Abstract
Background: Fatigue is reported in COPD and in heart disease; however, there are hardly any population based data on the relationship between these conditions. Aim: To describe fatigue in relation to COPD by disease severity and to evaluate the impact of respiratory symptoms and heart disease. Methods: Data were collected in 2007 from the OLIN COPD study; 564 subjects with COPD (FEV1/FVC < 0.70) and a distribution of disease severity representative for the general population, and 786 subjects without COPD. The Functional Assessment of Chronic Illness Therapy (FACIT)—Fatigue scale was used to assess fatigue (0–52); lower scores represent worse fatigue. Results: Median FACIT-F score was 44.0 in COPD defined by merely spirometric criteria and 42.0 in COPD also reporting respiratory symptoms, significantly lower compared to 46.0 in non-COPD (p = 0.006 and p < 0.001), and decreased by disease severity. The score was lower in COPD stage ≥ II and in COPD with respiratory symptoms already from stage I when compared to non-COPD. Subjects with heart disease reported lower scores than those without heart disease in COPD by all stages and in non-COPD. COPD with respiratory symptoms stage ≥II remained a significant risk factor for clinically significant fatigue also when adjusted for gender, age, heart disease and smoking habits (stage II OR 1.65, CI 1.17-2.31 and stage III-IV OR 2.66, CI 1.11-6.36). Conclusion: Fatigue is common in COPD, and is affected by respiratory symptoms and concomitant heart disease. In COPD with respiratory symptoms stage ≥ II, there is an increased risk for clinically significant fatigue.
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the most prevalent chronic diseases worldwide and predicted to be the third leading cause of death in 2030 (Citation1). COPD has a high symptom burden (Citation2, 3) and besides respiratory symptoms, fatigue is one of the most common (Citation2, Citation4, Citation5, 6). Fatigue is also reported by subjects with chronic heart failure (Citation3, Citation7) and it has been described that subjects with COPD have a high prevalence of cardiovascular diseases (Citation8, 9, Citation10).
It is suggested that the subjective feeling of fatigue is greater in subjects with COPD than in age-matched control subjects (Citation4, Citation11, Citation12), and that fatigue is related to reduced quality of life in moderate to severe COPD (Citation12, 13), but most studies report no gender difference (Citation4, Citation11, Citation14, 15). Respiratory symptoms, such as dyspnea, are also closely related to fatigue (Citation5, Citation12, Citation16), and it can be difficult for individuals to distinguish the symptoms (Citation17). It is well known that respiratory symptoms increase with COPD severity based on spirometric classification (Citation18) but when it comes to fatigue as a symptom, various findings have emerged. It has been reported that increased disease severity in COPD is associated with increased fatigue (Citation13), but in selected clinical samples no relationship has been found (Citation12). Others describe differences related to COPD severity, but only among selected dimensions of fatigue, as physical fatigue and reduced activity, and not in mental fatigue or general fatigue (Citation11). Varying results may be due to the use of different instruments when assessing fatigue and also due to highly selected study populations (Citation4, 5, Citation11, 12, Citation14). To better understand the experience of fatigue in relation to COPD it is important to describe fatigue in relation to all stages of COPD, thus data from population studies are needed.
From the epidemiological research program, the Obstructive Lung Disease in Northern Sweden (OLIN) studies, a cohort, including subjects with and without COPD, has been followed longitudinally since 2005. The distribution of disease severity in the COPD-population conforms well to what has been described in population samples from amongst all Sweden and Spain (Citation18, 19, Citation20). The aim of this study was to describe fatigue in relation to COPD, by disease severity, and in comparison with subjects without COPD. Further, to evaluate the impact of respiratory symptoms and heart disease.
