Abstract
Quadriceps muscle weakness and increased fatigability are well described in patients with chronic obstructive pulmonary disease (COPD). Whether these functional alterations also exist in distal leg muscles in patients with COPD is uncertain. Fifteen patients with COPD and 15 aged-matched healthy controls performed a 12-minute standardized treadmill exercise during which a fixed total expense of 40 Kcal was reached. The strength of i) dorsiflexors, ii) plantar flexors and iii) quadriceps was assessed at rest and after exercise using maximal voluntary contraction (MVC) and potentiated twitch force (Twpot). Resting MVC and Twpot were significantly lower in patients with COPD when compared with controls respectively for i) dorsiflexors (24.9 ± 8.4 vs. 31.2 ± 8.5 Nm, p < 0.05 and 4.3 ± 1.3 vs. 5.7 ± 1.8 Nm, p < 0.05), ii) plantar flexors (49.5 ± 11.8 vs. 62.1 ± 19.6 Nm, p < 0.05 and 10.8 ± 3.5 vs. 13.4 ± 2.7 Nm, p < 0.05), and iii) quadriceps muscles. There was a greater force loss in the distal leg muscles 15 minutes post-exercise in patients with COPD, while the strength of the quadriceps muscle remained stable in both groups. Patients with COPD had weaker dorsiflexor and plantar flexor muscles when compared to age-matched healthy controls. In addition, when exposed to the same absolute walking task, the fatigability of the distal leg muscles was higher in patients with COPD.
Introduction
Exercise intolerance is a hallmark feature in chronic obstructive pulmonary disease (COPD) and plays an important role in compromising quality of life in this disease (Citation1). Although ventilatory and gas exchange abnormalities are crucial to explain exercise limitation in patients with COPD, some studies also point to the contributing role of peripheral muscle function (Citation2, 3). For instance, quadriceps muscle strength, endurance and metabolic efficiency are often compromised in patients with COPD when compared with healthy controls (Citation4, 5).
The quadriceps has been the main focus in the characterization of the peripheral muscle alterations occurring in COPD (Citation6, 7). However, leg muscles such as the tibialis anterior and the gastrocnemius may also be involved in the process of limb muscle dysfunction associated with COPD (Citation8). Therefore, despite their importance in walking activities, a more complete characterization of the function of these additional leg muscles in patients with COPD is still awaiting. This information could be relevant in providing a better understanding of limb muscle dysfunction in COPD and also contribute to optimizing pulmonary rehabilitation programs.
Most of the work investigating the issue of quadriceps fatigue in COPD was done using cycle ergometry (Citation3, Citation9, Citation10) because quadriceps is intensely solicited during this form of exercise. As opposed to walking, cycling does not elicit an important demand for the distal leg muscles such as the dorsi and plantar flexors, and is therefore not the exercise modality of choice to investigate their function. In fact, cycling and walking have different consequences in the development of muscle fatigue (Citation11). Although cycling can easily induce quadriceps fatigue in the context of COPD (Citation7, Citation12), walking is likely to be more appropriate to study leg muscle fatigue susceptibility (Citation8). Moreover, a walking-based exercise protocol is more likely to reflect daily life activities of patients with COPD than cycling.
Consequently, we aimed to characterize the strength and fatigability of distal leg muscles following a standardised walking effort in patients with COPD. We hypothesized that dorsi and plantar flexors would show weakness and greater fatigability following walking exercise in patients with COPD compared to healthy controls. To test this hypothesis, volitional and non-volitional strength of i) the dorsiflexors, ii) the plantar flexors, and iii) the quadriceps where measured and compared before and after a treadmill walking exercise inducing similar energy expenditure in patients with COPD and healthy controls.
Methods
See the supplementary material for additional information.
