the management of COPD.
In conclusion, CanCOLD is the first prospective longitudinal study for COPD with population sampling. The CanCOLD study will attempt to extensively characterize subjects, men and women with COPD, to identify specific phenotypes and identify how factors other than airflow obstruction (FEV1) can affect outcomes. CanCOLD will assess other risk factors, in addition to smoking which might impact on disease progression, and the study will assess co-morbidities. CanCOLD provides a standardized research platform with access to a large data set (including patient reported outcomes, lifestyle, work exposure, cardiopulmonary exercise function, lung imaging and co-morbidities) and a centralized tissue bank (blood storage for measuring biomarkers including DNA and RNA); it will contribute to address challenging and important research questions on disease evolution and disease management (in particular for those at-risk and with mild disease) in a more concerted and collaborative (transdisciplinary from epidemiology, clinical to basic research), high-impact and patient-oriented fashion. Because of similarities in collecting data but also it many distinctive features from other large COPD cohort studies worldwide, CanCOLD will be extremely valuable in enhancing longitudinal information in COPD.
Declaration Of Interest
J. Bourbeau reports receiving research funding via the Research Institute of the McGill University Health Centre, from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck, Novartis, Nycomed, Pfizer and Theratechnologies; and has served on speakers, consultation panels and/or advisory boards for the above listed pharmaceutical companies. W.C. Tan has no potential conflict of interest relevant to this article. A. Benedetti has no potential conflict of interest relevant to this article. SD Aaron has no potential conflict of interest relevant to this article. K.R. Chapman has received compensation for consulting with AstraZeneca, Boehringer-Ingelheim, CSL Behring, GlaxoSmithKline, Merck Frosst, Novartis, Nycomed, Pfizer, Roche, Shering Plough and Talecris; he has undertake research funded by AstraZeneca, Boehringer-Ingelheim, CSL Behring, Forest Labs, GlaxoSmithKline, Novartis, Parangenix, Roche and Talecris; and has participated in continuing medical education activities sponsored in whole or in part by AstraZeneca, Boehringer-Ingelheim, CSL Behring, Grifols, Merck Frosst, Novartis, Nycomed, Pfizer, and Telacris. H. Coxson has served on the steering committee for ECLIPSE project for GSK. In addition, he was the co-investigator on two multicentre studies sponsored by GSK and has received travel expenses to attend meeting related to the project. He had three contract service agreements with GSK to quantify the CT scans in subjects with COPD and has a service agreement with Spiration Inc. to measure changes in lung volume in subject with severe emphysema. He has received a fee for speaking at a conference and related travel expenses from AstraZeneca. He was the recipient of a GSK Clinical Scientist Award (2010–2011). R. Cowie has no potential conflict of interest relevant to this article. J.M. Fitzgerald has no potential conflict of interest relevant to this article. R. Goldstein has no potential conflict of interest relevant to this article. P. Hernandez has participated in industry-sponsored clinical trials and continuing education events and medical advisory boards for the following companies: AstraZeneca, Boehringer-Ingelheim, Eli Lilly, GlaxoSmithKline, Novartis, Nycomed, Pfizer, CSL Behring, Actelion, Merck Frosst. J. Leipsic has no potential conflict of interest relevant to this article. F. Maltais has no potential conflict of interest relevant to this article. D. Marciniuk has no potential conflict of interest relevant to this article. D. O'Donnell has no potential conflict of interest relevant to this article. D.D. Sin has no potential conflict of interest relevant to this article. The authors are entirely responsible for the content and writing of this paper.
Acknowledgments
The successful completion of this study will only be possible through the commitment of the participants and the dedication of the study personnel from the national coordination and each participating site. The authors wish to thank the Laboratoire de Télématique Biomédicale du Réseau en Santé Respiratoire du Fonds de la Recherche en Santé du Québec (FRSQ).
References
- Chapman KR, Mannino DM, Soriano JB, Vermeire PA, Buist AS, Thun MJ, Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J 2006 Jan;27(1):188–207.
- Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J 2006 Sep;28(3):523–32.
- Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970–2002. JAMA 2005 Sep 14;294(10):1255–9.
- Connors AF, Jr., Dawson NV, Thomas C, Harrell FE, Jr., Desbiens N, Fulkerson WJ, Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996 Oct;154(4 Pt 1):959–67.