Methods
The OLIN COPD study
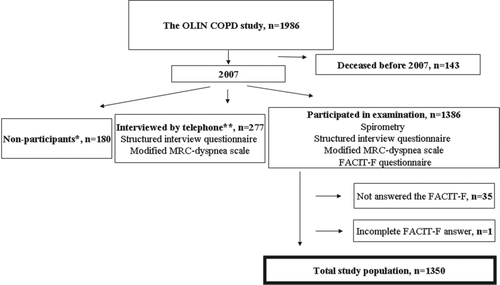
In the years of 2002–2004, four adult OLIN cohorts, randomly selected from the general population, were invited to re-examination. All subjects with COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) (Citation21) spirometric criteria, FEV1/FVC < 0.70, were identified (n = 993) together with a similar size age- and gender matched population without obstructive lung function impairment. The study population (n = 1986) has been invited to yearly examinations since 2005 (Citation19). The current paper is based on cross-sectional data collected in 2007, and the study population consists of all participants at examination with fully completed FACIT-F questionnaires, n = 1350 (). Nine subjects in the study population did not perform spirometry in 2007 due to medical reasons, in these cases, spirometry data from the previous year, 2006, were used.
Questionnaires
The included questions in the structured interview questionnaire are well validated and have been used in several studies (Citation18, Citation22), covering respiratory symptoms, smoking habits and co-morbidities including heart diseases (Citation19). Dyspnea was recorded during the structured interview using the modified Medical Research Council (MRC)- dyspnea scale, including scores from 0 to 4, higher score indicate more dyspnea (Citation23).
Subjective fatigue was measured with the 13-item self-completed questionnaire FACIT-F, designed for chronic diseases (Citation12, Citation24, Citation25, 26, Citation27), translated and validated into Swedish by a FACIT translation project (Citation24). FACIT-F consists of items with a 5-point Likert scale format, and questions are assumed from statements that fit the persons experience during the last seven days asking about the intensity of fatigue as well as its impact on daily life. The items were pooled together to generate a total score (0-52), lower scores represent worse fatigue (Citation24, 25). Three to four units change in score has been used to identify clinically significant differences or Minimally Important Difference, MID in FACIT-F (Citation28), and has also been used in a cross-sectional study of COPD (Citation27).
Spirometry and COPD classification
Spirometry was performed according to the ATS guidelines (Citation19), using a dry spirometer, the Dutch Mijnhardt Vicatest 5. Swedish reference values by Berglund (Citation29) were applied for FEV1. COPD was defined according to the GOLD-spirometric criteria (Citation21), FEV1/FVC < 0.70, including spirometric classification of disease severity based on post-bronchodilator FEV1; stage I: FEV1 ≥80% predicted, stage II: ≤50% <FEV1 <80% predicted, stage III: ≤30% <FEV1 <50% predicted and stage IV: <30% predicted.
Definitions
Smoking habits were divided into three categories; non-smokers, ex-smokers and current smokers. Age was categorized into the groups <65 and ≥ 65 years. Body Mass Index (BMI) was calculated as weight (kg) divided by squared height (m2) and classified according to the World Health Organization (WHO); underweight (<Citation18.Citation5), normal weight (Citation18.Citation5–24.Citation9), overweight (25.0–29.9) and obesity (≥ 30.0) (Citation30). ‘Heart disease’ was defined as at least one of the following; self-reported history of angina pectoris, myocardial infarction, cardiac insufficiency, coronary artery bypass or Percutaneous Coronary Intervention (PCI) procedure. Clinically significant dyspnea was defined as mMRC-dyspnea score ≥ 2. ‘Any respiratory symptom’ was defined as at least one of the following; mMRC-dyspnea ≥ 2, chronic cough, chronic productive cough or recurrent wheeze. COPD subjects reporting any respiratory symptoms are hereafter labelled ‘COPD with respiratory symptoms’.
Analyses
Statistical analyses were performed using the Statistical Package for the PASW Statistics (version 18.0; SPSS Inc, Chicago IL, USA). Univariate comparisons between COPD and non-COPD were assessed using Chi-square tests and Independent sample t-test. Chi-square was used for test for trend. Mann–Whitney and Kruskal–Wallis tests were used to test for differences in FACIT-F score. When significant differences were found by the Kruskal–Wallis test, post hoc analysis was carried out using Mann–Whitney tests with Bonferroni correction. FACIT-F scores are presented in the text by median values and in also as mean (sd). Due to a low number of subjects in GOLD stage III and IV, they were pooled together. A p-value < 0.05 was considered statistically significant for all tests.
