Abstract
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation; from a pathophysiological point of view it involves many components, including mucus hypersecretion, oxidative stress and inflammation.
N-acetylcysteine (NAC) is a mucolytic agent with antioxidant and anti-inflammatory properties. Long-term efficacy of NAC 600mg/d in COPD is controversial; a dose-effect relationship has been demonstrated, but at present it is not known whether a higher dose provides clinical benefits. The PANTHEON Study is a prospective, ICS stratified, randomized, double-blind, placebo-controlled, parallel-group, multi-center trial designed to assess the efficacy and safety of high-dose (1200 mg/daily) NAC treatment for one year in moderate-to-severe COPD patients. The primary endpoint is the annual exacerbation rate. Secondary endpoints include recurrent exacerbations hazard ratio, time to first exacerbation, as well as quality of life and pulmonary function. The hypothesis, design and methodology are described and baseline characteristics of recruited patients are presented. 1006 COPD patients (444 treated with maintenance ICS, 562 ICS naive, aged 66.27±8.76 yrs, average post-bronchodilator FEV1 48.95±11.80 of predicted) have been randomized at 34 hospitals in China. Final results of this study will provide objective data on the effects of high-dose (1200 mg/daily) long-term NAC treatment in the prevention of COPD exacerbations and other outcome variables.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation. Pathophysiologically it involves many components, including mucus hypersecretion, oxidative stress, and inflammation of the airways and lungs (Citation1).
Mucus hypersecretion is an important phenotype of COPD. It is associated with disease exacerbations (Citation2), accelerated decline in FEV1 (Citation3) and inflammatory cell infiltration (Citation4). Therefore, mucus clearance and sterility maintenance are important in COPD. In addition, COPD is associated with oxidative stress, which results in the inactivation of antiproteinases, airspace epithelial injury, mucus hypersecretion, increased influx of neutrophils into the lungs, transcription factor activation, and gene expression of proinflammatory mediators.
Potentially active agents possessing anti-inflammatory and anti-oxidative properties, as well as mucolytic activity, might be effective in the treatment of COPD. This concept has been validated in our previous study with carbocisteine (PEACE study) (Citation5).
N-acetylcysteine (NAC) is an effective mucolytic agent that reduces sputum viscosity and elasticity. The modulation of the inflammatory response (Citation6–8) and direct/indirect anti-oxidant properties may be more important than mucolysis itself for long-term COPD management.
The NAC thiol-group serves both as a direct antioxidant and as a cysteine-donor for glutathione-synthesis in the cell resulting in indirect antioxidant activity (Citation9). The antioxidant effects of NAC are well documented both in in vitro and in vivo studies (Citation10). NAC is able to attenuate the lesions induced by elastase in rats. This supports the idea that oxidant injury may contribute to the development of elastase-induced emphysema and that NAC treatment may attenuate or slow down the process (Citation11). Reduction in sputum eosinophilic cation protein (ECP) concentrations, improvement in the phagocytic ability of polymorphonuclear neutrophils (PMNs) (Citation12), inhibition of bacterial adherence to oropharyngeal epithelial cells (Citation13) as well as of influenza virus replication (Citation14) might be further NAC properties that could reduce the frequency of COPD exacerbations. Moreover, there is evidence of increased ROS in the airways of COPD patients (Citation15).
However, clinical studies designed to assess the benefits of NAC in patients with chronic bronchitis and COPD treated with the usual dose (600mg/d) of NAC have yielded inconsistent results (Citation16,17). NAC, at the currently recommended dosage, is not recommended by some guidelines (such as NICE 2010 [Citation18]) for regular COPD treatment.
Can an increase in the dose of NAC improve the efficacy of treatment? Gerrits et al. demonstrated that the reduction in the risk of exacerbation produced by NAC was dose-dependent (Citation19), Zuin et al. (Citation20) found that treatment of GOLD II-III stage patients with NAC 1200 mg/d achieved greater improvement in biological markers and clinical outcomes than 600 mg/d; however, their study lasted for only 10 days.
