Abstract
COPD is defined by airflow limitation that is not fully reversible and is usually progressive. Thus, airflow obstruction (measured as FEV1) has traditionally been used as the benchmark defining disease modification with therapy. However, COPD exacerbations and extrapulmonary effects are common and burdensome and generally become more prominent as the disease progresses. Therefore, disease progression should be broader than FEV1 alone. Interventions that reduce the frequency or severity of exacerbations or ameliorate extrapulmonary effects should also be considered disease modifiers. A narrow focus on FEV1 will fail to capture all the beneficial effects of therapy on disease modification. Although smoking cessation has been unequivocally demonstrated to slow the rate of FEV1 decline, inhaled corticosteroid–long-acting bronchodilator therapy may also have modest effects according to post hoc analysis. Maintenance pharmacotherapy with inhaled long-acting anti-muscarinic or ®-adrenergic agents or combined ®-adrenergic—inhaled corticosteroid reduces symptoms, improves lung function, reduces the frequency of exacerbations, and improves exercise capacity and HRQL. Pulmonary rehabilitation reduces symptom burden, increases exercise capacity, improves HRQL, and reduces health care utilization, probably through reducing the severity of exacerbations. Smoking cessation, lung volume reduction surgery, inhaled maintenance pharmacotherapy, and pulmonary rehabilitation administered in the post-exacerbation period may reduce mortality in COPD. These improvements over multiple outcome areas and over relatively long durations suggest that disease modification is indeed possible with existing therapies for COPD. Therefore, therapeutic nihilism in COPD is no longer warranted.
Introduction
Chronic obstructive pulmonary disease (COPD) is defined by the American Thoracic Society (ATS) and European Respiratory Society (ERS) as “a preventable and treatable disease state characterized by airflow limitation that is not fully reversible. The airflow limitation is usually progressive and is associated with an abnormal inflammatory response of the lungs to noxious particles or gases, primarily caused by cigarette smoking” (Citation1).
Practical diagnosis of COPD requires spirometric demonstration of post-bronchodilator airflow limitation, usually defined as a forced expiratory volume in 1 sec/forced vital capacity (FEV1/FVC) ratio < 0.70 (Citation2). Severity of airflow limitation is traditionally categorized by expressing the FEV1 as a percent of the predicted value for age, height, and ethnicity () (Citation2).The 2011 Global Initiative for Obstructive Lung Disease (GOLD) guidelines (Citation2) emphasize the role of symptom assessments and exacerbation frequency, in addition to the degree of spirometric obstruction in determining the severity of COPD; this is important because patients at similar levels of FEV1/FVC may have widely varying clinical presentations.
Table 1. GOLD spirometric classification of COPD according to FEV1.2
Smoking is the primary cause of COPD, although biomass smoke and industrial exposures also confer risk (Citation2, 3). Fletcher and Peto originally postulated that ∼15% of smokers were susceptible to developing COPD (Citation4), but it has recently been suggested that a higher proportion of smokers will eventually develop COPD if they live long enough (Citation5, 6). A longitudinal population-based Swedish study (Citation7) found that although the prevalence of GOLD COPD was ∼14% in all participants >45 years of age, about 50% of smoking enrollees who survived to old age eventually developed COPD. Approximately 80 million people worldwide have moderate-to-severe COPD (Citation8). In the United States, approximately 12 million people have diagnosed COPD (Citation9). However, because COPD often goes undiagnosed, its real prevalence is estimated to be closer to 24 million (Citation10). Mortality caused by COPD in the United States increased by 67% between 1980 and 2000 (Citation10). COPD is the third leading cause of death in the United States (Citation11) and is projected to become the third-leading cause of death worldwide by 2020 (Citation3).
The health care costs of COPD are substantial. In the United States in 2010, total costs of COPD were estimated at $49.9 billion (Citation12); in the European Union in 2004, the total direct costs of respiratory disease represented ∼6% of the total health care budget, with COPD accounting for 56% of respiratory costs (Citation13).
Despite the substantial burden of COPD on the individual and society, it often goes undiagnosed and is undertreated. This reflects, in part, an implicit perception that no currently available treatments favorably modify the course of COPD. This is shortsighted, as our review will illustrate.
The Complex Nature of COPD
Demonstrating the presence and categorizing the degree of spirometric airflow limitation is necessary but not sufficient to characterize COPD. Disease severity has many dimensions not captured in FEV1 (Citation14, 15). Dyspnea dominates patients’ experience of COPD (Citation16) but correlates poorly with measured airflow limitation (Citation17). Mortality is more strongly predicted by dyspnea (Citation18), lung hyperinflation (Citation19), functional status (Citation20), 6-min walk distance (Citation21), body mass index (Citation21), or mid-thigh cross-sectional area (Citation22) than by FEV1. To fully appreciate the pervasive effects of COPD requires looking beyond FEV1. The creation of a multidimensional staging index for COPD represents a step in this direction.
The BODE index, a multidimensional severity metric for COPD, combines information from four variables, body mass index, airway obstruction (FEV1), dyspnea (Medical Research Council dyspnea rating), and exercise capacity (6-min walk distance) into a 10-point severity rating, which predicts mortality in COPD better than any of its components (Citation21). Additional aspects of COPD severity beyond FEV1 or the BODE index include lung hyperinflation, systemic inflammation, extrapulmonary pathology, and frequency and severity of COPD exacerbations.
Hyperinflation as well as airflow limitation contributes to dyspnea in COPD. Static hyperinflation results, in part, from loss of lung elastic recoil. Dynamic hyperinflation, which occurs earlier in COPD than static hyperinflation, results when high respiratory rates (e.g., during exercise) are required to increase minute ventilation. In airflow-limited COPD patients requiring prolonged expiratory time, rapid breathing empties the lungs incompletely, progressively increasing end expiratory volume and markedly increasing elastic work of breathing and dyspnea. Hyperinflation not only contributes to dyspnea in COPD (Citation23) but independently predicts all-cause and respiratory mortality (Citation19).
Systemic inflammation is a feature common to COPD (Citation24–26), its extrapulmonary manifestations, and its frequent co-morbidities such as cardiovascular and metabolic diseases (Citation27–30). Inflammatory morbidities such as cachexia and cardiovascular disease (Citation31) may reflect systemic spillover (Citation32) from local inflammatory responses (e.g., to cigarette smoke)—a relation reflected in the ATS-ERS statement on COPD (Citation33), which states that COPD is characterized by inflammation in the lungs, and this has systemic consequences. Other authors conceptualize COPD as a systemic disease with respiratory consequences (Citation24).