Table 1. Basic characteristics of the study population (n = 1350), p value for comparing COPD vs. non-COPD
Table 2. FACIT-F scores in non-COPD, COPD and COPD with respiratory symptoms, by disease severity
Odds ratio (OR) and 95% confidence intervals (CI) were calculated using multiple logistic regression analysis. The dependent variable was the FACIT-F score 43, corresponding to MID using the score three units below median in the non-COPD group. The independent variables included in the model were gender, age, heart disease, smoking categories and FEV1 or COPD with respiratory symptoms by stages I, II and III-IV.
Results
Non-participation
Subjects not able to attend to the examination were telephone interviewed (n = 277), they had significantly higher mean age and proportion of females compared to the study population (n = 1350, non-COPD n = 786, COPD n = 564) (72.0 vs. 66.9 years, p < 0.001 and 51.8% vs. 44.6%, p = 0.009) but there were no differences regarding smoking habits, presence of heart disease or COPD. Non-participants (n = 180) had significantly higher mean age compared to the study population (74.6 vs. 66.9 years, p < 0.001) but there were no differences regarding gender ratio or COPD prevalence.
Basic characteristics
Basic characteristics of the study population are shown in . The distribution by GOLD stages was: stage I n = 294 (52.1%), stage II n = 242 (42.9%) and stage III-IV n = 28 (5.0%). All respiratory symptoms were significantly more common in COPD, and the proportion with respiratory symptoms increased by disease severity. The prevalence of any respiratory symptoms was significantly higher already in COPD stage I, 69.0% compared to in non-COPD, 49.7% (p < 0.001). The prevalence of heart disease was non-significantly higher in COPD 19.7% compared to in non-COPD 17.2% (p = 0.240), but increased by disease severity in COPD; stage I, 17.0%, stage II, 20.0% and stage III-IV, 46.9% (test for trend, p = 0.025).
Fatigue in COPD and non-COPD
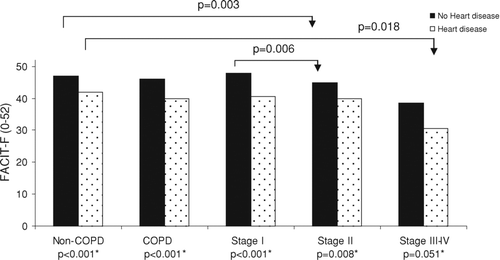
The FACIT-F score was significantly lower in COPD and in COPD with respiratory symptoms compared to in non-COPD (44.0 and 42.0 vs 46.0, p = 0.006 and p < 0.001) (). Among males, the score was significantly lower in COPD compared to non-COPD, 44.5 vs. 47.0 (p = 0.005), while there were no significant differences among females, 44.0 vs. 45.5 (p = 0.373). Comparing gender within the groups COPD, COPD with respiratory symptoms, and non-COPD respectively, females had non-significantly lower scores compared to males in all groups.
In both COPD and non-COPD the scores were lower in older subjects (≥ 65 years) reaching significant difference in COPD only, 43.0 vs. 47.0 (p < 0.001). Comparing COPD and non-COPD by BMI-groups, the scores were identical in normal weight (47.0) while in underweight, overweight and obesity the score was lower in COPD (31.0, 44.0 and 43.0) compared to in non-COPD (43.0, 47.0 and 44.0), significantly so in overweight (p = 0.016). When comparing COPD and non-COPD by smoking categories, there were no significant differences in fatigue scores. In subjects with COPD, current smokers had significantly lower score than non-smokers, 43.0 vs. 46.5 (p = 0.006), while there were no significant differences between smoking categories in non-COPD.
Fatigue by disease severity in COPD
The FACIT-F scores in COPD stage II and III-IV, 43.7 and 37.5, were significantly lower compared to in non-COPD, 46.0 (p < 0.001 and p < 0.001), and in COPD with respiratory symptoms all stages had significantly lower score compared to non-COPD (). Fatigue score distribution and MID in non-COPD and in COPD with respiratory symptoms by disease severity are illustrated by .
Figure 2. Box plots showing median and quartiles of FACIT-F score with a line depicting the clinical significant fatigue based on Minimally Important Difference, MID (defined as minus 3 units difference in median score from non-COPD). Non-COPD, COPD with respiratory symptoms (COPD-rs) stage I, II and III-IV.