Because of certain pitfalls in study design, such as small sample size, low dose of NAC, lack of double-blinding and placebo control, or short duration of the study, clinical trials with a reliable research study design are therefore warranted to clarify whether COPD patients can benefit from prolonged anti-inflammatory and anti-oxidant therapy, such as NAC.
The Placebo-controlled study on efficAcy and safety of N-acetylcysTeine High dose in Exacerbations of chronic Obstructive pulmoNary disease (PANTHEON Study) was designed by a panel of respiratory experts. The hypothesis tested in this trial is that high-dose (1200 mg/d) long-term (1 year) NAC treatment can reduce the exacerbation rates. As the BRONCUS study (Citation16), showed a reduction in exacerbations only in patients not using inhaled corticosteroids (ICS), the benefits of high-dose NAC for patients with and without concomitant treatment of ICS will also be tested.
Methods
PANTHEON study design
This was a multi-center, prospective, stratified, randomized, double-blind, placebo-controlled, parallel-group, one-year trial. The study protocol was designed by the investigator steering committee and presented to all study sites thereafter. All investigators were trained before the trial to ensure reliable study quality, with special emphasis on understanding the protocol, performing spirometry tests, blinding of allocation, drug supply management and compliance with Good Clinical Practice (GCP). Medkey Co., Ltd (Contract Research Organization, Shanghai, China), acting as a third party, was exclusively responsible for randomization, data management, data analysis and data quality control. The CONSORT Statement (Citation21) was followed to ensure proper reporting of this study.
All participants were stratified according to chronic use of ICS or ICS naive status at baseline as follows: Stratum I, ICS naive group: No use or irregular use of ICS during the last 3 months; Stratum II, ICS group: Regular daily use of ICS in the last 3 months. The ratio of ICS positive to ICS naïve status was set to about 4:6.
Stratified randomization was conducted using the predetermined computer-generated randomization list provided by a statistician from the third party not involved in this study. At each centre, the enrolled participants were allocated to the NAC group or the placebo group according to their assigned numbers.
Both NAC and placebo tablets were provided by Hainan Zambon Pharmaceutical Co., Ltd. The placebo was identical in composition, shape, color, and size, but did not contain any active ingredients. NAC and placebo tablets were packaged and labeled in such a way that they could not be distinguished from each other.
Supplies of tablets for every patient were identified with a four-digit number. A sealed envelope containing the randomization code for each patient was kept by the investigator and was not to be opened during the study, unless a serious life-threatening adverse event occurred. Opening of any envelope, whether intentional or accidental, was to be carefully recorded on the Case Report Form (CRF), and the patient was to be withdrawn from the study. The steering committee and the statisticians in charge of the analysis were blinded to the treatment allocations during the study.
After a 2-week run-in period, eligible patients with COPD were randomly assigned to receive NAC (600 mg, twice daily) or placebo (one tablet, twice daily) for 1 year. A visit was planned on the first and on the third month after randomization, then every three months until the end of the study; a monthly phone call interview was requested. Any unscheduled visit was to be recorded as such.
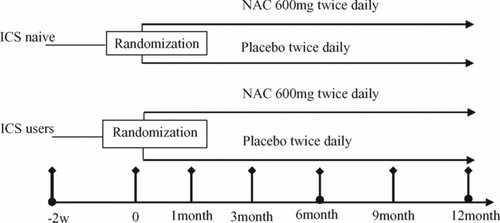
At every visit, the adherence to the study regimen was assessed by collecting and counting the number of remaining tablets, the investigational products were refilled and adverse events were recorded. The study flowchart is shown in . No follow-up study was planned.
Figure 1. Study Flowchart, • indicates pulmonary function test was performed; ♦ indicates SGRQ, St. George's Respiratory Questionnaire was completed, Diary card was dispensed and reclaimed. NAC: N-acetylcysteine.
