Abstract
Background: Chronic bronchitis (CB) is a risk factor in chronic obstructive pulmonary disease (COPD) for accelerated lung function decline and increased mortality. The lung and systemic inflammatory and immunological profile of COPD patients with CB which acutely experience respiratory failure upon a disease exacerbation is unknown. Methods: In this study, we explored the expression of Foxp3 by western blot analysis, TLR4 by immunocytochemistry and the concentrations of IP-10 and IL-8 by ELISA in the mini-bronchoalveolar lavages (mini-BAL) and in the peripheral blood of patients with respiratory failure requiring intubation and mechanical ventilation. The recruited subjects were separated into three different groups: smokers with CB and COPD (COPD, n = 18), smokers with CB but without COPD (S, n = 8) and patients without CB and without COPD (C, n = 10). Results: In mini-BAL of COPD group, Foxp3 and IP-10 were significantly reduced while TLR4 was significantly increased in comparison to C. TLR4 was also increased in mini-BAL of S. In COPD peripheral blood, Foxp3 was reduced in comparison to C but no significant differences were observed for TLR4 and for IP-10. No significant differences were observed for IL-8 concentrations in the mini-BAL and in the blood of the recruited patients. The mini-BAL TLR4 expression correlated with the Clinical Infective Pulmonary Score. Conclusions: In exacerbated COPD patients with respiratory failure, lung and systemic reduced immune regulatory events (low Foxp3 expression) and lung increased innate immunity responses (high TLR4 expression) occur. These events may contribute to the increased inflammatory events leading to respiratory failure.
| Abbreviations | ||
| COPD Chronic obstructive pulmonary disease | = | |
| C Controls | = | |
| CB chronic bronchitis | = | |
| S smokers | = | |
| Mini-BAL mini-bronchoalveolar lavage | = | |
| TLR Toll like receptor | = | |
| Foxp3 f forkhead box P3 | = | |
| IP-10 interferon gamma ind uced protein 10 | = | |
| IL-8 interleukin 8. | = | |
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous disease, is associated with pulmonary and extra-pulmonary clinical manifestations and includes different clinically relevant subtypes (Citation1). One of the COPD subtypes is characterized by chronic bronchitis (CB). CB is a risk factor for accelerated lung function decline in COPD, increased hospitalization, and increased mortality (Citation2).
Cigarette smoke represents the most important risk factor for CB (Citation3). Chronic oxidative stress of cigarette smoking induces mucus secretion and the increased mucus viscosity renders the airways susceptible to bacterial infections, a hallmark of CB (Citation3). A key component of the innate immunity and of the innate defence mechanisms against infections is represented by the toll like receptor (TLR) family (Citation4).
A recent hypothesis regarding COPD pathogenesis suggests as “step 1 of the disease” the activation of innate responses by injured tissue components (Citation5). Products derived from epithelial cell injury can act as ligands for TLR4 and TLR2, thus amplifying inflammatory responses within the airways. Cigarette smoke is able to increase the expression of TLR4 and to orientate the activation of TLR4 toward an increased release of IL-8 and a reduced release of interferon gamma-induced protein 10 (IP-10) in bronchial epithelial cells (Citation6).
In the airways of COPD patients mechanically ventilated due to acute respiratory failure, there is an increased expression of TLR4 and an increased chemotactic activity toward neutrophils but a reduced concentration of IP-10 with a reduced chemotactic activity toward lymphocytes (Citation7). The increased airway inflammation in the airways of COPD patients may also be sustained by the impairment of immune regulatory events and in particular may be linked to the reduced expression of the forkhead box P3 (Foxp3), a transcription factor crucially involved in T regulatory activities. COPD patients have, in small airways, decreased numbers of Foxp3 positive cells that negatively correlate with airflow obstruction (Citation8,9). Although COPD is associated with lung and systemic inflammation, it is unknown whether the alteration in the innate and immune regulatory events observed in the airways of COPD patients are also present in the systemic compartment.