Subjects
Fifteen GOLD stage II-III patients with COPD (post-bronchodilator FEV1 > 30% and < 80% predicted and FEV1/FVC < 0.70) (Citation13) and 15 sedentary healthy subjects paired for gender and age were included in the study. Patients were excluded if they had a recent exacerbation (≤ 4 weeks), diabetes mellitus, neoplasia or any medical condition, other than COPD, likely to influence muscle and exercise testing (i.e., cardiovascular, neurological, musculoskeletal, locomotor or other respiratory diseases as well as ß-blockers therapy). Subjects were asked to avoid alcohol, caffeine and heavy meals 3 hours before the visit and high intensity physical activity for at least 24 hours before testing. The research protocol was approved by the institutional ethics committee and a signed informed consent was obtained from every subject prior to enrollment.
Study design
The study consisted in three distinct visits. The initial visit included anthropometric measurements, complete pulmonary function test and familiarization with the incremental maximal walking test to be performed during the second visit. During the second visit, a progressive maximal walking test was performed on a treadmill. During the same visit, parameters (slope, speed) of the standardized 40 Kcal-expense walking exercise were determined and patients were familiarized with endurance walking exercise. The second and third visits were seven days apart, a period during which the level of daily physical activity was directly quantified using a portable activity monitor.
The third visit included resting strength measurements of i) the dorsiflexors, ii) the plantar flexors, and iii) the quadriceps. Finally, following a standardized 40 Kcal-expense walking exercise, fatigue of the aforementioned muscle groups was again assessed by measuring muscle strength at 15 minutes post-exercise and by calculating the difference between the resting and the respective post-exercise value. In a small subset of patients (8 patients with COPD and 6 healthy subjects), fatigue was also quantified at 40 minutes post-exercise.
Pulmonary function testing
Standard pulmonary function tests, including spirometry, lung volumes, and diffusion capacity were obtained according to previously described guidelines (Citation14) and related to predicted normal values (Citation15). The predicted value for inspiratory capacity was obtained by subtracting the functional residual capacity predicted value from total lung capacity predicted value. Maximum voluntary ventilation was estimated by multiplying FEV1 by 35 (Citation16).
Physical activity level
Physical activity in daily life was monitored during 7 consecutive days for at least 12 hours a day via a portable device (SenseWear® ArmBand, Bodymedia inc., Pittsburgh, USA) worn on the right upper arm. The device measures the number of daily steps and estimates the total caloric expense for moderately intense activities, i.e., beyond a fixed metabolic equivalent (>3 METs). We reported the mean daily values for these variables over the whole measurement period.
Exercise testing
Incremental walking test (IWT)
The test was performed on a treadmill to determine peak exercise capacity. Accordingly, we used an algorithm designed to yield a linear increase in workload on a treadmill until exhaustion (Citation17). The test was built on a 10-minute ramp-fashion (20 steps of 30 seconds) with a prior 3-minute warm-up period. During the test, subjects were connected to a portable gas exchange analyzer (Oxycon Mobile, Viasys Healthcare, Jaeger, Germany) to monitor ventilation (E), oxygen consumption (O2), CO2 output (CO2) and respiratory exchange ratio (RER). Oxygen pulse saturation (SpO2) was measured at baseline and during exercise using a pulse oxymeter (Nellcor N-395, Covidien, Boulder, CO, USA). Blood pressure was measured manually at baseline and at end-exercise. Dyspnea and leg fatigue Borg scores (Citation18) were obtained at baseline, every 2 minutes and at the end of exercise. A 12-lead electrocardiogram (JECG 12ch, Viasys Healthcare, Jaeger, Germany) was used to monitor cardiac electrical activity throughout exercise.
Standardized endurance walking test (EWT)
The EWT was realized in the same setting as the IWT. Parameters of the endurance test were set to obtain a 40 Kcal expense in every subject during a fixed walking duration of 12 minutes, according to prediction equations (Citation19). The required treadmill slope to obtain the predetermined 40 Kcal energy expenditure was determined according to the weight of the patient, after choosing his own comfortable walking pace. The fixed energy expenditure of 40 Kcal was chosen mainly to reproduce a realistic daily living task performed by patients with COPD. The 40 Kcal energy expenditure was calculated to represent a 12-minute walking exercise at 4 km/h, a pace that can be maintain by most patients with moderate-to-severe patients with COPD (Citation20) and reflecting daily physical tasks such as a walk to the grocery. Cardiorespiratory response was monitored using the same setup as for IWT. Dyspnea and leg fatigue Borg scores (Citation18) were obtained every 2 minutes during exercise and blood pressure was measured manually at the beginning and at the end of EWT.