Extrapulmonary morbidities in COPD may be mechanistically related to tobacco smoking and/or systemic inflammation whether they are COPD manifestations (e.g., muscle dysfunction) or co-morbid diseases (e.g., lung cancer). (Citation34) lists common extrapulmonary conditions contributing to morbidity and mortality in patients with COPD. Airflow limitation is independently associated with risk of cardiovascular disease and lung cancer, even after taking smoking history into account (Citation35). Additionally, about two-thirds of patients with mild to moderate COPD die of lung cancer and cardiovascular complications rather than of COPD itself (Citation36).
Table 2. Systemic manifestations and common co-morbidities in COPD (34).
Exacerbations of COPD markedly worsen patients’ respiratory and physical condition on multiple timescales: acutely during the exacerbation, during several weeks after recovery, and cumulatively over successive exacerbations. Although exacerbations do leave behind detectable decrements in FEV1 (Citation37), they also affect additional aspects of disease such as muscle strength (Citation38), systemic inflammation (Citation39), and mood (Citation40). Decreased physical and social activity in particular may persist for several weeks after an acute exacerbation. Exacerbation frequency tends to increase as FEV1 declines but is not tightly coupled to FEV1 (Citation41). Frequent exacerbators bear disproportionate burdens of functional and health-related quality of life (HRQL) (Citation42) losses, health care utilization, and costs (Citation43); and accelerated decline of FEV1 has been demonstrated as well (Citation37). Exacerbations are important determinants of the COPD trajectory and an aspect of the disease meriting modification efforts.
Progression of COPD: Thinking Outside the Fletcher-Peto Curve
The progression of COPD is traditionally viewed as gradually declining lung function over decades, as depicted in (Fletcher-Peto curve) (Citation4). This oversimplified graph demonstrates a greater rate of decrease in FEV1 in susceptible smokers compared with non-susceptible smokers or non-smokers. Inexorable decline in lung function eventually causes symptoms, disability, and death.
Figure 1. Progression of airflow limitation in COPD (4). Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 sec. Reproduced from Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977 Jun 25; 1(6077)1645–1648, with permission from BMJ Publishing Group Ltd.
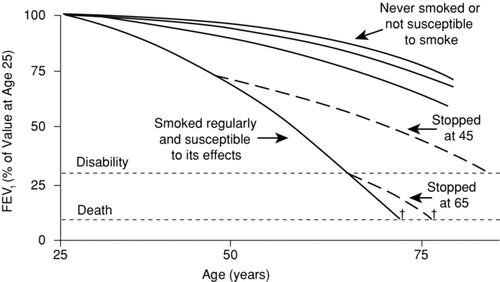
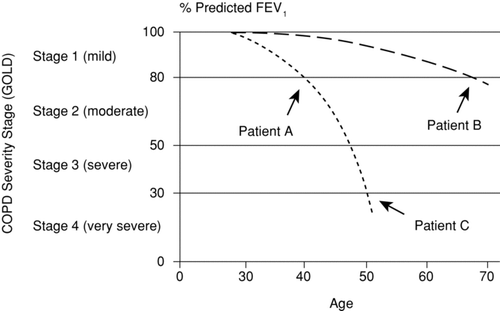
Although this simple graph may prove useful in smoking cessation counseling, the reality of progressive airways obstruction is much more complex. Rates of FEV1 decline vary markedly among individuals and may change within an individual's lifetime. “Snapshot” spirometric categories ignore differences in disease trajectory, as illustrated in (Citation44). Clearly, grouping a 40-year-old and 70-year old patient by FEV1 (in this case 80% of predicted) misses the rapid downhill course of the former. Also, the Fletcher-Peto curve does not consider developmental differences in lung function or major respiratory infections before age 20, which may also affect the adult trajectory of lung function (Citation4).
Figure 2. Severity staging and natural history of COPD.44 Grouping by FEV1 would put together patient A (40 years old with a rapid rate of decline) and patient B (70 years old with a slower rate of decline). Conversely, patients of different ages with rapid disease progression (patients A and C) may have more features in common than patients with the same FEV1 percent of predicted and different rates of progression. Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 sec; GOLD, Global Initiative for Obstructive Lung Disease. Reprinted from Rennard SI, Vestbo J. Natural histories of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008 Dec 15; 5(9):878–883, with permission of the American Thoracic Society. Copyright © 2012 American Thoracic Society. An official publication of the American Thoracic Society.
Even a perfect conceptualization of lung function decline would not completely reflect the natural history of COPD. Thus, increasing effects of hyperinflation, higher rate of exacerbations, greater likelihood of co-morbidities, progressive abnormalities of body composition, increasing dyspnea and fatigue, decreasing exercise capacity and physical activity levels, impaired functional status, propensity toward anxiety and depression, worsening quality of life, and greater risk of death all characterize progression of COPD (Citation45). Deteriorations in these areas probably follow diverse trajectories, further complicating our assessment of disease progression. But this complexity creates multiple opportunities to modify the course of COPD by interventions affecting its diverse aspects.
What is disease modification?
Assessment of potential disease-modifying interventions is complicated by a lack of consensus on what constitutes disease modification and which markers can be used to track it. Surrogate markers are needed because structural modifications in pulmonary pathology can be difficult to demonstrate directly. In COPD these surrogate markers may include pathobiological markers (from induced sputum or exhaled breath), physiological variables (e.g., FEV1), patient-centered outcomes (e.g., dyspnea, exercise capacity, or HRQL), exacerbations, or mortality (Citation46). One proposed definition of disease modification in COPD is as follows: “an improvement in, or stabilization of, structural or functional parameters as a result of reduction in the rate of progression of these parameters, which occurs whilst an intervention is applied and may persist even if the intervention is withdrawn” (Citation46). Importantly, this definition does not confine disease modification to one single dimension (Citation47, 48). The minimal duration and degree of clinically important improvement qualifying as disease modification remain to be determined.
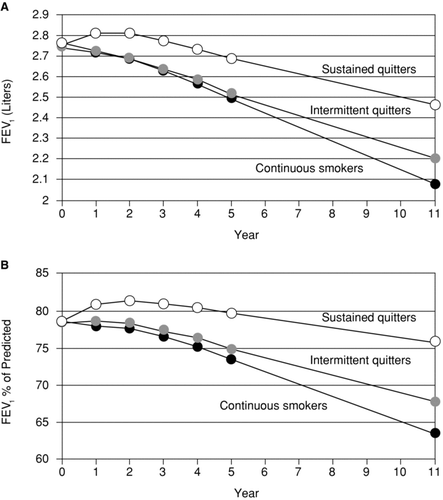
Disease modification in COPD may either alter the rate of disease progression, (e.g., the downward slope of FEV1 over time), or affect the status of the patient without changing the rate of progression. A rate-altering disease modification is illustrated in (Citation49). In the Lung Health Study (Citation49), smoking cessation reduced the subsequent rate of FEV1 decline. In contrast, a status-altering disease modification may not affect the rate of disease progression but may “turn back the clock” through sustained functional or symptomatic improvements (e.g., prolonged improvements in FEV1 over controls or baseline levels) (Citation46).