The objectives of this study were to investigate the immune regulatory events and the inflammatory and the host defence responses in the airways and in the systemic compartment of COPD patients with CB. Exacerbated COPD patients who experienced an acute respiratory failure requiring endotracheal intubation and mechanical ventilation were compared to patients requiring also intubation and mechanical ventilation but without COPD and without CB. In the recruited patients the expression of TLR4, the expression of Foxp3 and the release of specific chemokines (IP-10 and IL-8) were investigated.
Materials and Methods
Patient population
This study was conducted at the ICU of the Department of Anestesiology, Reanimation and Emergency of the University of Palermo, Italy. Local ethic committee permission and informed written consent from either the patient or closest relatives were obtained. The patients were classified into the following groups: 1) subjects without smoking history, without history of previous CB or chronic pulmonary diseases including asthma and COPD and with acute respiratory failure upon surgery for abdominal or thoracic aneurysm, (Controls = C; n = 10); 2) smoking subjects (>15 pack-year) with CB, without COPD and with acute respiratory failure upon surgery for abdominal or thoracic aneurysm (S; n = 8); 3) smoking subjects (>15 pack-years) with CB with COPD (GOLD-1-2) and with acute respiratory failure upon treatment failure of an acute exacerbation (COPD; n = 18).
The COPD patients fulfilled the diagnostic criteria of COPD (10) with a post-bronchodilator obstruction (FEV1 < 80% predicted, and FEV1/FVC ratio < 70%). Patients with x-ray or clinical evidence of sepsis or pneumonia at the time of mini-bronchoalveolar lavage (mini-BAL) collection were not included. CBs were defined on the basis of symptoms and in particular productive cough lasting more than three months in more than 2 years. All recruited subjects required mechanical ventilation and underwent therapy with antibiotics and systemic corticosteroids (no significantly different doses among the patients included in the three groups). The antibiotics were adjusted to cover any identified pathogens on the basis of antibiograms.
COPD patients, before COPD exacerbation, were undergoing therapy with bronchodilators but not with corticosteroids. Exacerbations were defined as previously reported (Citation10) and were treated with antibiotics and systemic corticosteroids. At the ICU admission, data for Clinical Pulmonary Infective Score (CPIS), the simplified Acute Physiology Score (SAPS II) and sepsis-related organ failure assessment (SOFA) were collected from each recruited patient. Paired mini-BAL and blood samples were collected from all participants. Microbiology of mini-BAL was also assessed.
Mini-BAL collection and processing
Distal lung fluid samples (mini-BAL) were obtained using BAL Cath system (by Kimberly Clark) within 1 h from the intubation. The protected catheter was blindly advanced through the endotracheal tube until it was wedged into a distal airway and aliquots of 10 ml of sterile 0.9% NaCl were instilled and gently suctioned (recovered volume about 70% of the instilled volume). Mini-BAL samples were filtered through a sterile gauze and then centrifuged at 1300 rpm for 10 min to separate cells from supernatants. Total and differential (diff-quick staining) cell counts were assessed. The cell fraction was used for immunocytochemistry and western blot experiments. The supernatants were assessed for cytokine levels.
Blood samples
Blood samples (10 ml) were collected from C, S, COPD subjects and then processed for obtaining plasma and peripheral blood mononuclear cells (PBMC). PBMC were isolated from blood s by Ficoll-Hypaque (Pharmacia) gradient centrifugation. The cells were suspended in RPMI 1640 tissue culture medium (Invitrogen Life Technologies) supplemented with 1% heat-inactivated FCS (Invitrogen Life Technologies), 2 mM L-glutamine, 20 mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin, 5 x 10–5 M 2-ME and 85 μg/ml gentamicin. Purity and viability were tested using trypan blue exclusion.