Salbutamol, a short-acting bronchodilator (SABA), was to be administered as needed to relieve symptoms, and its use was to be recorded in the patient's diary. Maintenance treatment for COPD started prior to the study, such as short-acting bronchodilators (SABA), long-acting bronchodilators (LABA), inhaled corticosteroids (ICS) and theophylline, was permitted to continue, but had to be continued during the study period. Systemic administration of corticosteroids, antibiotics, mucolytics or antitussive agents was not permitted, unless treatment for an exacerbation was needed. Patients were advised to avoid concomitant use of antioxidant vitamins and any other antioxidant dietary supplement. In addition, some non-pharmacological therapies, including pulmonary rehabilitation and drainage of phlegm, were not allowed during the study.
Patient participation
Inclusion criteria
Patients had to meet all the following criteria to be included: 1) Out-patient, male or female, aged between 40 and 80 years, smoking or non-smoking; 2) Patients clinically diagnosed with COPD, classified as GOLD II or III in terms of severity 1, post-bronchodilator forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio (FEV1/FVC)<0.7, and FEV1 between 30% and 70% of predicted; 3) Patients with a history of at least 2 exacerbations within the previous 2 years and who were clinically stable for at least 4 weeks prior to the study.
Exclusion criteria
Patients were not eligible for the trial if they met one or more of the exclusion criteria listed here:
a diagnosis of bronchial asthma, cystic fibrosis, active pulmonary tuberculosis, pneumonia, bronchial pneumonia, bronchiectasis, lung cancer or lung metastases, other progressively fatal diseases;
a diagnosis of severe cardiovascular disease, severe neurological disease or severely impaired hepatic or renal function;
need for mechanical airway management or long-term oxygen therapy (12 hours or more per day) or pulmonary rehabilitation;
treatment with systemic corticosteroids at inclusion;
Hospitalized patients and patients from institutional care facilities;
Immunocompromised patients;
suspected or known hypersensitivity to the study product or any of its excipients: rare hereditary problems of fructose intolerance (glucose-galactose malabsorption or sucrase-isomaltase insufficiency);
conditions affecting study drug absorption or history of peptic ulcer;
pregnant or lactating women, and absence of a medically acceptable method of contraception in women of child-bearing potential or in post-menopause for less than 1 year;
poor reliability (e.g., history of alcohol or drug abuse, mental disorder) and poor compliance;
Patients already enrolled in this study or patients who had received any other investigational drug in the last 3 months prior to study entry.
The study was approved by local ethics committees and conducted in compliance with the Declaration of Helsinki (Citation22) and Good Clinical Practice Guideline (Citation23). All patients gave their written informed consent before participating in the study.
Efficacy outcome measures
Primary endpoint
The primary endpoint is exacerbation rate over 1 year. Exacerbations were defined according to Anthonisen et al. (Citation24), i.e. at least 2-day persistence of two or all major symptoms (worsening dyspnea, increase in sputum purulence and/or volume), or of any single major symptom plus more than one minor symptoms (upper airway infection, fever unexplained by other causes, and increased wheezing). The severity was graded as follows: severe—exacerbation included the presence of all three of these symptoms; moderate—exacerbation included two of the symptoms; mild—exacerbation included one symptom plus at least one of the following: 1) an upper respiratory tract infection in the previous 5 days; 2) increased wheezing; 3) increased cough; 4) fever without an obvious source; and, 5) a 20% increase in respiratory rate or heart rate above baseline. When symptoms worsened, patients were asked to contact the Investigator. The interval between any two independent exacerbations had to last more than 7 days.
A specific exacerbation case record form was included in the Diary Card to capture all aspects of the exacerbation definition. For determination of exacerbations in this study, clinical data such as dyspnea, the amount and characteristics of sputum, fever and presence of upper airway infection were followed up using patient diary cards. The data were discussed during each visit and were validated by the steering committee.