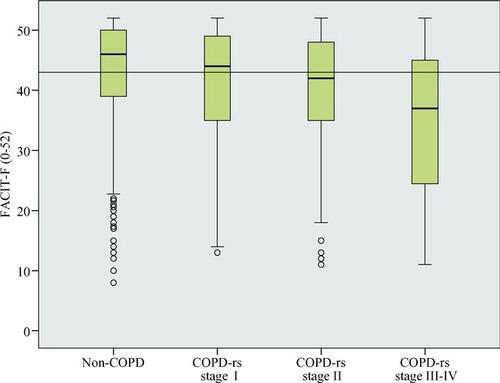
The proportion of subjects with fatigue corresponding to MID, was higher in COPD than in non-COPD, and increased by disease severity (). In COPD with respiratory symptoms the proportion was even higher, 53.5%, corresponding to 49.8%; 55.1% and 70.3% in stage I, II and III-IV, respectively. In COPD, all stages of COPD and in non-COPD, the score was significantly lower in subjects with mMRC-dyspnea ≥ 2 compared with subjects with mMRC-dyspnea <2.
Fatigue and self-reported heart disease
Subjects with heart disease reported lower FACIT-F score than those without heart disease in all groups; non-COPD, COPD and all stages of COPD (). Within the groups, with and without heart disease respectively, the score was lower in COPD compared to non-COPD but did not reach statistical significance.
Multivariate analyses
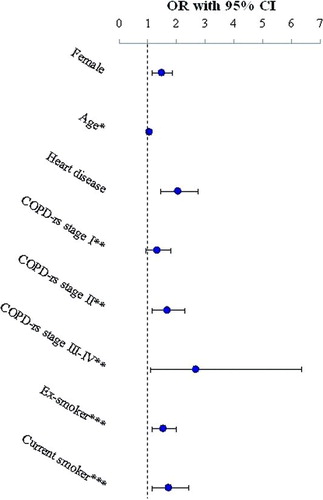
The risk for clinically significant fatigue increased by decreasing FEV1 (OR 1.01, CI 1.01-1.02) in a multivariate model adjusting for gender, age, heart disease and smoking categories. When FEV1 was replaced by COPD with respiratory symptoms, stage II and III-IV significantly increased the risk for a clinically significant fatigue compared to non-COPD, OR 1.65 (CI 1.17-2.31) and OR 2.66 (CI 1.11-6.36), respectively, while stage I did not reach statistical significance, OR 1.30 (CI 0.94-1.81) (). BMI was not a significant risk factor when included in the model and the effect on the OR's was negligible (data not shown).
Figure 4. Multiple logistic regression analysis of risk factors for clinically significant fatigue, Minimally Important Difference, MID (defined as minus 3 units difference in median score from non-COPD) including the co-variates gender, age, COPD with respiratory symptoms (COPD-rs) by stage, heart disease and smoking habits, presented as odds ratio (OR) and 95% confidence intervals (CI). *Entered like a continuous variable (age OR 1.02, CI 1.01-1.03). **Reference = non-COPD. ***Reference = non-smoker.
Discussion
This is the first population based study describing fatigue in COPD compared to in non-COPD, considering the impact of respiratory symptoms and concomitant heart disease. Fatigue was worse in COPD compared to in non-COPD, and fatigue increased with disease severity in COPD. The presence of heart disease and also the symptom dyspnea each increased fatigue in both subjects with COPD and without COPD. COPD with respiratory symptoms stage II and higher had an increased risk for clinically significant fatigue when compared to non-COPD.
In a primary care population (Citation27), including COPD subjects with a high level of respiratory symptoms, fatigue assessed by FACIT-F was increased already in GOLD stage I when compared to a US healthy population (Citation25), similar to our results when comparing COPD stage I with respiratory symptoms and non-COPD. Respiratory symptoms are common in the general population, but significantly more common in COPD (Citation31). A strong relationship between dyspnea and fatigue has been reported (Citation5, Citation12, Citation16), and respiratory symptoms, particularly dyspnea, had a negative impact on quality of life among subjects with chronic diseases (Citation32). Not only disease severity in COPD based on spirometric classification but also respiratory symptoms may be related to quality of life (Citation33) and also prognosis (Citation34). Our results confirm that dyspnea has an impact on fatigue not only in COPD. However, in COPD with respiratory symptoms fatigue was greater than in subjects identified by merely spirometric criteria, suggesting that fatigue is an important aspect related to clinically relevant disease.