Immunocytochemistry
The expression of TLR4 was evaluated using a rabbit polyclonal antibody (Santa Cruz Biotechnology). Immunocytochemistry was performed using AP-LSAB2 (DAKO, Glostrup, Denmark) kit following the manufacturer's instructions and new Fuchsin as chromogenic substrate (DAKO) (cytoplasmic red staining). Negative controls were performed using rabbit or mouse negative control immunoglobulins (DAKO). Data are expressed as percentage of positive cells.
Western blot analysis
The expression of Foxp3 was evaluated by western blot analysis as previously described (Citation11) with minor modifications. Then, 40 μg of total protein were loaded in the gel. All blots were probed using a goat polyclonal antibody anti-Foxp3 (1:100) (Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were then stripped and incubated with goat polyclonal anti–ß-actin (Sigma) as housekeeping protein to normalize differences in protein loading. Revelation was performed with an enhanced chemioluminescence system (GE Healthcare, Chalfont St. Giles, UK) followed by autoradiography. Negative controls were performed in the absence of primary antibody or including an isotype control antibody. Data are expressed as densitometric arbitrary units by correction with the density of the bands obtained for beta-actin.
Measurement of IL-8 and IP-10
The concentrations of IL-8 and IP-10 in plasma from C, S, CB-COPD subjects were determined by an enzyme-linked immunosorbent assays (ELISA) following the manufacturer's instructions (Quantikine; R&D Systems, Minneapolis, MN).
Statistics
Data are expressed as median (25-75 percentiles). The Kruskal–Wallis test was performed for comparisons among patient groups. A non-parametric Mann–Whitney test was then applied as the initial Kruskal–Wallis test was significant. The Wilcoxon test was used for comparisons between mini-BAL and autologous peripheral blood in each recruited patient. The Spearman test was used for correlations. P < 0.05 was accepted as statistically significant.
Results
Demographic characteristics of the subjects
The demographic characteristics of the three study groups are shown in . SAPS II and SOFA scores revealed no significant differences in the recruited patients. The CPIS score was significantly higher in COPD than in C (). No significant differences for CPIS score were shown in S in comparison to C and to COPD. The total and the differential cell counts of mini-BAL are shown in . Significantly higher numbers of total cells and of neutrophils were present in COPD patients. Microbiology of mini-BAL was shown in .
Table 1. Demographic and clinical characteristics of the subjects
Table 2. Total and differential cell counts of mini-BAL
Table 3. Microbiology of mini-BAL
Expression of TLR4 in cells from mini-BAL and from peripheral blood cells
The percentage of TLR4 positive cells was significantly higher in mini-BAL cells from COPD and from S in comparison to mini-BAL from C. The percentage of TLR4 positive cells was significantly higher in mini-BAL cells from COPD in comparison to mini-BAL from S and to autologous peripheral blood () (). No significant differences in TLR4 expression were observed in peripheral blood cells among C, S and COPD () ().
Figure 1. Increased expression of TLR4 in mini-BAL but not in the peripheral blood of S and of COPD. Mini-BAL cells and paired blood samples were recovered from C (n = 10), from S (n = 8) and from COPD (n = 18) patients. The expression of TLR4 was assessed by immunocytochemistry using an anti-TLR4 polyclonal antibody; *p < 0.05 vs C; **p < 0.05 vs S; #p < 0.05 vs autologous peripheral blood. Data are expressed as median (25-75 percentiles).