Secondary endpoints
Time to the first exacerbation and time to recurrent exacerbations were considered as secondary endpoints. Quality of life (QoL) was determined by St. George's Respiratory Questionnaire (SGRQ) (Citation25) total score as well as its 3 domain sub-scores (symptoms, activities and impacts). Patients completed SGRQ (using a validated Chinese version) before randomization and did so on 5 further occasions during the study while they were clinically stable, under the supervision of clinical staff well trained in questionnaire administration. In order to standardise the conditions under which the questionnaires were administered, a training session was organised for the trial coordinator in charge of instructing the patients.
Pulmonary function was assessed at the start, at 6 months and at the end of study. Post-bronchodilator spirometry was performed according to American Thoracic Society and European Respiratory Society (ATS/ERS) recommendations for acceptability and reproducibility (Citation26). FEV1 was measured on the test day in the morning, before and 20 minutes after inhalation of 400ug Salbutamol MDI via a spacer. Predicted FEV1 equations were selected from the European Committee of Coal and Steel (ECCS) predictions (Citation27) and adjusted for Chinese ethnicity according to recommendations by Zheng and Zhong (Citation28) to minimize variations due to ethnic differences, which have been published elsewhere5. Predicted FEV1 was calculated using the following formula (Citation28): Male: [–2.49 + 0.043 × height in cm–0.029 × age in year]×0.95; Female: [–2.60 + 0.040 × height in cm–0.025 × age in year] × 0.93. Forced vital capacity (FVC) and forced expiratory volume in 6 seconds (FEV6) were also measured for evaluation of lung volume change. No short-acting bronchodilator was allowed in the last 4 hours prior to spirometry; long-acting bronchodilators were not allowed in the last 12 hours.
Clinical safety profiles
All adverse events or concurrent illnesses that occurred during the study were to be documented in the CRF, focusing on gastric problems to assess the long-term safety of high doses.
Statistical analysis
Considerable attention was dedicated to the accuracy of sample size calculation during the planning of the trial, based on the currently available literature. In particular, reference was made to the BRONCUS study (Citation16) designed to compare NAC with placebo in treating COPD, but at a dose equivalent to only half the dose used in the present study, and to the PEACE Study (Citation5), where a significant reduction in exacerbation rate with carbocisteine compared to placebo (risk ratio: 0.75, p = 0.004) was found with 709 randomized patients. Consequently, based on the latter result, it was assumed that to detect a reduction of at least 20% in the yearly exacerbation rate, with a standard deviation of about 85% the placebo yearly rate, a Wilcoxon-Mann-Whitney rank-sum test at a 5% two-sided significance level will provide 95% power.
Yearly exacerbation rate is planned to be analyzed using a negative binomial regression model including, apart from ICS status, additional covariate candidates, such as COPD stages, smoking status age, BMI and concomitant medications: the final model is to be decided blind to treatment, on the basis of the covariate contribution to the model. Risk ratio (RR) and 95% confidence interval in the NAC group versus placebo group will be calculated. Regression models for recurrent data as well as the Cox proportional hazard model for the time to first episode will be used to analyze the occurrence of exacerbations. A mixed model for repeated measurements will be carried out for SGRQ scores, whereas spirometric assessments at 6 and 12 months will be analyzed using a covariance analysis (ANCOVA).
The primary efficacy analysis population is selected according to the intention-to-treat principle. In addition a per-protocol set is defined, before unblinding, excluding patients with protocol deviations that could influence the evaluation of efficacy. Adverse events, coded using the MedDRA dictionary, and other safety outcomes were summarized by treatment group.
Baseline characteristics
Recruitment was started on June 25, 2009 and completed on December 29, 2010. A total of 1297 patients were screened, among whom 1006 were randomized at 34 hospitals in China, which were academic hospital-based pulmonary clinics; 10 sites were located in northern China, 12 sites in eastern China, 7 sites in southern China and 5 sites in western China.
