Abstract
Background: Although hospitalization is recognized as an important cause of reduction in physical activity in daily life (PADL) in COPD, there is only one study evaluating this effect, and it was performed in European COPD patients who have a lower PADL than that of South American COPD patients. Objectives: To investigate the effect of hospitalization due to acute exacerbation of PADL in Brazilian COPD patients and to evaluate the factors that determines the physical activity levels during hospitalization and after discharge. Methods: PADL was quantified using a 3-axis accelerometer on the 3rd day of hospitalization and 1 month after discharge in Brazilian COPD patients who were hospitalized due to disease exacerbation. Six-minute walking distance (6MWD), lower limb strength and pulmonary function were also evaluated. Results: A total of 20 patients completed the study. During hospitalization, patients spent most of the time (87%) lying down or sitting; however, 1 month after they were walking >40 min/day. In addition, patients with prior hospitalization had a lower level of physical activity compared to those without a previous history of hospitalization. The time spent walking during hospitalization was significantly explained by the quadriceps strength (r2 = 0.29; p < 0.05), while 1 month after, the time spent walking was only significantly explained by the 6MWD (r2 = 0.51; p = 0.02). Conclusions: Brazilian COPD patients are inactive during hospitalization but become active 1 month after discharge. Previously hospitalized are more inactive both during and after exacerbation. The quadriceps strength and 6MWD explain the physical activity levels during hospitalization and at home, respectively.
Introduction
The exacerbation of chronic obstructive pulmonary disease (COPD) is defined as “an event in the natural course of the disease characterized by a change in baseline dyspnea, cough and/or secretion that goes beyond the daily variations, is acute and may require a change in the medication of these patients” (Citation1,2). Exacerbation is recognized as an important cause of the increased health care costs (Citation3) and risk of mortality (Citation4) among these patients as well as the reduction of health-related quality of life (HRQL) (Citation5), muscle strength (Citation6), lung function and the level of physical activity in daily life (PADL) (Citation7).
The decline in PADL during hospitalization in patients with COPD leads to a decrease in peripheral muscle strength over 5 days of hospitalization (Citation6) and appears to influence the development of the systemic consequences of COPD (Citation7).
To our knowledge, there is only one study that evaluated the effects of hospitalization on PADL levels in patients with COPD, which found that after 5 days of hospitalization, the patients showed a reduction in PADL for up to 1 month after discharge and that these patients have lower PADL levels compared to clinically stable COPD patients (Citation7). However, this study was conducted in Belgium, a country with a Caucasian population with high socio-economic and educational levels (Citation7), and there is evidence that the level of physical activity in daily life depends on several factors, such as ethnicity, educational level and socioeconomic status in adults and the elderly (Citation8–12).
In this sense, Pitta et al. (Citation13,14) showed that stable South American COPD patients have a higher level of PADL when compared with European patients.
In light of this, we speculate that perhaps the effect of hospitalization on the reduction of PADL in South American patients with COPD who have a low socioeconomic and cultural status, such as the residents of Brazil, is not as significant as has been previously described. Thus, the objective of this study was to quantify the effect of hospitalization due to acute exacerbation on the PADL of Brazilian COPD patients. Furthermore, we evaluated outcomes associated with PADL during hospitalization and after hospital discharge.
Methods
Patients
This longitudinal study evaluated 76 consecutive patients with a clinical diagnosis of COPD who experienced exacerbation of the disease characterized by an increased and/or changed appearance of the pulmonary secretions, cough and worsening dyspnea and who were admitted to the ward of a University Hospital.
After admission, patients who met the criteria for diagnosis of COPD based on Global Initiative for Chronic Obstructive Lung Disease (GOLD) (Citation15) and exacerbation of Anthonisen (Citation16) criteria were invited to participate in the study. We excluded the following patients: (i) those who responded inadequately to the initial drug treatment, defined as patients who presented signs of respiratory failure (respiratory rate > 35 bpm, paradoxical chest wall movements and worsening or onset of central cyanosis) despite treatment with corticosteroids, short-acting bronchodilators and oxygen on the 2nd day of hospitalization; (ii) those with altered mental status (confusion, stupor or coma); (iii) those with persistent or worsening hypoxemia (oxygen arterial pressure <40 mmHg) and/or worsening respiratory acidosis (pH < 7.25) despite supplemental oxygen and the use of noninvasive ventilation; (iv) those who experienced hemodynamic instability with the need for vasopressor drugs; and, (v) those who required invasive mechanical ventilation.