Concomitant heart failure and COPD stage III-IV is associated with a worse prognosis (Citation35), and in our study those subjects were the most fatigued. Previous studies indicate that heart disease does not affect fatigue in COPD (Citation27), and in a study on the relationship between COPD exacerbations and fatigue, ischemic heart disease was not an independent risk factor for fatigue (Citation12). However, in our study heart disease increased fatigue in both COPD and non-COPD, and doubled the risk for clinically significant fatigue. This difference, compared to previous studies, may be due to factors as prevalence of heart disease and different characteristics of COPD cases revealed by population based studies. Further, different definitions of heart disease may also affect the results.
Our results confirm that there are no gender differences regarding fatigue in COPD (Citation4, Citation11, Citation14, 15, Citation36). However, women in healthy populations reported more fatigue than men (Citation37, 38), and in our study female gender was a risk factor for clinically significant fatigue. As expected, and previously reported (Citation25), also ageing increased the risk for clinically significant fatigue. BMI was not a risk factor for clinical significant fatigue even though COPD subjects with overweight or obesity were more fatigued than normal weighted. For comparison, obese COPD patients in a pulmonary rehabilitation group were more fatigued and had poorer exercise performance compared to normal weighted patients. Rehabilitation at an earlier stage of disease for obese was suggested (Citation39), and maybe a similar approach could be considered already in overweight. Further, smoking is a well-known risk factor for COPD and heart disease, but tobacco smoke exposure was also an independent risk factor for clinical significant fatigue. The shown relationships between different subjects’ characteristics, diseases and symptoms, as fatigue, in the general population are important for the understanding of the clinical presentation even though the mechanisms behind fatigue remain unclear.
Several factors complicate comparison between studies on fatigue in COPD; different tools for assessing fatigue have different scales, there is no common general definition of high and low fatigue, and most studies are made in selected study populations, often recruited from health care (Citation4, 5, Citation6, Citation11, Citation12, Citation14). For example, the SF-36 was used in a study of subjects participating in a pulmonary rehabilitation program (Citation6), the Fatigue Impact Scale was used in a COPD population without information on spirometric classification (Citation4) and the FACIT-F was used in an epidemiological setting including a primary care population with a high level of respiratory symptoms (Citation27). However, also the well-known large under diagnosis of COPD (Citation18, Citation20) must be taken into account when interpreting data from selected study population. To the best of our knowledge, there are, besides our study, no population based studies on fatigue in COPD using validated instruments.
The strength of this epidemiological study is the large sample size, the distribution of disease severity representative for COPD in the general population, and the use of a validated instrument for assessment of fatigue. The FACIT-F is comparable and briefer than other instruments measuring fatigue, which make it easy to administer and score, and further, subjects do not necessarily have to experience fatigue to be able to answer the questions (Citation24). The external validity of fatigue assessment can be considered high as the FACIT-F score in the control group without obstructive lung function impairment was on a similar level as in a random sample of the US general population (Citation25).
There is support in the literature to regard a difference in score of 3 to 4 as clinically significant, MID, for the FACIT-F in cross sectional settings (Citation27, 28). Further, a cut off at 43 has been used in other studies to distinguish increased fatigue in a population affected by disease compared to a general population sample (Citation25). The proportion of stage I and II is about 95% in a general population sample of COPD (Citation18, Citation20) and our large COPD population has the statistical power to contribute to new and generalizable data for these stages. The low proportion of stage III-IV in a general population sample affects the power to indicate statistical difference even though being the most fatigued. However, similar results are reported (Citation27) and support the generalizibility also for severe disease. When discussing limitations of this study it must be pointed out that the FACIT-F was not distributed to subject's interviewed by telephone. Other study limitations are lack of data on other factors that may affect fatigue as for example anaemia, physical activity level, depression and exacerbations.
Our study highlights the importance for care and nursing care. Health care professionals in both primary health care and institutional care need to be aware of and recognise fatigue to meet their patient's needs. Questions about fatigue can represent a proxy for patient's well-being and provide healthcare professionals with a therapeutic goal. For example, pulmonary rehabilitation can greatly reduce the fatigue of patients with COPD (Citation6).