Figure 3. An example of a rate-altering disease modification: smoking cessation. In the Lung Health Study (49), sustained quitters had a reduced rate of the loss of lung function in comparison to intermittent quitters. Reprinted from Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med 2002 Sep 1; 166(5):675–679, with permission of the American Thoracic Society. Copyright © 2012 American Thoracic Society. Official Journal of the American Thoracic Society.
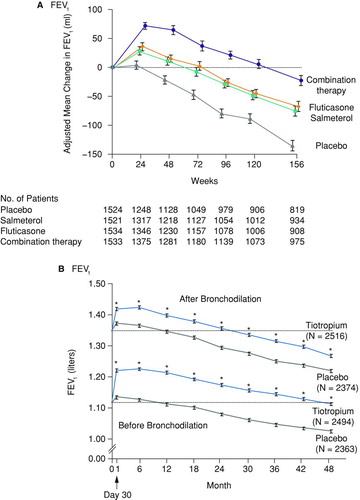
Status-altering disease modification is illustrated in (TORCH study) and 4b (UPLIFT study). shows the FEV1 over 3 years in COPD patients treated with the inhaled corticosteroid (ICS) fluticasone, the long-acting β-adrenergic agent (LABA) salmeterol, their combination, or placebo (Citation50). shows the peak and trough FEV1 over 4 years in patients with COPD receiving usual care and randomized to receive either tiotropium or placebo additionally (Citation51). In TORCH (Citation50), a pre-specified analysis showed significantly different rates of FEV1 decline between fluticasone/salmeterol and placebo groups; the clinical significance of this finding is unclear because TORCH was not powered to assess FEV1 decline. In UPLIFT (Citation51), the rate of FEV1 decline did not change significantly with tiotropium versus control in the main analysis population (pre-specified analysis did show a slowed rate of FEV1 decline in GOLD II participants). In both UPLIFT and TORCH, FEV1 did increase in response to therapy, and this increase remained above the level of controls throughout the course of the study. Thus, maintenance therapies (fluticasone/salmeterol or tiotropium) alter COPD patients’ status through sustained improvements in lung function irrespective of any effect on the rate of lung function decline.
Figure 4. Examples of status-altering disease-modifying therapies. Lung function in the TORCH50 (a) and UPLIFT51 (b) studies. Abbreviation: FEV1, forced expiratory volume in 1 sec. Reproduced by permission from (a) Calverley et al. (50) and (b) Tashkin et al. (51). Copyright © 2010 Massachusetts Medical Society. All rights reserved.
How successful are we at modifying the course of COPD?
Comprehensive COPD treatment combines non-pharmacological and pharmacological therapies as needed. Non-pharmacological therapies include smoking cessation interventions (Citation2, Citation33), physical activity promotion, self-management education, and palliative care planning (advance directives). Supplemental oxygen administration is usually considered under non-pharmacological therapy. Many of these non-pharmacological therapies can be conveniently and effectively bundled in pulmonary rehabilitation. Lung volume reduction surgery (LVRS) for patients with predominantly upper lobe emphysema and decreased exercise capacity has shown positive results over multiple outcome areas (Citation52).
Pharmacological therapies include vaccinations, short-acting bronchodilators, long-acting antimuscarinic agents (LAMAs) or LABAs, ICS, and combinations consisting of different bronchodilator classes or bronchodilators with inhaled corticosteroids.
The following will discuss the effectiveness of non-pharmacological and pharmacological therapeutic interventions in modifying the course of COPD. Areas of potential disease modification include lung function, exercise capacity, dyspnea, HRQL, physical activity, exacerbations, and mortality. summarizes findings in each of these areas (Citation47, Citation49-77). We will categorize interventions into those that change trajectory, those that change status (i.e., “set the clock back”) without changing trajectory, and those that reduce mortality.
Table 3. How good are we at modifying COPD?
Airflow limitation
Smoking cessation is currently the only intervention unequivocally demonstrated to reduce the longitudinal rate of FEV1 decline in COPD (Citation53), as shown in the Lung Health Study, a randomized trial of 5887 smokers who had mild COPD at enrollment and a reanalysis of the data after 11 years (Citation49). The study had three arms: 1) smoking intervention and the short-acting antimuscarinic agent ipratropium, 2) smoking intervention and placebo, and 3) usual care. We will focus on the effect of smoking cessation on the rate of decline in FEV1. Participants who sustained smoking cessation had a significantly lessened rate of decline in FEV1 that persisted for 11 years. At year 11 only 10% of sustained quitters had an FEV1 < 60% of predicted, contrasted with 38% of continuing smokers (Citation78).
In general, pharmacological treatments, including short-acting bronchodilators, maintenance LABAs, LAMAs, ICS, or LABA/ICS combinations, directly increase FEV1 in response to treatment but have not been conclusively proven to modify its downhill trajectory; thus, these therapies are largely status-modifying (Citation50, 51, Citation56). Three large COPD trials compared the effect of pharmacological treatment versus placebo or usual care on FEV1 (acute response and rate of decline): 1) the 4-year UPLIFT trial (Citation51) of 5993 patients evaluating the LAMA tiotropium plus usual care versus placebo plus usual care; 2) the 1-year SUN (Citation56) trial of 1964 patients evaluating the ICS budesonide, the LABA formoterol, and their combination; and 3) the 3-year TORCH study of 6112 patients evaluating salmeterol, fluticasone, and their combination (Citation50). In each study, treatment significantly increased FEV1 compared with controls throughout the study period.
In UPLIFT (Citation51) and SUN (Citation56), the rate of decline of FEV1 was not affected by therapy. Although TORCH was not powered to assess rate of FEV1 decline, a pre-specified analysis showed significantly different rates with salmeterol/fluticasone compared with placebo. A pre-specified subgroup analysis in UPLIFT found reduced rates of FEV1 decline in GOLD II participants (Citation79); and post hoc analyses suggested reduced rates in participants aged ≤ 50 years (Citation80) and in participants previously untreated with maintenance therapies (Citation81). A TORCH post hoc analysis reported that the rate of decline in FEV1 in patients receiving combination treatment was reduced by 16 mL/year compared with placebo (Citation82), although it has been suggested that regression to the mean affected this analysis (Citation83). TORCH participants with FEV1 < 60% (a subset of GOLD II and later stages) had slowed rates of decline in a severity-stratified post hoc analysis (Citation84). Therefore, whether pharmacological treatment modifies the course of lung function may depend on which patients are considered and how modification is defined.
Pulmonary rehabilitation and supplemental oxygen are generally believed not to affect FEV1 directly (Citation2). Pulmonary rehabilitation works through both increasing patients’ capacity for exercise and decreasing their dyspnea (Citation85). Supplemental oxygen used during rehabilitation allows patients to train more intensely (Citation67); and long-term domiciliary oxygen in hypoxemic patients with very severe COPD improves survival (Citation86).