Muscle strength measurements
Strength of the dominant lower extremity was measured at rest and 15 minutes after EWT. Subjects were seated in a recumbent chair (N-K 330 Exercise Table; N-K Products, Elsinore, CA) with 90° knee flexion and the ankle attached to a strain gauge (Hewlett-Packard, Houston, TX). Maximal voluntary contraction (MVC) and potentiated twitch force (Twpot) of the quadriceps were measured as previously reported (Citation3). To measure the strength of the dominant dorsi and plantar flexors, the ankle was fixed into a custom-made ankle foot orthosis (AFO) specifically designed to measure torque production at the ankle (). Muscle force was transmitted to a load cell (Transducer Techniques, model MLP-150) inserted in a rod of the orthosis: plantar flexors contraction produces load cell compression, although dorsiflexors contraction produces tension.
Figure 1. Custom-made ankle foot orthosis designed to measure dorsi and plantar flexors muscle force.
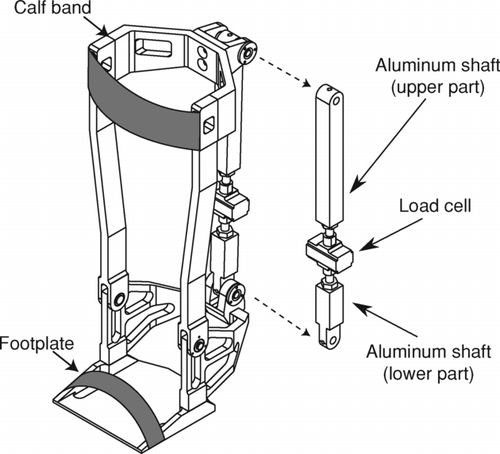
The foot was always parallel to the ground during torque measurements. Volitional strength of the dorsi and plantar flexors were measured during a set of 3 isometric MVCs sustained for 3 seconds, and subjects were asked to produce their maximum effort. During this manoeuver, verbal encouragements were provided and each MVC was separated by 1 minute of rest. The greatest value sustained over 2 seconds was defined as MVC. Twpot force of the dorsi and plantar flexors was obtained with the foot in the same device, using magnetic stimulation (Magstim 200; Magstim Co Ltd; Whitland, Dyfed, Wales, UK) connected to a 42 mm figure-of-eight coil to stimulate the peroneal and tibial nerves, respectively. A Tw/power output relationship was obtained for each patient and for each muscle to evaluate whether the magnetic stimulation was supramaximal and to ensure an optimal localization of the coil. Reported values for twitch measures are the mean of the two strongest contractions, and torque is reported in Newton meters (Nm).
In 8 patients with COPD and 6 controls, strength measurements were repeated 40 minutes post-exercise. Additional details on the AFO, validity, reproducibility of this method and supramaximality in distal leg muscles in COPD are available on the online supplementary material.
Statistical analysis
Results are expressed as mean ± S.D. Baseline characteristics of the subjects were compared using unpaired t-test. End-exercise values for ventilation (E), oxygen consumption (O2), carbon dioxide output (CO2), E/CO2, tidal volume (VT), breathing frequency (Bƒ), respiratory exchange ratio (RER), heart rate (HR), O2 pulse, pulse oxygen saturation (SpO2) and Borg scores were compared between the two groups using one-way ANOVA analysis. Time courses of muscle strength data before and after exercise were analyzed by a mixed model using two fixed experimental factors, one associated with the between-group comparisons and the other with the within-group comparisons. Pearson correlations coefficients corrected for multiple testing were performed to examine the association between quadriceps and ankle muscles strength and markers of physical activity in daily life. A statistical level of significance of 0.05 was used for all analysis. The data were analyzed using JMP statistical package (Version 8.0.1, SAS Institute Inc., Cary, NC).