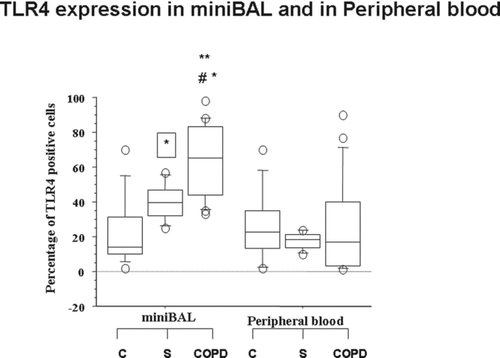
Table 4. Total and differential cell counts of mini-BAL
Concentrations of IL-8 and of IP-10 in mini-BAL and in peripheral blood cells
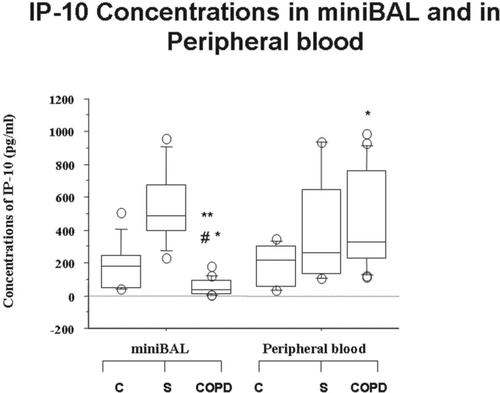
The concentrations of IL-8 were significantly higher in mini-BAL of all the recruited patients (C, S and COPD patients) in comparison to autologous peripheral blood (). No significantly different concentrations of IL-8 were observed in mini-BAL and in peripheral blood among C, S and COPD (). The IP-10 concentrations were significantly reduced in mini-BAL from COPD in comparison to C and S and in comparison to autologous peripheral blood (). The IP-10 concentrations were significantly increased in peripheral blood from COPD in comparison to mini-BAL from C. No significantly different concentrations of IP-10 were observed in peripheral blood between S and C ().
Figure 2. Absence of differences in the concentrations of IL-8 in mini-BAL and in the peripheral blood of S and of COPD. Mini-BAL supernatants and paired blood samples were recovered from C (n = 10), from S (n = 8) and from COPD (n = 18) patients. IL-8 concentrations were measured by ELISA as described in “materials and methods” and are expressed as pg/ml. Data are expressed as median (25-75 percentiles).
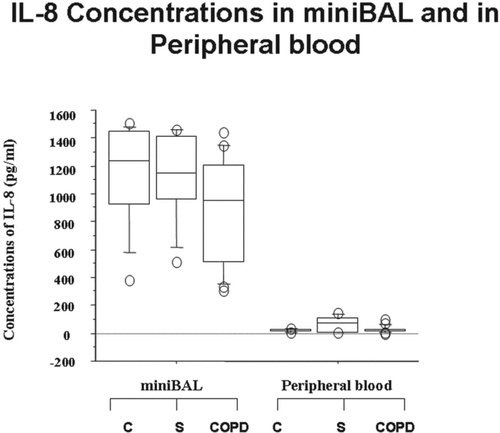
Figure 3. Reduced concentrations of IP-10 in mini-BAL but not in the peripheral blood of COPD. Mini-BAL supernatants and paired blood samples were recovered from C (n = 10), from S (n = 8) and from COPD (n = 18) patients. IP-10 concentrations were measured by ELISA as described in “materials and methods” and are expressed as pg/ml. *p < 0.05 vs C; **p < 0.05 vs S; #p < 0.05 vs autologous peripheral blood. Data are expressed as median (25-75 percentiles).
Expression of Foxp3 in mini-BAL and in peripheral blood cells.
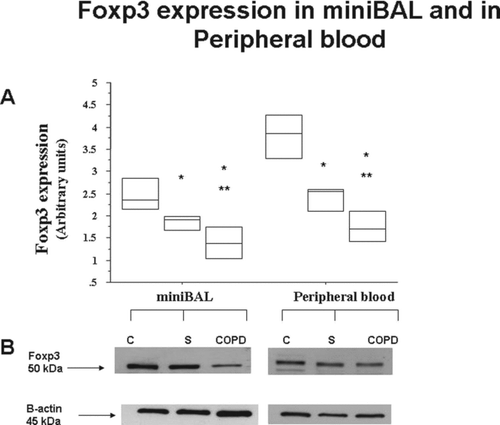
In COPD and in S the expression of Foxp3 in mini-BAL and in peripheral blood was significantly lower than in C () (). In COPD the expression of Foxp3 in mini-BAL and in peripheral blood was significantly lower than in S () (). No significant differences were observed in the expression of Foxp3 in mini-BAL and in autologous peripheral blood in all the recruited patients.