Exercise capacity
Decreased exercise capacity in COPD results from several factors, including intrinsic lung disease (airways obstruction, hyperinflation, gas exchange abnormalities); peripheral muscle dysfunction (from systemic inflammation, corticosteroids, hypoxia, deconditioning, and sarcopenia); and co-morbidities (e.g., heart disease or peripheral vascular disease, osteoporosis, anxiety, and depression). In a substantial proportion of patients with COPD the locus of symptom limitation during exercise testing is leg fatigue, not breathlessness (Citation87).
Both pulmonary rehabilitation and supplemental oxygen therapy increase exercise capacity. For example, a systematic review of pulmonary rehabilitation demonstrated a mean increase in 6-min walk distance of 48 meters, which was statistically significant but slightly below what the systematic review authors considered the threshold for clinical significance (Citation54 meters (Citation68); a more recent study (Citation88) suggests that 35 meters may represent a clinically important 6-min walk difference in patients with moderate to severe COPD.) Post-exacerbation pulmonary rehabilitation is also effective (Citation73). The benefit of pulmonary rehabilitation on exercise capacity typically tends to decrease after 1 year (Citation89). It is unclear whether rehabilitation affects status rather than trajectory, or whether its effects merely fade when activity is not maintained.
Supplemental oxygen may enhance exercise capacity in hypoxemic and non-hypoxemic COPD patients (Citation90, 91). The benefit of oxygen on exercise capacity in COPD may be mediated in part through a reduction in dynamic hyperinflation: reduction of perceived dyspnea by oxygen therapy lowers respiratory rates during exercise, prolonging expiratory time and allowing more complete lung emptying during exhalation. The brevity of both these studies is a possible limitation of their findings.
LVRS for emphysematous severe COPD was shown to improve maximal exercise capacity (peak work rate achieved in incremental cycle ergometry) by ≥10 watts in significantly more surgical patients than in controls in the National Emphysema Treatment Trial (NETT) (Citation52).
Many pharmacotherapeutic trials show drug-induced improvements in exercise parameters in patients with COPD. Most trials have been brief in duration (≤8 weeks), enrolling patients in GOLD stages III–IV, and using diverse exercise testing methods. Tiotropium significantly increased endurance shuttle walk time (Citation92), 6-min walk distance (Citation93), and cycling endurance time under various protocols (Citation62, Citation94). Adding tiotropium to pulmonary rehabilitation (Citation61) or to formoterol (Citation95) increased treadmill walking endurance time at submaximal speed more than the respective therapies alone. Similarly, tiotropium plus fluticasone/salmeterol and pulmonary rehabilitation increased the 6-min-walk distance gains beyond the effect of fluticasone/salmeterol plus rehabilitation without tiotropium.
Budesonide/formoterol increased constant load cycling endurance time more than its component monotherapies or placebo (Citation96). Salmeterol increased endurance time in constant load cycling (Citation97) and endurance shuttle walking (Citation98), despite its inconsistent effect on 6-min walk distance (Citation97). Drug effects on exercise largely reflect reduced dynamic hyperinflation. Thus, the inspiratory capacity and its ratio to total lung capacity at rest and during exercise may be important parameters for disease modification.
Dyspnea
Dyspnea is the most bothersome COPD symptom for many patients (Citation16). Pharmacotherapies, supplemental oxygen, and pulmonary rehabilitation have been shown to improve dyspnea.
Although pulmonary rehabilitation does not directly affect lung function, strong evidence supports its benefits on dyspnea in COPD (Citation89). In fact, the effect size of rehabilitation generally exceeds that of pharmacotherapy. Pulmonary rehabilitation effectively ameliorates systemic consequences of COPD such as ambulatory muscle deconditioning (Citation99). Rehabilitation improves muscle efficiency and oxidative capacity (Citation99), reducing ventilatory requirements while exercising (permitting slower breathing and more time for exhalation); therefore, exercise training in pulmonary rehabilitation may reduce dyspnea through both improved muscle function and reduced dynamic hyperinflation.
LVRS for severe emphysematous COPD in NETT (Citation52) improved dyspnea in a majority of recipients. Dyspnea scores improved in 66% of surgery recipients versus 34% of controls (p < 0.001) at 6 months, in 58% of surgery recipients versus 29% of controls (p < 0.001) at 12 months, and in 50% of surgery recipients versus 21% of controls (p < 0.001) at 24 months.
Improvements in dyspnea have been demonstrated with the LABAs salmeterol (Citation54) and formoterol (Citation56), the LAMA tiotropium (Citation58), the ICS-LABA combinations salmeterol-fluticasone (Citation54) and formoterol-budesonide (Citation56), and ICS monotherapy (Citation64, Citation66).
Health-related quality of life
HRQL in COPD reflects the impacts of the respiratory disease, its systemic effects, its co-morbidities, and its therapy on the ability of the patient to perform or enjoy activities of daily living. Health status gradually declines in COPD over time and is profoundly affected by exacerbations. Two questionnaires assessing HRQL in COPD are the Chronic Respiratory Questionnaire (CRQ) (Citation100), St. George's Respiratory Questionnaire (Citation101), both disease-specific HRQL instruments that can be used in various settings and trials. The CRQ has four subscales: dyspnea, fatigue, emotion, and mastery. A 0.5 unit change (increase) per question is considered clinically meaningful. The SGRQ has three domains: symptoms, activities, and impacts. Decreasing score means improved HRQL, and a 4-unit change is considered clinically meaningful.
Strong evidence indicates that pulmonary rehabilitation improves HRQL in COPD (Citation85). Interestingly, little evidence exists for HRQL improvement with supplemental oxygen, although methodological issues and insufficient sample size may affect the evidence base.
Numerous LABA, LAMA, ICS, and LABA/ICS trials demonstrate HRQL benefits in COPD, although often the effects are statistically significant but fall short of the clinically meaningful threshold. In the 4-year UPLIFT (Citation51) study of tiotropium, the 3-year TORCH (Citation50), and the 1-year TRISTAN (Citation54) studies of salmeterol/fluticasone or its components, and the 1-year SUN (Citation56) trial of budesonide/formoterol, the mean changes in SGRQ generally were somewhat less than the 4-unit clinical significance threshold. Nevertheless, the improvements were well maintained over the 3- to 4-year trial periods.
In NETT (Citation52) comparing LVRS with medical care for emphysema, patients with upper lobe disease and low exercise capacity receiving LVRS were more likely to have an 8-point SGRQ improvement after 2 years than those receiving medical care.
Activity
Patients with COPD are markedly less active than healthy elderly subjects, with an almost 50% reduction in daily walking time, 35% less standing time, and three times more time spent lying down (Citation74). Low activity levels are associated with poor outcomes in several areas, including health care utilization and mortality risk (Citation77). Few data are available regarding treatment effects on activity levels in COPD. Pulmonary rehabilitation in a few studies has improved physical activity in daily life as well as exercise capacity in the laboratory (Citation75–77, Citation102).