- Schiele F, Meneveau N, Seronde MF, Caulfield F, Fouche R, Lassabe G, Compliance with guidelines and 1-year mortality in patients with acute myocardial infarction: a prospective study. Eur Heart J 2005 May;26(9):873–80.
- Tan WC, Bourbeau J, FitzGerald JM, Cowie R, Chapman K, Hernandez P, Can age and sex explain the variation in COPD rates across large urban cities? A population study in Canada. Int J Tuberc Lung Dis 2011 Dec;15(12):1691–8.
- Hakkaart-van Roijen L, Bouwmans CAM. Manual: Short Form - Health and Labour Questionnaire (SF-HLQ)The Health and Labour Questionnaire. Http://www imta nl/publications/0052 pdf 2007Rotterdam
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988 Mar;93(3):580–6.
- Partridge MR, Karlsson N, Small IR. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an internet survey. Curr Med Res Opin 2009 Aug;25(8):2043–8.
- Partridge MR, Miravitlles M, Stahl E, Karlsson N, Svensson K, Welte T. Development and validation of the Capacity of Daily Living during the Morning questionnaire and the Global Chest Symptoms Questionnaire in COPD. Eur Respir J 2010 Jul;36(1):96–104.
- Shatenstein B, Nadon S, Godin C, Ferland G. Development and validation of a food frequency questionnaire. Can J Diet Pract Res 2005;66(2):67–75.
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc 2001 Jul;33(7):1126–41.
- Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992 Jun;30(6):473–83.
- Orbon KH, Schermer TR, van der Gulden JW, Chavannes NH, Akkermans RP, Schayck OP, Employment status and quality of life in patients with chronic obstructive pulmonary disease. Int Arch Occup Environ Health 2005 Jul;78(6):467–74.
- Pakhale S. Validation of McGill COPD Quality of life questionnaire McGill University; 2008.
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992 Jun;145(6):1321–7.
- Blanc PD, Eisner MD, Trupin L, Yelin EH, Katz PP, Balmes JR. The association between occupational factors and adverse health outcomes in chronic obstructive pulmonary disease. Occup Environ Med 2004 Aug;61(8):661–7.
- Kremer AM, Pal TM, van Keimpema AR. Employment and disability for work in patients with COPD: a cross-sectional study among Dutch patients. Int Arch Occup Environ Health 2006 Oct; 80(1):78–86.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983 Jun; 67(6):361–70.
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am Rev Respir Dis 1987 Jul; 136(1):225–44.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007 Sep 15; 176(6):532–55.
- Buist AS, Vollmer WM, Sullivan SD, Weiss KB, Lee TA, Menezes AM, The Burden of Obstructive Lung Disease Initiative (BOLD): rationale and design. COPD 2005 Jun; 2(2):277–83.
- ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002 Jul 1; 166(1):111–7.
- Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985 Apr 15; 132(8):919–23.
- ATS/ACCP. Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003 Jan 15; 167(2):211–77.
- O'Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001 Sep 1; 164(5):770–7.
- Richard R, Lonsdorfer-Wolf E, Charloux A, Doutreleau S, Buchheit M, Oswald-Mammosser M, Non-invasive cardiac output evaluation during a maximal progressive exercise test, using a new impedance cardiograph device. Eur J Appl Physiol 2001 Aug; 85(3–4):202–7.
- Tordi N, Perrey S, Harvey A, Hughson RL. Oxygen uptake kinetics during two bouts of heavy cycling separated by fatiguing sprint exercise in humans. J Appl Physiol 2003 Feb; 94(2):533–41.
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008 Mar; 246(3):697–722.
- Nakano Y, Wong JCDe Jong PA, Buzatu L, Nagao T, Coxson HO, The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med 2005 Jan 15; 171(2):142–6.
- Hedeker D, Gibbons RD, Waternaux C. Sample size estimation for longitudinal designs with attrition: Comparing time-related contrasts between two groups. J Educ Behav Statist 1999; 24(1):70–93.
- Bridevaux PO, Gerbase MW, Probst-Hensch NM, Schindler C, Gaspoz JM, Rochat T. Long-term decline in lung function, utilisation of care and quality of life in modified GOLD stage 1 COPD. Thorax 2008 Sep; 63(9):768–74.
- Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010 Sep 1; 182(5):693–718.