Because no data are available on dorsi and plantar flexors strength in patients with COPD, the study sample size calculation was based on the expected difference in baseline MVC of the quadriceps as previously reported by our laboratory (Citation3). Eleven subjects in both groups were necessary to detect an 11 ± 10 Nm difference in quadriceps strength between controls and patients with COPD, with α = 0.05 and 1–β = 0.90.
Results
Subjects
Characteristics of the subjects are presented in . Groups were well matched for age, gender distribution and body mass index. Patients with COPD had, on average, moderate to severe airflow obstruction with evidence of gas trapping and reduced diffusion capacity. They also exhibited decreased exercise capacity and lower levels of daily physical activity when compared with healthy controls.
Table 1. Subject characteristics.
Resting muscle strength assessment
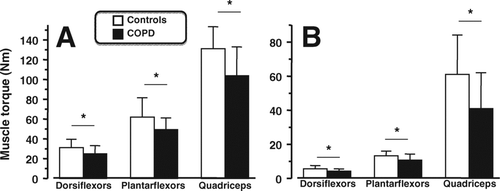
Volitional and non-volitional resting strengths of the dorsiflexors, plantar flexors and quadriceps were significantly lower in patients with COPD than in controls (, panels A, and B). Significant correlations were found between resting MVC and Twpot for the dorsiflexors (r = 0.67, p < 0.001), plantar flexors (r = 0.39, p < 0.05) and quadriceps (r = 0.76, p < 0.001). The number of daily steps per day (r = 0.44, p < 0.05) and the energy expenditure > 3 METs (r = 0.54, p < 0.01) were related to resting plantar flexors MVC. Finally, the peak workload reached during the IWT was related to the plantar flexors (R = 0.45, p = 0.01) and dorsiflexors (R = 0.51, p = 0.003) MVCs.
Figure 2. Baseline strength of i) dorsiflexors, ii) plantar flexors and iii) quadriceps for maximal voluntary contraction (MVC –panel A) and potentiated twitch force (Twpot –panel B) in patients with COPD (filled bars) and healthy control subjects (open bars). Values are mean ± SD. * p < 0.05 vs. controls.
Exercise response to standardized endurance walking test
To reach the targeted 40 Kcal expense during the fixed walking time of 12 minutes, patients with COPD and healthy controls respectively completed the exercise with a similar mean treadmill speed (2.9 ± 0.3 vs. 3.1 ± 0.4 km h-1, p = 0.12) and slope (1.8 ± 1.8 vs. 1.4 ± 1.5%, p = 0.54). Patients with COPD reached the predetermined energy expenditure at the expense of a higher ventilatory requirement (the consequence of lower ventilatory efficiency) and a lower ventilatory reserve (higher E/MVV ratio) in comparison to controls (). Patients with COPD were also more short of breath and perceived more leg fatigue at the end of exercise, as indicated by a higher Borg score. Heart rate was higher at the end of exercise in COPD.
Table 2. End-exercise cardiorespiratory response to standardized endurance walking test.
Muscle fatigue assessment
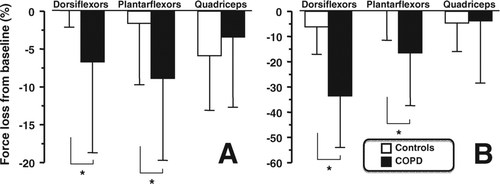
Both, volitional and nonvolitional strength of the dorsi and plantar flexors were significantly reduced following walking in patients with COPD (, panels A and B). The fall in dorsi and plantar flexors MVC and Twpot after walking was more pronounced in patients with COPD than in controls (, panels A and B). There was no significant decline in quadriceps strength following walking in both groups. Finally, fatigue at 15 minutes post-exercise as assessed by the fall in Twpot expressed in% resting value was inversely related to the peak workload determined during IWT for plantar flexors (R = –0.47, p = 0.009) and dorsiflexors (R = –0.57, p = 0.001).
Figure 3. Post-exercise loss in strength for the i) dorsiflexors, ii) plantar flexors and iii) quadriceps for maximal voluntary contraction (MVC –panel A) and potentiated twitch force (Twpot –panel B) in patients with COPD (filled bars) and healthy control subjects (open bars). Values are mean ± SD and expressed as the% fall from resting values. * p < 0.05 vs. controls.