Exacerbations
Exacerbations are common in COPD and may result in increased health care utilization, decline in function, and increase in mortality (Citation20, Citation103–106). Numerous treatments have been shown to reduce the frequency and/or severity of exacerbations.
ICS, LABA, LAMA, and ICS-LABA therapies have all been demonstrated to reduce exacerbations. In UPLIFT (Citation51), tiotropium significantly delayed the time to the first exacerbation and to the first hospitalization for an exacerbation and reduced the mean number of exacerbations by 14%. The Prevention of Exacerbations with Tiotropium (POET) study (Citation107) evaluated time to the first exacerbation as a primary outcome in 7376 patients with GOLD II–IV and recent exacerbations randomized to receive tiotropium (n = 3707) or salmeterol (n = 3669) for 1 year.
Tiotropium significantly delayed the first exacerbation versus salmeterol (187 vs 145 days) with a 17% reduction in risk (hazard ratio (HR) 0.83; 95% confidence interval 0.77, 0.90; p < 0.0001). Tiotropium significantly delayed the first severe exacerbation and reduced annual numbers of moderate or severe exacerbations. In TORCH (Citation50) and TRISTAN (Citation54), combined salmeterol/fluticasone reduced the annual rate of exacerbations versus placebo from 1.13 to 0.85 and from 1.30 to 0.97, respectively. Monotherapy with either agent reduced annual exacerbation rates significantly versus placebo but to a significantly lesser degree than the combination (Citation50, Citation54). Combined budesonide/formoterol in the 1-year SUN trial (Citation56) reduced the annual rate of exacerbations by 37% versus placebo and by 25% versus formoterol alone. Fluticasone monotherapy in ISOLDE reduced the median yearly exacerbation rate by 25% versus placebo (Citation63).
Pulmonary rehabilitation reduces subsequent health care utilization, presumably through reducing the impact or severity of exacerbations. Griffiths and colleagues compared health care utilization in COPD patients given either pulmonary rehabilitation or usual care (Citation47). Although the number of patients hospitalized for exacerbations was similar in both groups, hospital length of stay was significantly lower in the rehabilitation patients. Pulmonary rehabilitation also substantially reduced health care utilization in a collaborative California study; utilization savings were attributed to a decreased impact of COPD exacerbations (Citation108).
Mortality
To date, only smoking cessation (Citation36, Citation53), long-term supplemental oxygen therapy for hypoxemic patients (Citation69, 70, Citation86), and LVRS for selected emphysematous patients (Citation52), have been demonstrated to reduce mortality from COPD. Most studies in COPD have not been sufficiently powered to detect a survival benefit from the intervention, so the lack of a demonstrable effect on mortality does not necessarily mean it is not present. Mortality can be difficult to assess in practice, and surrogate predictors may be valuable in this regard such as the BODE index (Citation109–111) and the inspiratory capacity to total lung capacity ratio (Citation19).
Mortality reduction by smoking cessation was shown in the 14.5-year follow-up of the Lung Health Study, which assessed the long-term effect of a 10-week smoking cessation program on mortality in 5887 subjects with asymptomatic airway obstruction (Citation36). At the end of the study, all-cause mortality was significantly lower in the group given smoking cessation intervention compared with the usual care group. A systematic review of 17 studies on morbidity and mortality in patients with COPD or airway obstruction not attributed to asthma revealed that all-cause and COPD-attributed mortality rates declined progressively after smoking cessation compared with continued smoking; however, ex-smokers still had higher mortality risk than never-smokers, even after many years of smoking abstinence (Citation53).
A Cochrane review assessed the effect of domiciliary oxygen therapy on survival in patients with COPD in 6 randomized controlled trials (Citation86). Analysis of two trials (continuous vs nocturnal oxygen; domiciliary oxygen therapy vs none) revealed significantly reduced 24-month and 5-year mortality (odds ratio [OR] 0.45 and 0.42, respectively). However, two other trials in mild to moderate COPD patients with nocturnal arterial desaturation (nocturnal oxygen therapy vs none) revealed no treatment difference in mortality when the trials were analyzed separately or together. Finally, in two trials of mildly to moderately hypoxemic COPD patients (long-term oxygen therapy vs none), there was no effect on survival out to 3 years (Citation86). Thus, long-term oxygen therapy has only been shown to improve survival in severely hypoxemic COPD patients (arterial PaO2 < 55 mmHg).
The 3-year TORCH study, designed with mortality as the primary endpoint, came closest to demonstrating a survival benefit in COPD (Citation50), with the combination of salmeterol and fluticasone having a HR of 0.825 for all-cause mortality compared with placebo (p = 0.052). In addition, a post hoc factorial analysis of the TORCH data indicated a statistically significant reduction in all-cause mortality of 19% in patients receiving salmeterol compared with those who did not receive salmeterol (Citation112). In the UPLIFT trial (Citation51), mortality, which was not the primary endpoint, was significantly lower in the tiotropium group compared with the control group in the on-treatment analysis and in the analysis including dropouts at day 1440, which was the predefined treatment period, but not at day 1470, which included a 30-day follow-up period.
Pulmonary rehabilitation in the stable patient with COPD has not been demonstrated to benefit survival, possibly because its trials have been underpowered to demonstrate this effect. However, pulmonary rehabilitation given after a hospitalized exacerbation (during the post-discharge period of high mortality risk) does appear to reduce mortality (Citation73). Exercise capacity, functional status, and dyspnea all predict mortality in COPD, and all are improved with pulmonary rehabilitation. Therefore, pulmonary rehabilitation may indeed measurably reduce mortality.
LVRS reduces mortality in emphysematous COPD patients with predominantly upper lobe emphysema and low exercise capacity (Citation52).
Observational studies provide evidence that treatments indicated for common co-morbidities of COPD such as statins and angiotensin-converting enzyme (ACE) inhibitors for cardiovascular disease may reduce morbidity and/or mortality in patients with COPD (Citation113, 114), likely by affecting systemic manifestations. A recent systematic review concluded that outcomes associated with statin treatment include decreased all-cause and COPD-related mortality, fewer exacerbations, and an attenuated decline in lung function (Citation113). In a retrospective US Veterans Affairs cohort study using administrative data from subjects aged ≥ 65 years, hospitalized with a COPD exacerbation, current statin use (OR 0.51), and ACE inhibitors or angiotensin II receptor blocker use (OR 0.55) were significantly associated with decreased 90-day mortality (Citation114).
Conclusions
Although FEV1 is clearly a valuable marker of COPD status, using a single marker for a complex, multi-factorial disease risks overlooking valuable effects of treatments. In contrast to the perceptions of many physicians and lay people, both pharmacological and non-pharmacological treatments can meaningfully modify the health status of patients with COPD. We suggest that these treatments be regarded as disease-modifying. COPD is a chronic, progressive disease that physicians can and should actively identify and treat to improve patients’ health status and prognosis.