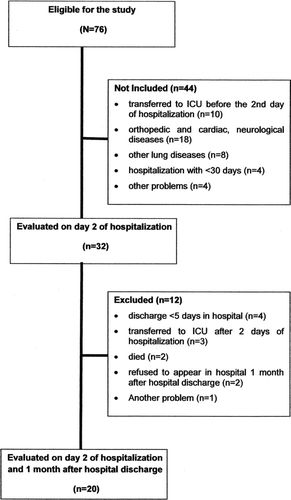
Patients were also excluded if they had been hospitalized during the previous 30 days, had a previous history of other lung diseases, were clinically diagnosed with unstable cardiovascular, neurological or orthopedic diseases that would preclude them from walking. Furthermore, we excluded patients who, even after inclusion, were referred to the intensive care unit due to the worsening of the clinical profile or who had a hospital stay of less than 5 days and those who refused to return to the hospital 1 month after being discharged.
During the study, all patients received optimal pharmacological treatment according to the recommendations of GOLD (Citation15) and supplemental oxygen or were otherwise maintained at pulse oxygen saturation (SpO2) >88% breathing room air. All patients received chest physiotherapy aiming mucous secretion clearance and breathing exercises, but no formal exercise therapy was offered. The patients were duly informed of the purpose of the study and agreed to sign a consent form approved by the Ethics and Research Committee of the University Hospital, protocol 727/07.
Study design
After inclusion in the study, patients were evaluated for prior medical history, anthropometric data, pulmonary function test, 6-minute walk distance (6MWT), BODE score, peripheral muscle strength of the lower limbs, C reactive protein (CRP) and arterial blood gases tests on the 2nd day of hospitalization and 1 month after hospital discharge. The inpatient physical activity in daily life (PADL) was evaluated on the 3rd or 4th day of hospitalization and re-evaluated during 2 consecutive days 1 month after being discharged from the hospital. Days of hospitalization, corticosteroid use and the number of hours on non-invasive ventilation were also quantified.
Analyzed variables
Pulmonary function test
Spirometry (Microquark, Cosmed, USA) was performed according to the criteria of the American Thoracic Society (Citation17) and the obtained values were calculated in relation to predicted values for the Brazilian population (Citation18).
Submaximal physical capacity
To measure sub-maximal physical capacity, we conducted a 6MWT. Briefly, the test was performed in a 30-meter long corridor with little traffic, and every minute, standardized phrases of encouragement were spoken. Patients were instructed to walk as fast as possible during the test and were allowed to rest if they presented limiting dyspnea or any other discomfort that made them unable to continue walking. The patient received oxygen via nasal cannula if they presented a SpO2 <88% during the test. The cardiac and respiratory rates, PaO2 and subjective sensation of effort (modified Borg scale) (Citation19) were monitored before and after testing.
Peripheral muscle strength of the lower limbs
The strength of maximal voluntary isometric contraction of the lower limbs was measured using a load cell (EMG System, Brazil) (Citation20). The signals received by the load cell were transmitted to a digital signal conditioning module using software for data acquisition storage (EMG100, EMG System, Brazil). Isometric muscle strength of the dominant member of the following muscle groups was evaluated: hip flexors, knee flexors and knee extensors. The exercises were performed as previously described (Citation20).
Briefly, the patient was asked to sustain maximum force for 5 seconds with verbal encouragement, and the largest amount from three attempts was used, provided that there was a difference of less than 10%. Between each attempt there was a 1-minute rest period, and to minimize the learning curve, the patient made an attempt before starting the test.
Monitoring of physical activity in daily life
PADL monitoring was performed using a DynaPort Moviemonitor (McRoberts, The Netherlands) developed to evaluate PADL and validated in patients with COPD (Citation21). This small (54 × 84 × 8 mm), lightweight (45 g), high resolution, tri-axial sensor quantifies activities such as walking, changes in body position and the time spent and caloric expenditure of sitting, lying or standing activities. The device was inserted into an elastic band positioned on the lower back in the region of the second lumbar vertebra.