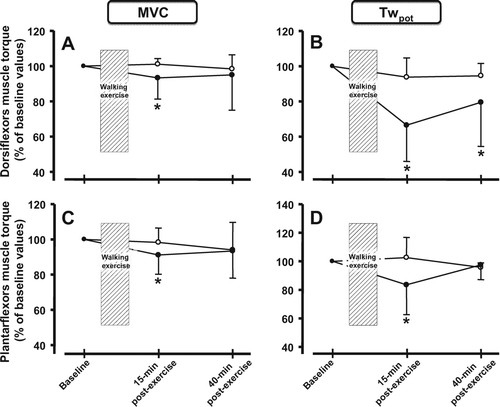
Dorsiflexors Twpot was still reduced at 40 minutes post-exercise in patients with COPD, yet values in healthy controls had returned to resting values (, panel B). At the same time post-exercise, plantar flexors’ strength had recovered in both groups (, panel D).
Figure 4. Post-exercise loss in strength for the i) dorsiflexors and ii) plantar flexors for maximal voluntary contraction (MVC –panel A and C) and potentiated twitch force (Twpot –panel B and D) in patients with COPD (filled circles) and healthy control subjects (open circles) at 15 minutes and 40 minutes post-exercise. Values are mean ± SD. The values at 40 minutes post-exercise are only available for 8 patients with COPD (n = 8) and 6 healthy controls (n = 6). * p < 0.05 vs. baseline force values within groups.
Discussion
The novel contribution of the present study is to provide a specific characterization of the strength and fatigue susceptibility of the leg dorsi and plantar flexors in patients with COPD. To this extent, our results emphasize that patients with COPD presenting weaker quadriceps muscles strength also have weaker dorsi and plantar flexors muscles when compared to age-matched healthy controls. Similarly, these patients exhibit an increased susceptibility to fatigue in the distal leg muscles following a walking exercise of similar energy expenditure. These findings are particularly relevant considering that walking is a central activity in daily living for patients with COPD.
Weakness of the leg muscles in COPD
Quadriceps muscle dysfunction has been well described in patients with COPD, as much for its functional (Citation4, Citation21), structural (Citation22) and metabolic properties (Citation23). Interestingly, very few studies focused on the distal leg muscles in COPD, probably because the quadriceps is readily accessible for study and that it is considered to be representative of all the lower limb muscles. Our results highlight that distal leg muscles fatigue may also occur in conjunction with quadriceps weakness in patients with COPD. This is likely to be clinically relevant given the important contribution of distal leg muscles during walking (Citation24) and the cardinal importance of walking for daily living (Citation25).
Our results are at variance with those of Seymour and colleagues, who did not find weakness of the dorsiflexors in COPD patients with similar fat-free mass to healthy controls (Citation26). Body composition was not assessed in the present study, and as such we cannot comment on whether leg muscle weakness in our subjects could be the result of an atrophying process. Along those lines, we previously reported atrophy of the calf muscle in COPD (Citation8).
Susceptibility to fatigue of the distal leg muscles in COPD
The development of fatigue after walking only in the distal leg muscle groups and not in quadriceps underlines the important solicitation of the former muscles during walking. These findings are important because they illustrate that fatigue of the distal limb may be an issue in patients during activity of daily living. Although the vastus lateralis and the rectus femoris may express electrical evidence of fatiguing contraction during self-paced walking using electromyographic analysis in COPD patients (Citation8), we only found trivial loss in muscle force of the quadriceps after the treadmill exercise. The absence of fatigue in the quadriceps is in line with other experiments involving walking exercises in patients with COPD (Citation7, Citation12).
The recovery time of fatigue may be indicative of the origin of fatigue that is developing during walking and also a reflection of the metabolic profile of the muscle involved in the fatiguing process. The prolonged muscle fatigue seen in patients with COPD for dorsiflexor muscles suggests low-frequency fatigue likely to engage excitation-contraction coupling (Citation27).