Declaration of interest: Dr. ZuWallack discloses institutional funding from Boehringer-Ingelheim for advisory board participation and a Boehringer-Ingelheim grant for clinical research testing; he also discloses Boehringer-Ingelheim/Pfizer and GlaxoSmithKline speakers’ bureau participation. Dr. Nici declares that she has no conflicts of interest. This work was supported by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI) and Pfizer. Editorial assistance was provided by Linda Merkel, PhD, Andrew Cooper, CMPP, and Kim Coleman Healy, PhD, CMPP, of Envision Scientific Solutions, which was contracted by BIPI and Pfizer for these services. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE) and were involved at all stages of manuscript development. The authors exerted scientific control and are solely responsible for the content of the paper. Apart from the provision of editorial assistance, the authors received no compensation related to the development of the manuscript.
References
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004 Jun; 23(6):932–946.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. [Internet]. Bethesda, MD; 2011. Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007 Sep 15; 176(6):532–555.
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977 Jun 25; 1(6077):1645–1648.
- Marsh S, Aldington S, Shirtcliffe P, Weatherall M, Beasley R. Smoking and COPD: What really are the risks? Eur Respir J 2006 Oct; 28(4):883–884.
- Rennard SI, Vestbo J. COPD: the dangerous underestimate of 15%. Lancet 2006 Apr 15; 367(9518):1216–1219.
- Lundback B, Lindberg A, Lindstrom M, Ronmark E, Jonsson AC, Jonsson E, Larsson LG, Andersson S, Sandstrom T, Larsson K. Not 15 but 50% of smokers develop COPD?–Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med 2003 Feb; 97(2):115–122.
- World Health Organization. Chronic obstructive pulmonary disease. Fact sheet No. 315. [Internet]. 2008. Available from: http://www.who.int/mediacentre/factsheets/fs315/en
- American Lung Association. Chronic obstructive pulmonary disease (COPD). [Internet]. Washington, DC; 2008. Available from: http://www.lungusa.org/assets/documents/publications/lung-disease-data/ldd08-chapters/LDD-08-COPD.pdf
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007 Sep 1; 370(9589):765–773.
- Minino AM, Xu J, Kochanek KD. Deaths: preliminary data for 2008. Natl Vital Stat Rep 2010; 59(2):1–52.
- US National Heart Lung and Blood Institute. Morbidity and Mortality: the 2009 Chart Book on Cardiovascular, Lung, and Blood Diseases. Bethesda, MD: National Institutes of Health; 2009.
- The ASPECT Consortium. Tobacco or Health in the European Union: Past, Present, and Future. In: European Bureau for Action on Smoking Prevention Brussels, Belgium: European Commission; 2004.
- Agusti AG. COPD, a multicomponent disease: implications for management. Respir Med 2005 Jun; 99(6):670–682.
- Celli BR, Cote CG, Lareau SC, Meek PM. Predictors of survival in COPD: more than just the FEV1. Respir Med 2008 Jun; 102 Suppl 1:S27–35.
- Jones PW. Health status and the spiral of decline. COPD 2009 Feb; 6(1):59–63.
- Wolkove N, Dajczman E, Colacone A, Kreisman H. The relationship between pulmonary function and dyspnea in obstructive lung disease. Chest 1989 Dec; 96(6):1247–1251.
- Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002 May; 121(5):1434–1440.
- Casanova C, Cote C, de Torres JP, Aguirre-Jaime A, Marin JM, Pinto-Plata V, Celli BR. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005 Mar 15; 171(6):591–597.
- Connors AF, Jr., Dawson NV, Thomas C, Harrell FE, Jr., Desbiens N, Fulkerson WJ, Kussin P, Bellamy P, Goldman L, Knaus WA. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996 Oct; 154(4 Pt 1):959–967.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004 Mar 4; 350(10):1005–1012.
- Marquis K, Debigare R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, Maltais F. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002 Sep 15; 166(6):809–813.
- O'Donnell DE, Laveniziana P. Physiology and consequences of lung hyperinflation in COPD. Eur Respir Rev 2006 Dec; 15(100):661–667.
- Agusti A. Thomas A. Neff lecture. Chronic obstructive pulmonary disease: a systemic disease. Proc Am Thorac Soc 2006 Aug; 3(6):478-481.
- Fogarty AW, Jones S, Britton JR, Lewis SA, McKeever TM. Systemic inflammation and decline in lung function in a general population: a prospective study. Thorax 2007 Jun; 62(6):515–520.
- Walter RE, Wilk JB, Larson MG, Vasan RS, Keaney JF, Jr., Lipinska I, O'Connor GT, Benjamin EJ. Systemic inflammation and COPD: the Framingham Heart Study. Chest 2008 Jan; 133(1):19–25.
- Hunninghake DB. Cardiovascular disease in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005; 2(1):44–49.
- Kohansal R, Martinez-Camblor P, Agusti A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med 2009 Jul 1; 180(1):3–10.
- Sin DD, Man SF. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc 2005; 2(1):8–11.
- Sin DD, Man SF. Chronic obstructive pulmonary disease: a novel risk factor for cardiovascular disease. Can J Physiol Pharmacol 2005 Jan; 83(1):8–13.
- Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003 Mar 25; 107(11):1514–1519.
- van Eeden SF, Yeung A, Quinlam K, Hogg JC. Systemic response to ambient particulate matter: relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005; 2(1):61–67.
- American Thoracic Society, European Respiratory Society. Standards for the diagnosis and management of patients with COPD [Internet]. New York, NY; 2004. Available from: http://www.thoracic.org/sections/copd/index.html
- Zuwallack R, Nici L. Integrated care of the COPD patient: a pulmonary rehabilitation perspective. Breathe 2010; 6(4):313–319.
- Young RP, Hopkins R, Eaton TE. Forced expiratory volume in one second: not just a lung function test but a marker of premature death from all causes. Eur Respir J 2007 Oct; 30(4):616–622.
- Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med 2005 Feb 15; 142(4):233–239.
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002 Oct; 57(10):847–852.
- Vilaro J, Ramirez-Sarmiento A, Martinez-Llorens JM, Mendoza T, Alvarez M, Sanchez-Cayado N, Vega A, Gimeno E, Coronell C, Gea J, Roca J, Orozco-Levi M. Global muscle dysfunction as a risk factor of readmission to hospital due to COPD exacerbations. Respir Med 2010 Dec; 104(12):1896–1902.
- Kersul AL, Iglesias A, Rios A, Noguera A, Forteza A, Serra E, Agusti A, Cosio BG. Molecular mechanisms of inflammation during exacerbations of chronic obstructive pulmonary disease. Arch Bronconeumol 2011 Apr; 47(4):176–183.