Figure 4. Reduced expression of Foxp3 in mini-BAL and in the peripheral blood of S and of COPD. Mini-BAL cells and paired blood samples were recovered from C (n = 10), from S (n = 8) and from COPD (n = 18) patients. Total proteins were extracted and analysed for Foxp3 expression by western blot analysis. Membranes were then stripped and incubated with goat polyclonal anti–ß-actin. A. Densitometric analysis of Foxp3 expression. Signals corresponding to Foxp3 on the various western blots were semiquantified by densitometric scanning, normalized and expressed after correction with the density of the band obtained for beta-actin (mean±SD). * p < 0.05. B. Representative Western blot analysis for Foxp3 expression from C, S and COPD subjects. Data are expressed as median (25-75 percentiles).
Correlations
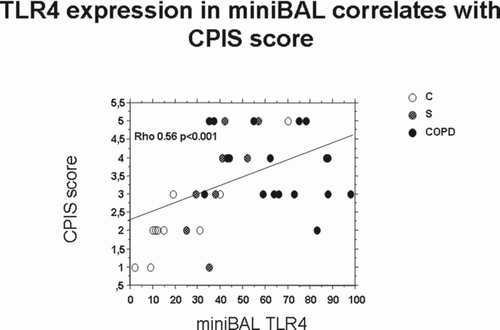
Finally, we tested whether the observed alterations in TLR4, Foxp3 and IP-10 in both lung and systemic compartments correlate with clinical scores of severity. CPIS correlates with mini-BAL TLR4 expression () but not with the other markers (Foxp3 and IP-10) (data not shown). No significant correlations were observed between SOFA or SAPS II score with any of the tested markers (data not shown).
Discussion
COPD is a heterogeneous disease and includes different clinically relevant subtypes. A divergent distribution of parenchymal (emphysema) and bronchial airway (chronic bronchitis) (CB) disease contributes to the phenotypic heterogeneity in COPD (Citation12). COPD is associated not only with an abnormal inflammatory response in the lung but also with systemic inflammation, including systemic oxidative stress, activation of circulating inflammatory cells and increased circulating levels of inflammatory cytokines (Citation12). The low-grade systemic inflammation present in COPD patients may be responsible for the systemic clinical manifestations of the disease including malnutrition, muscle wasting, osteoporosis, cardiovascular diseases, type II diabetes, anaemia and depression (Citation13).
This study explored whether lung and systemic inflammation occur concurrently and similarly in patients with acute respiratory failure with and without CB or COPD. We demonstrated for the first time that patients with both CB and COPD who underwent an acute exacerbation have a different inflammatory profile (IP-10 levels) and a different alteration in the innate immune responses (TLR4 expression) in the airways and in the systemic compartment. Differently, in these patients a similar alteration in the immune regulatory events (low Foxp3 expression) was observed in the airways and in the systemic compartment. The novel aspects underlined by the present study are related to the accurate selection of patients with a similar phenotype (CB) and a similar exposure to risk factor (cigarette smoke) in order to limit the variability of the obtained results and to better identify the role of the observed alterations in airway obstruction. Most of the studies report data from smoker or COPD patients with different phenotypes.
CB is defined on the basis of chronic cough and sputum due to mucus hypersecretion and histologically, it is characterised by airway inflammation, hypertrophy of submucosal mucus secreting glands, and goblet cell hyperplasia (Citation14). CB is a risk factor in COPD for accelerated lung function decline, increased hospitalization, and increased mortality (Citation2). In this regard it has been demonstrated that the presence of CB may compromise the sterility of distal airway supporting the hypothesis of natural progression from the simple mucus hypersecretion to purulent hypersecretion and obstructive bronchitis (Citation15).