In conclusion, in this population based study fatigue was commonly perceived and reported by subjects with COPD, and the presence of heart disease increased fatigue not only in COPD. Fatigue increased by COPD disease severity, the proportion with clinically significant fatigue was high and increased in the presence of respiratory symptoms. COPD with respiratory symptoms stage II and higher was an independent risk factor for clinically significant fatigue.
Ethics approval
The Regional Ethics Committee at University Hospital of Northern Sweden and Umeå University approved the study.
Declaration of Interest
The authors declare that they have no conflict of interest. Hana Muellerova is an employee of GlaxoSmithKline, R&D, a producer of pharmaceuticals and owns shares and stock options of GlaxoSmithKline plc. The authors alone are responsible for the content and writing of the paper.
Acknowledgment
First, the authors thank Professor Bo Lundbäck the initiator and still active scientific senior advisor of the OLIN studies, and Professor Eva Rönnmark the present head of the OLIN studies for their support. Further, the authors thank the research assistants Ann-Christine Jonsson, RN, Sigrid Sundberg, RN, and Linnea Hedman, PhD, for collecting the data. The authors also thank Helena Backman for statistical advice, Ola Bernhoff for work with creating the data base of the study and Viktor Johansson for computerising the data.
References
- WHO. World Health Statistics. 2008. Available from: www.who.int/respiratory.
- Blinderman CD, Homel P, Billings A, Tennstedt S, Portenoy, RK. Symptom distress and quality of life in patients with advanced chronic obstructive pulmonary disease. J Pain Symptom Manage 2009; 38:115–123.
- Walke LM, Byers AL, Tinetti ME, Dubin JA, McCorkle R, Fried TR. Range and severity of symptoms over time among older adults with chronic obstructive pulmonary disease and heart failure. Arch Intern Med 2007; 167:2503–2508.
- Theander K, Unosson M. Fatigue in patients with chronic obstructive pulmonary disease. J Adv Nur 2004; 45:172–177.
- Kapella MC, Larson JL, Patel MK, Covey MK, Berry JK. Subjective fatigue, influencing variables, and consequences in chronic obstructive pulmonary disease. Nurs Res 2006; 55:10–17.
- Baltzan MA, Scott AS, Wolkove N, Bailes S, Bernard S, Bourbeau J, Fatigue in COPD: Prevalence and effect on outcomes in pulmonary rehabilitation. Chron Respir Dis 2011; 8:119–128.
- Tang WR, Yu CY, Yeh SJ. Fatigue and its related factors in patients with chronic heart failure. J Clin Nurs 2010; 19:69–78.
- Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and Asthma in primary care. Chest 2005; 128:2099–2107.
- Lange P, Mogelvang R, Marott JL, Vestbo J, Jensen JS. Cardiovascular morbidity in COPD: A study of the general population. COPD 2010; 7:5–10.
- Lindberg A, Larsson LG, Rönmark E, Lundbäck B. Co-morbidity in mild-to-moderate COPD: Comparison to normal and restrictive lung function. COPD 2011; 8:421—428.
- Lewko A, Bidgood PL, Garrod R. Evaluation of psychological and physiological predictors of fatigue in patients with COPD. BMC Pulm Med 2009; 9:47.
- Baghai-Ravary R, Quint JK, Goldring JJP, Hurst JR, Donaldson GC, Wedzicha JA. Determinants and impact of fatigue in patients with chronic obstructive pulmonary disease. Respir Med 2009; 103:216–223.
- Breslin E, van der Schans C, Breukink S, Meek P, Mercer K, Volz W, Perception of fatigue and quality of life in patients with COPD. Chest 1998; 114:958–964.
- Oh EG, Kim CJ, Lee WH, Kim SS. Correlates of fatigue in Koreans with chronic lung disease. Heart Lung 2004; 33:13–20.
- Gift AG, Shepard CE. Fatigue and other symptoms in patients with chronic obstructive pulmonary disease: Do women and men differ? J Obstet Gynecol Neonatal Nurs 1999; 28:201–208.
- Woo K. A pilot study to examine the relationships of dyspnoea, physical activity and fatigue in patients with chronic obstructive pulmonary disease. J Clin Nurs 2000; 9:526–533.