- Kessler R, Stahl E, Vogelmeier C, Haughney J, Trudeau E, Lofdahl CG, Partridge MR. Patient understanding, detection, and experience of COPD exacerbations: an observational, interview-based study. Chest 2006 Jul; 130(1):133–142.
- O'Reilly JF, Williams AE, Holt K, Rice L. Defining COPD exacerbations: impact on estimation of incidence and burden in primary care. Prim Care Respir J 2006 Dec; 15(6):346–353.
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998 May; 157(5(Pt 1)):1418–1422.
- Halpern MT, Higashi MK, Bakst AW, Schmier JK. The economic impact of acute exacerbations of chronic bronchitis in the United States and Canada: A literature review. J Manag Care Pharm 2003 Jul-Aug; 9(4):353–359.
- Rennard SI, Vestbo J. Natural histories of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008 Dec 15; 5(9):878–883.
- Cazzola M, Hanania NA, Jones PW, Mahler DA, Make B, Ohar J, Rennard S. It's about time–directing our attention toward modifying the course of COPD. Respir Med 2008 Jun; 102 Suppl 1:S37–48.
- Halpin DM, Tashkin DP. Defining disease modification in chronic obstructive pulmonary disease. COPD 2009 Jun; 6(3):211–225.
- Griffiths TL, Burr ML, Campbell IA, Lewis-Jenkins V, Mullins J, Shiels K, Turner-Lawlor PJ, Payne N, Newcombe RG, Ionescu AA, Thomas J, Tunbridge J. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet 2000 Jan 29; 355(9201):362–368.
- Kaplan RM. The minimally clinically important difference in generic utility-based measures. COPD 2005 Mar; 2(1):91–97.
- Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med 2002 Sep 1; 166(5):675-679.
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007 Feb 22; 356(8):775–789.
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008 Oct 9; 359(15):1543–1554.
- Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood DE. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003 May 22; 348(21):2059–2073.
- Godtfredsen NS, Lam TH, Hansel TT, Leon ME, Gray N, Dresler C, Burns DM, Prescott E, Vestbo J. COPD-related morbidity and mortality after smoking cessation: status of the evidence. Eur Respir J 2008 Oct; 32(4):844–853.
- Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, Anderson J, Maden C. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003 Feb 8; 361(9356):449–456.
- van Noord JA, Aumann JL, Janssens E, Smeets JJ, Verhaert J, Disse B, Mueller A, Cornelissen PJ. Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. Eur Respir J 2005 Aug; 26(2):214–222.
- Rennard SI, Tashkin DP, McElhattan J, Goldman M, Ramachandran S, Martin UJ, Silkoff PE. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trial. Drugs 2009; 69(5):549–565.
- Tashkin DP, Rennard SI, Martin P, Ramachandran S, Martin UJ, Silkoff PE, Goldman M. Efficacy and safety of budesonide and formoterol in one pressurized metered-dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease: results of a 6-month randomized clinical trial. Drugs 2008; 68(14):1975–2000.
- Casaburi R, Mahler DA, Jones PW, Wanner A, San PG, ZuWallack RL, Menjoge SS, Serby CW, Witek T, Jr. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J 2002 Feb; 19(2):217–224.
- Tonnel AB, Perez T, Grosbois JM, Verkindre C, Bravo ML, Brun M. Effect of tiotropium on health-related quality of life as a primary efficacy endpoint in COPD. Int J Chron Obstruct Pulmon Dis 2008; 3(2):301–310.
- Niewoehner DE, Rice K, Cote C, Paulson D, Cooper JA, Jr., Korducki L, Cassino C, Kesten S. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med 2005 Sep 6; 143(5):317–326.
- Casaburi R, Kukafka D, Cooper CB, Witek TJ, Jr., Kesten S. Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest 2005 Mar; 127(3):809–817.
- Maltais F, Hamilton A, Marciniuk D, Hernandez P, Sciurba FC, Richter K, Kesten S, O'Donnell D. Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest 2005 Sep; 128(3):1168–1178.
- Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000 May 13; 320(7245):1297–1303.
- Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med 2000 Dec 28; 343(26):1902–1909.
- Pauwels RA, Lofdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB, Ohlsson SV. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med 1999 Jun 24; 340(25):1948–1953.
- Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 1999 May 29; 353(9167):1819–1823.
- Emtner M, Porszasz J, Burns M, Somfay A, Casaburi R. Benefits of supplemental oxygen in exercise training in nonhypoxemic chronic obstructive pulmonary disease patients. Am J Respir Crit Care Med 2003 Nov 1; 168(9):1034–1042.
- Lacasse Y, Lecours R, Pelletier C, Begin R, Maltais F. Randomised trial of ambulatory oxygen in oxygen-dependent COPD. Eur Respir J 2005 Jun; 25(6):1032–1038.
- Medical Research Council Working Party. Long-term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet 1981 Mar 28; 1(8222):681–686.
- Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 1980 Sep; 93(3):391–398.
- Benzo R, Farrell MH, Chang CC, Martinez FJ, Kaplan R, Reilly J, Criner G, Wise R, Make B, Luketich J, Fishman AP, Sciurba FC. Integrating health status and survival data: the palliative effect of lung volume reduction surgery. Am J Respir Crit Care Med 2009 Aug 1; 180(3):239–246.
- Steele BG, Belza B, Cain KC, Coppersmith J, Lakshminarayan S, Howard J, Haselkorn JK. A randomized clinical trial of an activity and exercise adherence intervention in chronic pulmonary disease. Arch Phys Med Rehabil 2008 Mar; 89(3):404–412.
- Puhan MA, Scharplatz M, Troosters T, Steurer J. Respiratory rehabilitation after acute exacerbation of COPD may reduce risk for readmission and mortality –a systematic review. Respir Res 2005; 6:54.
- Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005 May 1; 171(9):972–977.
- Sewell L, Singh SJ, Williams JE, Collier R, Morgan MD. Can individualized rehabilitation improve functional independence in elderly patients with COPD? Chest 2005 Sep; 128(3):1194–1200.
- Walker PP, Burnett A, Flavahan PW, Calverley PM. Lower limb activity and its determinants in COPD. Thorax 2008 Aug; 63(8):683–689.
- Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 2006 Sep; 61(9):772–778.
- Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, Conway WA, Jr., Enright PL, Kanner RE, O'Hara P, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 1994 Nov 16; 272(19):1497–1505.
- Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet 2009 Oct 3; 374(9696):1171–1178.
- Morice AH, Celli B, Kesten S, Lystig T, Tashkin D, Decramer M. COPD in young patients: a pre-specified analysis of the four-year trial of tiotropium (UPLIFT). Respir Med 2010 Nov; 104(11):1659–1667.