Exercise response to standardized walking exercise
The standardized walking effort elicited a significantly larger fraction of the total capacity in patients with COPD as reflected by higher ventilatory requirements, O2 consumption and heart rate response when compared to healthy controls. This was further supported by greater dyspnea and leg fatigue Borg scores in patients with COPD. These findings highlight that walking, as performed in the current protocol, imposes a considerable physiological demand in patients with COPD (Citation8). Competition for the available blood flow between the high working respiratory muscles and the limb muscles to the benefit of the former muscles was recently demonstrated during high intensity cycling exercise in patients with COPD (Citation28). However, such a phenomenon would be unlikely to occur under our experimental protocol during which the intensity of ventilatory stimulation was submaximal.
Methodological considerations
We report distal leg muscle fatigue assessment using magnetic stimulation, a validated and fatigue-sensitive technique (Citation10). Additionally, our validation data (see online supplementary material) confirmed highly reproducible measurements in MVC and Twpot. We refer to plantar and dorsiflexors as it was not possible to isolate the action of each muscle composing each of these muscle groups. We decided to impose a standardized walking effort of 40 Kcal for both groups in order to characterize muscle fatigue for a comparable task. This predetermined energy expenditure was used to reflect routine activities in daily life (i.e., walking to the grocery store). Although the slope imposed during endurance treadmill exercise differed slightly between groups, no statistically significant correlations were found between the extent of distal leg muscle fatigue and the corresponding treadmill slope, supporting the thesis that increased fatigability observed in patients with COPD was not due to differences in this parameter. Finally, it was beyond the scope of the study to explore the electrical, biochemical, metabolic and structural muscle modification that could underlie the susceptibility to distal leg muscle fatigue observed in COPD.
Clinical relevance of findings
Beyond the potential consequences for walking exercise intolerance, our results may have other important functional implications. For example, muscle weakness and fatigue are known to increase the risk of falls in elderly people (Citation29). The present study thus emphasizes the potential need for including specific leg muscles dorsi and plantar flexors exercises in pulmonary rehabilitation programs where only larger muscle groups are typically targeted.
Conclusion
We conclude that patients with COPD presenting weaker quadriceps also have distal leg muscles weakness when compared with healthy controls. Also, following a standardized walking exercise, dorsi and plantar flexors were much more prone to the development of fatigue in patients with COPD. Our results point to the relative contribution of fatigue of the distal limbs in daily physical activities in COPD.
Declaration of Interest
The authors report no conflicts of interest. All authors were all substantially involved contributed in design, acquisition, analysis and interpretation of the study and contributed to the intellectual content of the manuscript. Accordingly, we did not omit any other author that would fulfill these authorship requirements.
Table S1. Subject characteristics.
Definitions of abbreviations: BMI = body mass index; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity. Values are mean ± SD.
Acknowledgments
The authors acknowledge the help of Marthe Bélanger, Marie-Josée Breton, Brigitte Jean, Josée Picard in accomplishing this study and are grateful to Éric Nadreau for his technical support during the exercise testing. The authors are also thankful to Andréanne Guérin and Annie Lemelin for their contribution in the technical development of this project and to Serge Simard for his statistical assistance.
References
- Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am J Respir Crit Care Med 2003; 167(4):544–549.
- Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med 1996; 153(3):976–980.
- Gagnon P, Saey D, Vivodtzev I, Laviolette L, Mainguy V, Milot J, Impact of pre-induced quadriceps fatigue on exercise response in chronic obstructive pulmonary disease and healthy subjects. J Appl Physiol 2009; 107(3):832–840.
- Allaire J, Maltais F, Doyon JF, Noel M, Leblanc P, Carrier G, Peripheral muscle endurance and the oxidative profile of the quadriceps in patients with COPD. Thorax 2004; 59(8):673–678.
- Maltais F, Simard AA, Simard C, Jobin J, Desgagnés P, LeBlanc P. Oxidative capacity of the skeletal muscle and lactic acid kinetics during exercise in normal subjects and in patients with COPD. Am J Respir Crit Care Med 1996; 153(1):288–293.