Here, it is showed that the expression of TLR4 is increased in COPD patients with CB and acute exacerbation in the airways but not in the systemic compartment and this alteration is observed at lower extent also in smokers with CB but without COPD and without acute exacerbation. TLR4 expressed is associated in these patients with the presence of neutrophils which represent the predominant cell type in COPD patients. The data provided extend and integrate previous results from our group showing that in the airways of COPD patients with acute respiratory failure, there is an increased expression of TLR4 (Citation7).
Our data demonstrate that TLR4 expression is higher in mini-BAL cells in comparison to blood compartment suggesting an up-regulation of TLR4 at the transit from blood into the airway compartment. This phenomenon seems to be specific for TLR4 since it has been previously demonstrated that in COPD patients the expression of TLR2 is lower on sputum neutrophils in comparison to blood compartment indicating a down-regulation of TLR2 at the transit from blood into the airway compartment (Citation16).
TLR4 signaling, through MyD88 and IRAK1, plays a predominant role as a regulator of smoke-induced protease production (Citation17). Furthermore, CSE increase the expression of TLR4 but not of TLR2 and modify the functional activation of TLR4 generating an imbalance between cytokines with opposite functions such as IL-8 and IP-10 (Citation6). IP-10 concentrations in mini-BAL of smoker COPD who are mechanically ventilated for acute exacerbation are reduced in comparison to another group of patients mechanically ventilated but not smokers and without COPD (Citation7). We confirm here the presence of reduced concentrations of IP-10 within the airways of mechanically ventilated and exacerbated COPD patients and demonstrate for the first time that within the systemic compartment in the same patients an increased concentration of IP-10 is observed in comparison to patients with acute respiratory failure but without CB and COPD.
Nasal epithelial cells obtained from smokers create an overall cytokine microenvironment that after infection with influenza suppresses the concentrations of IP-10 (Citation18). When the bronchial epithelial cells were exposed to CSE, the release of IP-10 decreases while the release of IL-8 increases (Citation6). Although no significantly different concentrations of IL-8 were observed in mini-BAL and in peripheral blood between COPD, S and C within the airways the elevated concentrations of IL-8 are not balanced by elevated IP-10 concentrations.
The prevalence of IL-8 may in turn sustain the influx of neutrophils into the airways thus triggering innate immunity responses, while IP-10 attracts monocytes and lymphocytes (Citation19) thus promoting activation of adaptative responses to efficiently and specifically limit microbial invasion and to restrain the harmful effects of prolonged neutrophil activation. The findings that mini-BAL from COPD had reduced IP-10 concentrations and reduced chemotactic activities toward lymphocytes (Citation7) might contribute to explain why the differential cell counts of mini-BAL from COPD failed to have lymphocytes and prompted us to explore whether lymphocyte regulatory activities were altered in these patients.
CD4+ Foxp3+ regulatory T lymphocytes (Treg) are a subclass of CD4+ T cell receptor (TCR) αβ+ T cells that are essential to preserve immune homeostasis (Citation20), (Citation21). Stable expression of the transcription factor Foxp3 is a prerequisite for the maintenance of suppressive properties in CD4+ regulatory T cells. Foxp3 mRNA expression is not itself sufficient for stable Foxp3 protein expression (Citation22). The epigenetic modifications, such as histone modification or DNA methylation, control regulatory T cells by controlling Foxp3 gene expression through altering the accessibility of the Foxp3 locus and by acetylating or deacetylating Foxp3 protein, thereby enabling the epigenetic regulation of Foxp3 target genes. Absence of the Foxp3 transcription factor at systemic level leads to the rapid development of fulminant multi-organ autoimmunity. A decreased Foxp3 expression in COPD patients and smokers parallels the aggravation of the disease (Citation23).
Patients with moderate or severe COPD upon fluticasone and salmeterol combination therapy show an increased proportion of Foxp3+Tregs in the total peripheral blood CD4+T cell population (Citation24). Smokers with normal lung function and COPD patients have increased numbers of Foxp3-positive cells in large airways but they have decreased numbers of Foxp3-positive cells in small airways (Citation25). In the patients with acute respiratory failure recruited in the present study, the decreased expression of Foxp3 is present in smokers and in COPD in the distal airways and in the systemic compartment suggesting that the alterations in the immune regulatory activities are early events in the disease and may contribute to the systemic effects of the disease. It is conceivable that cigarette smoke may contribute to induce epigenetic modifications leading to the reduced expression of Foxp3 in smokers and in COPD smokers at both the airways and systemic compartment. No study has already specifically addressed this point yet.