Baseline characteristics are summarized in Tables to . On average, participants were 66.27 ± 8.76 yrs old (range: 39 ∼ 81 yrs) at inclusion, and male patients accounted for 81.91%. They had a diagnosis of COPD made on average 92.68 ± 95.20 months earlier and had 3.50 ± 1.98 exacerbations (Range: 2 ∼ 24) during the last 2 years prior to the study; 76% of subjects were smokers or ex-smokers. Average post-bronchodilator FEV1 was 48.95±11.80 of predicted. Then, 444 patients (44%) were routinely treated with ICS or ICS plus LABA, and 562 (56%) were not on ICS treatment or regular ICS treatment.
Table 1. Baseline characteristics of the patients
Table 2. History of COPD
Table 3. Baseline lung function and bronchial dilation tests and SGRQ scores
Out of the 1006 patients randomized, approximately 46% were classified, by postbronchodilator FEV1, as being at GOLD stage II, 52% at GOLD stage III and less than 2% as GOLD stage IV. Therapies for COPD indication, taken occasionally or routinely, recorded during the last three months prior to entry, are shown in .
Table 4. COPD medications at entry and during the past 3 months
Discussion
COPD exacerbations result in significant morbidity and mortality, higher health care costs, poor quality of life and more rapid decline in lung function (Citation29). The importance of recognizing and managing COPD exacerbations has been emphasized in recent COPD guideline statements. The reduction in exacerbations was reported in many studies, such as the PEACE (Citation5) (carbocisteine 1500 mg/d vs placebo), ISOLDE (Fluticasone 1000 ug/d vs placebo) (Citation30) and UPLIFT (Tiotropium 18ug/d vs placebo) (Citation31), with the exception of patients treated concomitantly with NAC and ICS in the BRONCUS study (NAC 600mg/d vs placebo) (Citation16). On the other hand, the decline in lung function, such as FEV1, was explored also in BRONCUS, UPLIFT and many other COPD clinical trials, but none of these studies achieved positive results, indicating that such a decline in FEV1 is not a good indicator for evaluating long-term treatment efficacy. On the contrary, prevention of exacerbations as primary endpoint appears to be more suitable. That is why we selected COPD exacerbations as the primary endpoint in this study.
In the BRONCUS Study, NAC 600mg/d decreased the exacerbation rate significantly by 22% in patients with COPD without concomitant ICS treatment, but not in patients taking ICS (Citation16). As a dose-effect relationship was demonstrated by Gerrits et al. (Citation19) and Zuin et al. (Citation20), an increase in dose of NAC might achieve a better outcome with and without concomitant use of ICS. To test this hypothesis, both patients on ICS treatment and ICS treatment naïve status patients have been enrolled and stratified.
In our previous PEACE study (Citation5), although the exacerbation rates significantly decreased in patients treated with carbocisteine, time to first exacerbation did not differ from placebo. Further analysis of the recurrent exacerbations disclosed a significant difference between the treatment and placebo arms (Citation32), indicating that carbocisteine exerts long-term treatment effects that are particularly beneficial for patients with recurrent exacerbations. New insights into exacerbations were provided by the ECLIPSE Study (Citation33,34), which showed that a history of two or more exacerbations in the prior year is a relatively stable phenotype predictive of future events. All these results suggest that recurrent exacerbations should be considered an important outcome, which was therefore adopted in the present study.
Dose selection has been carefully considered. From the published data, 1200 mg/d of NAC is superior to the regular dose 600 mg/d in reducing C Reactive Protein, IL-8 levels and subjective difficulty in expectoration, as well as in shortening hospital stay. A higher dose, such as 1800 mg/d, might provide greater benefits, but considering cost and safety (acidity of NAC might result in gastric discomfort), we preferred to select 1200mg daily as the treatment dose.
It is important to underline that some baseline demographic details as well as the disease profile of patients in the PANTHEON study are similar to those of important COPD mega trials (TORCH and UPLIFT) and also to PEACE study. In this trial 24% of participants were non-smoking patients, which is similar to the percentage in the PEACE study (25%). Although smoking is the most important risk factor for COPD as it consists of abundant noxious, oxidant radical-rich gas, not all patients develop COPD because of smoking. In rural areas of developing countries like China, biomass fuel is also an important source of indoor air pollution Citation35. Therefore, non-smoking patients were also to be recruited in the present study because they might be exposed to other inhaled oxidant agents from the environment.