The measurement was performed for 12 hours/day (from 08:00 to 20:00) on the 3rd and 4th days of hospitalization, and the patients were strictly instructed to carry on their daily physical activity as desired, and were not instructed specifically either to stay in bed or to perform extra physical activities in addition to what they desired to. Measurements of PADL at home (1 month after hospital discharge) were made on 2 consecutive weekdays. The signals were stored on a 512 Mb memory card and transmitted via the Internet to the manufacturer for analysis. For data analysis, we took the average of both days.
BODE index: It was quantified as previously described (Citation22). Briefly, the index ranges from 0 to 10 and takes into consideration the body mass index, the FEV1 (Forced Expiratory Volume in 1 second), dyspnea on a scale modified from the Medical Research Council (MMRC) and the 6MWT. The higher the value, the greater the risk of patient's mortality.
Statistical analysis
Quantitative variables were expressed as the means and standard deviations, except under specific conditions. The parametric distribution of the data was evaluated using the Shapiro-Wilk test, and the comparison between admission and discharge was made using the paired t-test (parametric data) or the Wilcoxon signed-rank test (non-parametric data). Based on our preliminary hypotheses, Spearman's correlation coefficient was used to determine the association of the time spent walking with those variables that could interfere with the physical activity in daily life. The variables with a significant association (p < 0.05) were used in a stepwise multiple regression analysis to identify the independent contributors to the time spent walking in the hospital and at home. The significance level was set at 5% (p ≤ 0.05) for all tests, and we used the statistical program SPSS 17.0.
A sample size of 20 patients was calculated based on a 12% increase in the average time spent during weight-bearing activities between hospitalization and after hospital discharge with a 19% standard deviation, as previously reported (Citation7). This analysis was considered to have an 80% power using a two-sided test and α = 0.05.
Results
Of the 76 patients eligible for the study, 44 patients were excluded. Some (n = 18) were excluded because of orthopedic, neurological or cardiac disorders, while others were excluded because they were transferred to the intensive care unit (ICU) before the 2nd day of hospitalization (n = 10), due to other lung diseases (n = 8), due to re-hospitalization (n = 4) and, lastly, due to other problems (n = 4) (). Additionally, 12 patients were excluded because they had a hospital stay of <5 days (n = 4) because they were transferred to the ICU (n = 3), died (n = 2), refused to come back to the hospital (n = 2) and other reasons (n = 1). The baseline clinical characteristics (age, FEV1, 6MWD and BMI) were similar between the studied participants and those individuals who refused to come back 1 month after their hospital discharge.
Of the 20 patients analyzed, all patients were able to perform tests to assess their muscle strength and PADL levels during the study. Five patients could not perform the 6MWT on day 2 due to worsening of their respiratory profiles, and the test was performed the next day. In addition, 6 patients could not perform the spirometry on the 2nd day of hospitalization that was repeated until the 4th day of hospitalization.
Patients had a mean age of 68.6 ± 10.7 years, the majority was male (70%) (), and the length of hospitalization during the study was 8.9 ± 3.1 days. No change was observed in body mass index (BMI) or quadriceps strength during the study period (p > 0.05); however, the patients showed an increase in FEV1, the 6MWD and the PaO2 and a reduction in dyspnea at 1 month post-hospital discharge compared with the 2nd day of hospitalization (p < 0.05) ().
Table 1. Clinical characteristics, quadriceps strength and BODE index of the studied patients
The patients showed high levels of co-morbidities, the most prevalent of which were hypertension (60%) and diabetes mellitus (35%), and 7 patients (35%) reported at least one hospitalization in the past year ().
Table 2. Co-morbidities and variables recorded during hospitalization
The time of in-patient and outpatient physical activity is described in . It was found that, during hospitalization, COPD patients spent most of the time (86.7%) inactive, either lying down (57.6%) or sitting (29.1%), and they walked only 7.2 minutes per day. After 1 month of hospitalization, the patients still remained inactive, but their time spent walking increased on average to 42 minutes/day (p < 0.001).