- Troosters T, Celli B, Lystig T, Kesten S, Mehra S, Tashkin DP, Decramer M. Tiotropium as a first maintenance drug in COPD: secondary analysis of the UPLIFT trial. Eur Respir J 2010 Jul; 36(1):65–73.
- Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, Vestbo J, Knobil K, Yates JC, Calverley PM. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med 2008 Aug 15; 178(4):332–338.
- Suissa S, Ernst P. Mega trials in COPD–clinical data analysis and design issues. Pneumonol Alergol Pol 2011; 79(3):227–231.
- Jenkins CR, Jones PW, Calverley PM, Celli B, Anderson JA, Ferguson GT, Yates JC, Willits LR, Vestbo J. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: Analysis from the randomised, placebo-controlled TORCH study. Respir Res 2009; 10:59.
- Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, Make B, Rochester CL, Zuwallack R, Herrerias C. Pulmonary rehabilitation: Joint ACCP/AACVPR Evidence-based clinical practice Guidelines. Chest 2007 May; 131(5 Suppl):4S–42S.
- Cranston JM, Crockett AJ, Moss JR, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005; (4):CD001744.
- Killian KJ. Limitation to muscular activity in chronic obstructive pulmonary disease. Eur Respir J 2004 Jul; 24(1):6–7.
- Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schunemann HJ. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J 2008 Sep; 32(3):637-643.
- Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med 1995 Jun 1; 122(11):823–832.
- O'Donnell DE, D'Arsigny C, Webb KA. Effects of hyperoxia on ventilatory limitation during exercise in advanced chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001 Mar; 163(4):892–898.
- Somfay A, Porszasz J, Lee SM, Casaburi R. Dose-response effect of oxygen on hyperinflation and exercise endurance in nonhypoxaemic COPD patients. Eur Respir J 2001 Jul; 18(1):77–84.
- Bedard ME, Brouillard C, Pepin V, Provencher S, Milot J, Lacasse Y, Leblanc P, Maltais F. Tiotropium improves walking endurance in chronic obstructive pulmonary disease. Eur Respir J 2012 Feb;39(2):265–271.
- Okudan N, Gok M, Gokbel H, Suerdem M. Single dose of tiotropium improves the 6-minute walk distance in chronic obstructive pulmonary disease. Lung 2006 Jul-Aug; 184(4):201–204.
- O'Donnell DE, Fluge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, Make B, Magnussen H. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004 Jun; 23(6):832–840.
- Berton DC, Reis M, Siqueira AC, Barroco AC, Takara LS, Bravo DM, Andreoni S, Neder JA. Effects of tiotropium and formoterol on dynamic hyperinflation and exercise endurance in COPD. Respir Med 2010 Sep; 104(9):1288–1296.
- Worth H, Forster K, Eriksson G, Nihlen U, Peterson S, Magnussen H. Budesonide added to formoterol contributes to improved exercise tolerance in patients with COPD. Respir Med 2010 Oct; 104(10):1450–1459.
- Grove A, Lipworth BJ, Reid P, Smith RP, Ramage L, Ingram CG, Jenkins RJ, Winter JH, Dhillon DP. Effects of regular salmeterol on lung function and exercise capacity in patients with chronic obstructive airways disease. Thorax 1996 Jul; 51(7):689–693.
- Brouillard C, Pepin V, Milot J, Lacasse Y, Maltais F. Endurance shuttle walking test: responsiveness to salmeterol in COPD. Eur Respir J 2008 Mar; 31(3):579–584.
- Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, Carone M, Celli B, Engelen M, Fahy B, Garvey C, Goldstein R, Gosselink R, Lareau S, MacIntyre N, Maltais F, Morgan M, O'Donnell D, Prefault C, Reardon J, Rochester C, Schols A, Singh S, Troosters T. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med 2006 Jun 15; 173(12):1390–1413.
- Guyatt GH, Townsend M, Keller J, Singer J, Nogradi S. Measuring functional status in chronic lung disease: conclusions from a randomized control trial. Respiratory medicine 1991 Sep; 85 Suppl B:17–21; discussion 33–37.
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992 Jun; 145(6):1321–1327.
- Pitta F, Troosters T, Probst VS, Langer D, Decramer M, Gosselink R. Are patients with COPD more active after pulmonary rehabilitation? Chest 2008 Aug; 134(2):273–280.
- Fuso L, Incalzi RA, Pistelli R, Muzzolon R, Valente S, Pagliari G, Gliozzi F, Ciappi G. Predicting mortality of patients hospitalized for acutely exacerbated chronic obstructive pulmonary disease. Am J Med 1995 Mar; 98(3):272–277.
- Higgins MW, Thom T. Incidence, prevalence and mortality: intra-and inter-country difference. In: Hensley MJ, Saunders NA, eds. Clinical Epidemiology of Chronic Obstructive Pulmonary Disease. New York: Marcel Dekker; 1990:23–43.
- Peters KD, Kochanek KD, Murphy SL. Deaths: final data for 1996. Natl Vital Stat Rep 1998 Nov 10; 47(9):1–100.
- Seneff MG, Wagner DP, Wagner RP, Zimmerman JE, Knaus WA. Hospital and 1-year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary disease. JAMA 1995 Dec 20; 274(23):1852–1857.
- Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten-van Molken MP, Beeh KM, Rabe KF, Fabbri LM. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011 Mar 24; 364(12):1093–1103.
- California Pulmonary Rehabilitation Collaborative Group. Effects of pulmonary rehabilitation on dyspnea, quality of life, and healthcare costs in California. J Cardiopulm Rehabil 2004 Jan-Feb; 24(1):52–62.
- Cote CG, Celli BR. BODE index: a new tool to stage and monitor progression of chronic obstructive pulmonary disease. Pneumonol Alergol Pol 2009; 77(3):305–313.
- Imfeld S, Bloch KE, Weder W, Russi EW. The BODE index after lung volume reduction surgery correlates with survival. Chest 2006 Apr; 129(4):873–878.
- Martinez FJ, Han MK, Andrei AC, Wise R, Murray S, Curtis JL, Sternberg A, Criner G, Gay SE, Reilly J, Make B, Ries AL, Sciurba F, Weinmann G, Mosenifar Z, DeCamp M, Fishman AP, Celli BR. Longitudinal change in the BODE index predicts mortality in severe emphysema. Am J Respir Crit Care Med 2008 Sep 1; 178(5):491–499.
- La Vecchia C, Fabbri LM. Prevention of death in COPD. N Engl J Med 2007 May 24; 356(21):2211–2212; author reply 2213–2214.
- Dobler CC, Wong KK, Marks GB. Associations between statins and COPD: a systematic review. BMC Pulm Med 2009; 9:32.
- Mortensen EM, Copeland LA, Pugh MJ, Restrepo MI, de Molina RM, Nakashima B, Anzueto A. Impact of statins and ACE inhibitors on mortality after COPD exacerbations. Respir Res 2009; 10:45.