- Mador MJ, Deniz O, Aggarwal A, Kufel TJ. Quadriceps fatigability after single muscle exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003; 168(1):102–108.
- Man WD, Soliman MG, Gearing J, Radford SG, Rafferty GF, Gray BJ, Symptoms and quadriceps fatigability after walking and cycling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;168(5):562–567.
- Marquis N, Debigaré R, Bouyer L, Saey D, Laviolette L, Brouillard C, Physiology of walking in patients with moderate to severe chronic obstructive pulmonary disease. Med Sci Sports Exerc 2009; 41(8):1540–1548.
- Mador MJ, Kufel TJ, Pineda L. Quadriceps fatigue after cycle exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161(2 Pt 1):447–453.
- Saey D, Debigaré R, LeBlanc P, Mador MJ, Côté CH, Jobin J, Contractile leg fatigue after cycle exercise: a factor limiting exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003; 168(4):425–430.
- Gottschall JS, Kram R. Energy cost and muscular activity required for leg swing during walking. J Appl Physiol 2005; 99(1):23–30.
- Pepin V, Saey D, Whittom F, LeBlanc P, Maltais F. Walking versus cycling: sensitivity to bronchodilation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172(12):1517–1522.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176(6):532–555.
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (ATS). Am J Respir Crit Care Med 1995; 152(5 Pt 2):S77–121.
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Eur Respir J Suppl 1993; 16:5–40.
- Gandevia B, Hugh-Jones P. Terminology for measurements of ventilatory capacity; a report to the thoracic society. Thorax 1957; 12(4):290–293.
- Porszasz J, Casaburi R, Somfay A, Woodhouse LJ, Whipp BJ. A treadmill ramp protocol using simultaneous changes in speed and grade. Med Sci Sports Exer 2003; 35(9):1596–1603.
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exer 1982; 14(5):377–381.
- Tipton CM, editor. ACSM's Advanced Exercise Physiology. Lippincott Williams & Wilkins, Baltimore, MD, 2006.
- Perrault H, Baril J, Henophy S, Rycroft A, Bourbeau J, Maltais F. Paced-walk and step tests to assess exertional dyspnea in COPD. COPD 2009; 6(5):330–339.
- Man WD, Kemp P, Moxham J, Polkey MI. Skeletal muscle dysfunction in COPD: clinical and laboratory observations. Clin Sci (Lond) 2009; 117(7):251–264.
- Gosker HR, Zeegers MP, Wouters EF, Schols AM. Muscle fibre type shifting in the vastus lateralis of patients with COPD is associated with disease severity: a systematic review and meta-analysis. Thorax 2007; 62(11):944–949.
- Saey D, Lemire BB, Gagnon P, Bombardier E, Tupling AR, Debigaré R, Quadriceps metabolism during constant workrate cycling exercise in chronic obstructive pulmonary disease. J Appl Physiol 2011; 110(1):116–124.
- Ericson MO, Nisell R, Ekholm J. Quantified electromyography of lower-limb muscles during level walking. Scand J Rehabil Med 1986; 18(4):159–163.
- Casaburi R, ZuWallack R. Pulmonary rehabilitation for management of chronic obstructive pulmonary disease. N Engl J Med 2009; 360(13):1329–1335.
- Seymour J, Ward K, Steier C, Jolley C, Reilly C, Polkey MI, Distribution of leg muscle weakness in chronic obstructive pulmonary disease (COPD). Eur Respir J 2008; (A1330):223s.
- Edwards RH, Hill DK, Jones DA, Merton PA. Fatigue of long duration in human skeletal muscle after exercise. J Physiol 1977; 272(3):769–778.
- Amann M, Regan MS, Kobitary M, Eldridge MW, Boutellier U, Pegelow DF, Impact of pulmonary system limitations on locomotor muscle fatigue in patients with COPD. Am J Physiol Regul Integr Comp Physiol 2010; 299(1):R314–324.
- Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: A systematic review and meta-analysis. J Am Geriatr Soc 2004; 52(7):1121–1129.