Table 3. Daily life physical activity during hospitalization and 1 month after hospital discharge
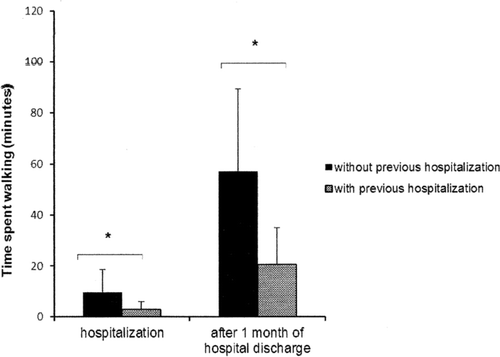
The time spent walking during hospitalization and 1 month after hospital discharge by the patients with (n = 7) or without (n = 13) a prior history of hospitalization during the past year is shown in . The patients with previous hospitalizations had a shorter time spent walking both during the hospitalization period (p = 0.02) and at home (p < 0.01) than those without a previous hospitalization. Interesting, no clinical difference was observed during hospital admission between COPD patients with or without previous hospitalization.
Figure 2. The bars represent the mean and standard deviation of the time spent walking during hospitalization and 1 month after hospital discharge of those patients with (n = 7) or without (n = 13) previous hospitalization in the last year. *p < 0.05 between groups.
The linear correlation between the time spent walking and the other outcomes analyzed in the study is shown in . We found that the time spent walking during the hospitalization period was positively correlated with the 6MWD (r = 0.57; p < 0.01), quadriceps strength (r = 0.54; p < 0.01) and PaO2 (r = 0.44; p = 0.05) and negatively correlated with the BODE index prognosis (r = −0.49; p = 0.02). A multivariate regression analysis was then performed and revealed that only the strength of the quadriceps muscle showed an association with the time spent walking during the hospitalization period (partial r2 = 0.29; p < 0.05).
Table 4. Linear correlation between walking time and other outcomes during hospitalization and 1 month after hospital discharge
The time spent walking at home 1 month after hospital discharge was positively correlated with FEV1 (r = 0.47; p = 0.03) and 6MWD (r = 0.71; p < 0.01) and negatively correlated with BODE, prior hospitalization and the dyspnea scale (). However, there was no significant linear correlation between quadriceps strength and time spent walking at home. In a multivariate regression analysis, the time spent walking at home was significantly explained by 6MWD (partial r2 = 0.51; p = 0.02).
Discussion
The results of the present study demonstrate that Brazilian COPD patients remain inactive during hospitalization but are active 1 month after being discharged from the hospital. We also demonstrated that patients with previous hospitalization were more inactive during both hospitalization and at home. Finally, we demonstrate that the PADL was significantly explained by the peripheral muscle strength and submaximal physical capacity during acute exacerbation and at home, respectively.
To our knowledge, only one study quantified the levels of PADL objectively during hospitalization, and the authors showed that European COPD patients remain inactive 91% of the time (7). Interestingly, our Brazilian COPD patients were similarly inactive during their hospitalization (86.7%). These results suggest that physical inactivity during hospitalization is increased in COPD patients independent of the socioeconomic conditions and ethnicity. The reasons for such inactivity are most likely multiple and are likely to include the severity of illness, fear of worsening of symptoms and the hospital environment. In our study, patient's inactivity was observed, despite the fact that subjects have received respiratory care treatment.
Although inactivity in hospitalized patients has been described in the elderly (Citation23) and patients who are not restricted to the hospital bed (Citation24), potential harmful effects of immobility have not been addressed. Therefore, strategies such as resistance exercises (Citation25) and neuromuscular electrical stimulation (Citation26) have potential for counteracting the inactivity of these patients, although further research is required on this subject.
The reduced levels of physical activity in daily life observed during hospitalization in our patients significantly improved 1 month after hospital discharge, and the patients were moving at levels near those of European patients with stable COPD without a history of hospitalization (Citation13). Our results differ from those of Pitta et al. (Citation7), which showed that European COPD patients remained physically inactive 1 month after they were discharged. This difference between both studies may be explained by the higher prevalence of comorbidities and the higher FEV1 and 6MWD observed in our patients and could favor the recovery after hospital discharge observed in our study.
Although we do not know the cause of this difference recovery of PADL, previous studies have revealed a difference in the level of physical activity in clinically stable COPD patients from different continents (Citation13). We may hypothesize that the increased levels of PADL in the Brazilian COPD patients can be at least partially explained by their lower socioeconomic status as well as the warmer temperatures and higher city dimensions. Despite these issues, the observation that our patients are physically active 30 days after their hospital discharge is a positive finding because there is evidence that a higher level of physical activity is associated with both a lower risk of mortality and hospitalization due to illness (Citation27) and with a healthier lifestyle (Citation28).