- Meek PM, Lareau SC. Critical outcomes in pulmonary rehabilitation: Assessment and evaluation of dyspnea and fatigue. J Rehabil Res Dev 2003; 40:13–24.
- Lindberg A, Bjerg-Bäcklund A, Rönmark E, Larsson LG, Lundbäck B. Prevalence and underdiagnosis of COPD by disease severity and attributable fraction of smoking. Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med 2006; 100:264–272.
- Lindberg A, Lundbäck B. The Obstructive Lung Disease in Northern Sweden chronic obstructive pulmonary disease study: design, the first year, participation and mortality. Clin Respir J 2008; 2:64–71.
- Miravitlles M, Soriano JB, Garcia-Rio F, Munoz L, Duran-Tauleria E, Sanchez G, Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax 2009; 64:863–868.
- Rabe K, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. GOLD executive summary. Am J Respir Crit Care Med 2007; 176:532–555.
- Lundbäck B, Eriksson B, Lindberg A, Ekerljung L, Muellerova H, Larsson LG, A 20-year follow-up of a population study-based COPD cohort-report from the obstructive lung disease in Northern Sweden studies. COPD 2009; 6:263–271.
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988; 93:580–586.
- Cella D. Manual of the Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System. Evanston, IL: Center on Outcomes, Research and Education (Core), Evanston Northwestern Healthcare and Northwestern University; 1997.
- Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer 2002; 94:528–538.
- Yennurajalingam S, Palmer LJ, Zhang T, Poulter V, Bruera E. Association between fatigue and other cancer-related symptoms in patients with advanced cancer. Support Care Cancer 2008; 16:1125–1130.
- Jones PW, Brusselle G, Dal Negro RW, Ferrer M, Kardos P, Levy ML, Health-related quality of life in patients by COPD severity within primary care in Europe. Respir Med 2011; 105:57–66.
- Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences. The FACIT experience. Eval Health Prof 2005; 28:172–191.
- Berglund E, Birath G, Grimsby G, Kjellmer I, Sandqvist L, Söderholm B. Spirometric studies in normal subjects. Forced expirograms in subjects between 7 and 70 years of age. Acta Med Scand 1963; 173:185–192.
- World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation (WHO Technical report series 894). Geneva; 2000.
- Lindberg A, Jonsson AC, Rönmark E, Lundgren R, Larsson LG, Lundbäck B. Prevalence of chronic obstructive pulmonary disease according to BTS, ERS, GOLD and ATS criteria in relation to doctor's diagnosis, symptoms, age, gender, and smoking habits. Respiration 2005; 72:471–479.
- Voll-Aanerud M, Eagan TML, Wentzel-Larsen T, Gulsvik A, Bakke PS. Changes in respiratory symptoms and health-related quality of life. CHEST 2007; 132:1890–1897.
- Voll-Aanerud M, Eagan TML, Wentzel-Larsen T, Gulsvik A, Bakke PS. Respiratory symptoms, COPD severity, and health related quality of life in a general population sample. Respir Med 2008; 102:399–406.
- de Marco R, Accordini S, Anto JM, Gislason T, Heinrich J, Janson C, Long-term outcomes in mild/moderate chronic obstructive pulmonary disease in the European community respiratory health survey. Am J Respir Crit Care Med 2009; 180:956–963.
- Mascarenhas J, Lourenco P, Lopes R, Azevedo A, Bettencourt P. Chronic obstructive pulmonary disease in heart failure. Prevalence, therapeutic and prognostic implications. Am Heart J 2008; 155:521–525.
- Al-shair K, Kolsum U, Berry P, Smith J, Caress A, Singh D, Development, dimensions, reliability and validity of the novel Manchester COPD fatigue scale. Thorax 2009; 64:950–955.
- Theander K, Unosson M. No gender differences in fatigue and functional limitations due to fatigue among patients with COPD. J Clin Nurs 2011; 20:1303–1310.
- Schwarz R, Krauss O, Hinz A. Fatigue in the general population. Onkologie 2003; 26:140–144.
- Ramachandran K, McCusker C, Connors M, ZuWallack R, Lahiri B. The influence of obesity on pulmonary rehabilitation outcomes in patients with COPD. Chron Respir Dis 2008; 5:205–209.