Finally, the relevance of the observed alterations in the severity of the recruited patients was assessed. Several commonly used scoring systems exist assessing the severity of the disease in critically ill patients by predicting mortality. In the present study the recruited patients were classified on the basis of their SOFA, SAPS II and CPIS score. The mortality in elderly patients was higher than that of the younger patients and SAPS II (Citation26) was an independent predictor of mortality in elderly patients with sepsis (Citation27). The SOFA score, widely used in many cardiac surgical intensive units, is used for grading organ dysfunction or failing organ system (Citation28). Prognostic relevance of the SOFA score in combination with inflammatory parameters was also found in a recent study conducted by Zügel et al. (Citation29).
The CPIS score is calculated on the basis of points assigned for various signs and symptoms of pneumonia (e.g., fever and extent of oxygenation impairment) and a CPIS >6 may serve as a surrogate tool to facilitate the diagnosis of ventilator-associated pneumonia (Citation30). In the present study, recruited patients have no significant different SOFA or SAPS II scores and these scores did not correlate with any of the tested markers. The only differences among the recruited patients were related to the CPIS score and CPIS score correlates with TLR4 mini-BAL expression. Future studies on a larger cohort of patients are needed to clarify whether the assessment of TLR4 expression in mini-BAL may improve the predictive value of CPIS to early identify patients with ventilator-associated pneumonia.
In conclusion, patients with both CB and COPD who underwent an acute exacerbation have a different alteration in the levels of IP-10 and in TLR4 expression in the airways and in the systemic compartment while they have a similar alteration in Foxp3 expression in the airways and in the systemic compartment. The inflammatory profile of the COPD patients in the present study may be the result of the failure to properly clear the inflammatory cells after the exacerbation of the disease.
Acknowledgments
This work was supported by the Italian National Research Council. Elisabetta Pace and Maria Ferraro designed the study, performed the statistical analysis of the data e wrote the manuscript and declares that they have had access to and takes responsibility for the integrity of the data and the accuracy of the data analysis. Chiara Cipollina performed all the experiments of the study and participated to the interpretation of the data. Antonino Giarratano contributed to the patient selection, collected and managed biological samples. Mark Gjomarkaj contributed to the interpretation of the data and to the writing out of the manuscript.
References
- Garcia-Aymerich J, Serra Pons I, Mannino DM, Maas AK, Miller DP, Davis KJ. Lung function impairment, COPD hospitalisations and subsequent mortality. Thorax 2011; 66(7):585–590.
- Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Am J Respir Crit Care Med 1996; 153:1530–1535.
- Mullen JB, Wright JL, Wiggs BR, Pare PD, Hogg JC. Structure of central airways in current smokers and ex-smokers with and without mucus hypersecretion: Relationship to lung function; Thorax 1987;42: 843–848.
- Zhang G, Ghosh S. Molecular mechanisms of NF-kappaB activation induced by bacterial lipopolysaccharide through Toll-like receptors. J Endotoxin Res 2000; 6:453–457.
- Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med 2009; 360:2445–2454.
- Pace E, Ferraro M, Siena L Melis M, Montalbano A, Johnson M, Bonsignore MR, Bonsignore G, Gjomarkaj M. Cigarette smoke increases TLR4 and modifies LPS mediated responses in airway epithelial cells. Immunology 2008; 124:401–411.
- Pace E, Giarratano A, Ferraro M, Bruno A, Siena L, Mangione S, Johnson M, Gjomarkaj M. TLR4 upregulation underpins airway neutrophilia in smokers with chronic obstructive pulmonary disease and acute respiratory failure. Hum Immunol 2011; 72:54–62.