Interestingly, our results also show that patients who had been hospitalized in the last year (35% of patients) were more sedentary than patients without prior hospitalization. It is important to reinforce that during hospital admission we did not observe any clinical differences between COPD with or without previous hospitalization suggesting the higher physical inactivity observed in CODP with previous hospitalization cannot be explained by patient´s weakness or worst disease´s condition. Our results differ from those obtained by Pitta et al. (Citation7), who did not observe difference in physical activity levels between patients with or without previous exacerbation during hospitalization.
We believe that this discrepancy may be explained at least in part by the worse FEV1 of European patients compared to ours (29% vs. 48%, respectively), which would cause them to have more dyspnea and increase limitations on their physical activities. Our findings that patients with previous hospitalization have greater physical inactivity even within the hospital environment reinforces the idea that hospitalization influences the development of systemic consequences of COPD, particularly with regard to physical activity7 and muscle strength (Citation6).
In evaluating the possible factors associated with physical activity levels during hospitalization, we found that the strength of the quadriceps muscle, blood oxygenation and walk test were linearly associated with physical activity levels. However, when we carried out a multivariate analysis, we found that only quadriceps strength was independently associated with the physical activity level of the patient. A previous study suggested that some inflammatory markers were associated with a reduction in the strength of the quadriceps muscle in COPD patients during the hospitalization period (Citation6), and we hypothesize that this issue could affect the PADL. However, the systemic levels of C reactive protein were not associated with either quadriceps strength or PADL.
Despite previous evidence that patients with low FEV1 are those with the greatest risk of hospitalization (Citation29–31), our data suggest that airway obstruction did not reflect the level of physical activity in this environment. Because dyspnea during hospitalization in our patients was at levels close to the maximum (MMRC 3.5 ± 0.7), we believe that the association between muscle strength and level of physical activity may be explained by the use of muscle strength as a reserve source for performing activities.
Moreover, 1 month after the hospitalization, our patients maintained muscle strength and increased the level of physical activity was associated with significantly improved performance on the 6MWD. This reinforces the importance of the 6MWD as a valuable element in the assessment of patients with COPD.
This study has limitations. First, although the sample size of 20 subjects was calculated based on a previous study (Citation7), a problem arising from this sample size is the limited generalization of the results. For example, care must be taken when generalizing these results to patients having other GOLD stages not included in our study. Second, our study design did not include activity monitoring before the exacerbation of interest. However, this problem is difficult to circumvent because it would be necessary to assess a large number of stable patients and wait for them to be hospitalized, what would happen in a different time interval for each patient. Therefore, repeated baseline measures would be required because patients may deteriorate activity level over time even without hospitalization.
Third, we did not use the Bonferroni correction to recalculate the significance value in our analysis of multiple comparisons. Although the Bonferroni correction has been proposed to avoid type I errors, its use has also been previously determined to be unnecessary in multiple comparisons to avoid type II errors (Citation32). Finally, the lack of a control group does not allow us to know how much the PADL patients recovered after hospitalization.
Conclusion
Our results show that hospitalized Brazilian COPD patients have a reduced level of physical activity that rises significantly 1 month after their hospital discharge. We also showed that patients with previous hospitalizations had a lower level of physical activity during and after hospitalization. Finally, the level of physical activity in daily life during the hospitalization period was significantly explained by the peripheral muscle strength, and the level of physical activity post-discharge was significantly explained by the submaximal physical capacity.
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest 2000; 117(5, Suppl 2):398S–401S.
- Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J Suppl 2003; 41:46s–53s.
- Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J 2007; 29:1224–1238.
- Wedzicha JA, Donaldson GC. Exacerbations of chronic obstructive pulmonary disease. Respir Care 2003; 48:1204–1213.
- Seemungal TAR, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157:1418–1422.
- Spruit MA, Gosselink R, Troosters T, Muscle force during an acute exacerbation in hospitalized patients with COPD and its relationship with CXCL8 and IGF – I. Thorax 2003; 58:752–756.
- Pitta F, Troosters T, Probst VS, Physical activity and hospitalization for exacerbation of COPD. Chest 2006; 129:536–539.
- Marshall SJ, Jones DA, Ainsworth BE, Reis JP, Levy SS, Macera CA. Race/ethnicity, social class, and leisure-time physical inactivity. Med Sci Sports Exerc 2007; 39:44–51.
- Ransdell LB, Wells CL. Physical activity in urban white, Africane American, and Mexicane American women. Med Sci Sports Exerc 1998; 30:1608–1615.
- Martinez-Gonzalez MA, Varo JJ, Santos JL, De Irala J, Gibney M, Kearney J, Prevalence of physical activity during leisure time in the European Union. Med Sci Sports Exerc 2001; 33:1142–1146.
- Crespo CJ, Ainsworth BE, Keteyian SJ, Heath GW, Smit E. Prevalence of physical inactivity and its relation to social class in U.S. adults: results from the Third National Health and Nutrition Examination Survey, 1988e1994. Med Sci Sports Exerc 1999; 31:1821–1827.
- Parks SE, Housemann RA, Brownson RC. Differential correlates of physical activity in urban and rural adults of various socioeconomic backgrounds in the United States. J Epidemiol Commun Health 2003; 57:29–35.
- Pitta F, Breyer MK, Hernandes NA, Teixeira D, Sant’Anna TJP, Fontana AD, Probst VS, Brunetto AF, Spruit MA, Wouters EFM, Burghuber OC, Hartl S. Comparison of daily physical activity between COPD patients from Central Europe and South America. Respiratory Medicine 2009; 103:421–426.
- Hernandes NA, Teixeira DC, Probst VS, Brunetto AF, Ramos EMC, Pitta F. Profile of the level of physical activity in the daily lives of patients with COPD in Brazil. J Bras Pneumol 2009; 35:949–956.
- Rabe KF, Hurd S, Anzueto A, Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease – GOLD Executive Summary. Am J Respir Crit Care Med. 2007: 176;532–555.
- Anthonisen NR, Manfreda J, Warren CP, Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106:196–204.
- American Thoracic Society Statement. Standards for the diagnosis and care of patients with COPD. Am J Respir Crit Care Med. 1995; 152:S77–S120.
- Pereira CAC, Barreto SP, Simões JG, Valores de referência para espirometria em uma amostra da população brasileira. J Pneumol 1992; 18:10–12.
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14:377–381.
- Hogrel JY, Payan CA, Ollivie Gr, Tanant V, Attarian S, Couillandre A, Development of a French isometric strength normative database for adults using quantitative muscle testing. Arch Phys Med Rehabil 2007; 88:1289–1297.
- Langer D, Gosselink R, Sena R, Validation of two activity monitors in patients with COPD. Thorax 2009; 64:641–642.
- Celli BR, Cote CG, Marin JM, The body – mass index, airflow obstruction, dyspneia, and exercise capacity index in Chronic Obstrutive Pulmonary Disease. N Engl J Med 2004; 350:1005–1012.
- Inouye SK, Wagner DR, Acampora D, A controlled trial of a nursing-centered intervention in hospitalized elderly medical patients: the Yale Geriatric Care Program. J Am Geriatr Soc 1993; 41:1353–1360.
- Suesada MM, Martins MA, Carvalho CRF. Effect of short-term hospitalization on functional capacity in patients not restricted to bed. Am J Phys Med Rehabil 2007; 86:455–462.
- Troosters T, Probst VS, Crul T, Pitta F, Gayan-Ramirez G, Decramer M, Gosselink R. Resistance training prevents deterioration in quadriceps muscle function during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 181:1072–1077.
- Neder JA, Sword D, Ward SA, Home based neuromuscular electrical stimulation as a new rehabilitative strategy for severely disabled patients with chronic obstructive pulmonary disease (COPD). Thorax 2002; 57:333–337.
- Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 2006; 61:772–778.
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 1995; 273:402–407.
- Garcia-Aymerich J, Farrero E, Felez MA, Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax 2003; 58:100–105.
- Garcia-Aymerich J, Monso E, Marrades RM, Risk factors for hospitalization for a chronic obstructive pulmonary disease exacerbation. EFRAM study. Am J Respir Crit Care Med 2001; 164:1002–1007.
- Gudmundsson G, Gislason T, Janson C, Risk factors for rehospitalisation in COPD: role of health status, anxiety and depression. Eur Respir J 2005; 26:414–419.
- Perneger TV. What's wrong with Bonferroni adjustments. BMJ 1998; 316:1236–1238.