- Isajevs S, Taivans I, Strazda G, Kopeika U, Bukovskis M, Gordjusina V, Kratovska A. Decreased FOXP3 expression in small airways of smokers with COPD. Eur Respir J 2009; 33(1):61–67.
- Roos-Engstrand E, Pourazar J, Behndig AF, Bucht A, Blomberg A. Expansion of CD4+CD25+ helper T cells without regulatory function in smoking and COPD. Respir Res 2011; 12:74.
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Am J Respir Crit Care Med 2001; 163:1256–1276.
- Pace E, Ferraro M, Uasuf CG, La Grutta S, Liotta G, Giarratano A, Johnson M, Gjomarkaj M. Cilomilast counteracts the effects of cigarette smoke in innate responses of airway epithelial cells. Cell Immunol 2011; 268:47–53.
- Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet 2003; 362:1053–1061.
- Agustí A. Systemic effects of chronic obstructive pulmonary disease: What we know and what we don't know (but should). Proc Am Thorac Soc 2007; 4(7):522–525.
- Reid L. Pathology of chronic bronchitis. Lancet 1954; i:275–279.
- Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004; 364:709–721.
- von Scheele I, Larsson K, Dahlén B, Billing B, Skedinger M, Lantz AS, Palmberg L. Toll-like receptor expression in smokers with and without COPD. Respir Med 2011; 105:1222–1230.
- Geraghty P, Dabo AJ, D'Armiento J. TLR4 protein contributes to cigarette smoke-induced matrix metalloproteinase-1 (MMP-1) expression in chronic obstructive pulmonary disease. J Biol Chem 2011; 286:30211–30218.
- Horvath KM, Brighton LE, Zhang W, Carson JL, Jaspers I. Epithelial cells from smokers modify dendritic cell responses in the context of influenza infection. Am J Respir Cell Mol Biol 2011; 45:237–245.
- Boodoo S, Spannhake EW, Powell JD, Horton MR. Differential regulation of hyaluronan-induced IL-8 and IP-10 in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2006; 291:L479–L486.
- Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol 2005; 6:331–337.
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 2010; 10:490–500.
- Hori S. Rethinking the molecular definition of regulatory T cells. Eur J Immunol 2008; 38:928–930.
- Chu S, Zhong X, Zhang J, Lao Q, He Z, Bai J. The expression of Foxp3 and ROR gamma t in lung tissues from normal smokers and chronic obstructive pulmonary disease patients. Int Immunopharmcol 2011; 11(11):1780–1788. Epub 2011 Jul 23.
- Yang L, Ma QL, Yao W, Zhang Q, Chen HP, Wang GS, Wang CZ. Relationship between the anti-inflammatory properties of salmeterol/fluticasone and the expression of CD4+CD25+Foxp3+ regulatory T cells in COPD. Respir Res 2011; 12:142.
- Isajevs S, Taivans I, Strazda G, Kopeika U, Bukovskis M, Gordjusina V, Kratovska A. Decreased FOXP3 expression in small airways of smokers with COPD. Eur Respir J 2009; 33(1):61–67.
- Le Gall Jr Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993; 270:2957–2963.
- Tiruvoipati R, Ong K, Gangopadhyay H, Arora S, Carney I, Botha J. Hypothermia predicts mortality in critically ill elderly patients with sepsis. BMC Geriatr 2010; 10:70.
- Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 1998; 26(11):1793–1800.
- Zügel NP, Kox M, Lichtwark-Aschoff M, Gippner-Steppert C, Jochum M. Predictive relevance of clinical scores and inflammatory parameters in secondary peritonitis. Bull Soc Sci Med Grand Duche Luxemb 2011; 1:41–71.
- Zilberberg MD, Shorr AF. Clin Infect Dis. Ventilator-associated pneumonia: the clinical pulmonary infection score as a surrogate for diagnostics and outcome. Clin Infect Dis 2010; 51 Suppl 1: S131–135.