In our clinical experience, GOLD IV patients usually develop more complications and have poorer health status. Consequently, treatment compliance and the long-term follow up could be problems for those patients and they were not enrolled to guarantee the quality of the study.
As showed by Tashkin et al. (Citation36) and our previous finding (Citation37), FVC, representing volume change, responds to bronchodilator treatment better then FEV1, especially in terms of severe airflow limitation. Thus, FVC probably is a more accurate indicator of treatment response in COPD and was therefore selected, as well as FEV6, as efficacy variable in this study.
In conclusion, the hypothesis, design and methodology, and baseline characteristics of recruited patients of the PANTHEON Study are presented. Final results of this study will provide objective data on the efficacy and safety of high-dose NAC in the prevention of COPD exacerbations and on other outcome variables.
Declaration of Interests
The authors would like to acknowledge that funding, medication, as well as payment for travel and hotel to attend to investigator's meeting for this study were supplied by Hainan Zambon Pharmaceutical Co., Ltd. Authors had full access to all data and were involved in data interpretation and preparation of the manuscript in collaboration with the sponsor. Corresponding authors had final responsibility for the decision to submit for publication.
Appendix: Trial Organization
The study was managed by the Steering Committee, Quality Control Committee, Contract Research Organization and participating sites.
Steering Committee
This committee was responsible for the development of the protocol and took all the decisions related to relevant important changes to this study The committee consisted of the following members: Nan-Shan Zhong (Chairman), Jin-Ping Zheng, Jian Kang, Fuqiang Wen, Chunxue Bai, Wanzhen Yao and Lijun Ma.
Quality Control Committee for Spirometry
Prof. Jinping Zheng (Chairman) and Dr. Yi Gao were the members of the Quality Control Committee for spirometry. Prof. Zheng represented the Asian-Pacific Society of Respirology (APSR) in the steering committee of ERS global lung initiative task force for Lung function prediction. This committee focused on spirometer performance training and spirometry data quality evaluation at all study sites. Spirometry data had to be validated before the study started. Any issue related to spirometry was be addressed by this committee.
Contract Research Organization
Medkey (Shanghai) Med-Tec Co., Ltd, an independent Contract Research Organization (CRO), works as a third party in this study for study quality control, data collection and management, and statistical analysis.
Participating Sites
State Key Laboratory of Respiratory Disease, First Affiliated Hospital of Guangzhou Medical College, Guangzhou, China (Prof J-P Zheng MD, Prof N-S Zhong MD, Doc. Yi Gao); Guangzhou First Municipal People's Hospital, Guangzhou, China (Prof Z-W Zhao MD); The First Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China (Prof C-M Xie MD); The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China (Prof T-T Zhang MD); The First Affiliated Hospital, Chongqing Medical University, Chongqing, China (Prof S-L Guo MD); Daping Hospital, Research Institute of Surgery Third Military Medical University, Chongqing, China (Prof S-H Cui MD); Huaxi Hospital, Sichuan university, Chengdu, China (Prof F-Q Wen); Sichuan Provincial People's Hospital & Sichuan Academy of Medical Sciences, Chengdu, China (Prof Y-J Liiu MD); Zhongshan Hospital, Fudan University, Shanghai, China (Pro C-X Bai MD); Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China (Prof H-Y Wan MD); Shanghai First People's Hospital, Shanghai, China (Prof X Zhou MD); Changhai Hospital, Shanghai, China (Prof Q Li MD); Jiangsu Province Hospital, Nanjing, China (Prof M Huang MD); Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, China (Prof D-P Zhang MD); Guangdong General Hospital, Guangzhou, China (Prof L Chen MD); Hangzhou First People's Hospital, Hangzhou, China (Prof Z-Y Ren MD); Second Affiliated Hospital Zhejiang University School of Medicine, Hangzhou, China (Prof H-H Shen MD); Sir Run Run Shaw Hospital, Hangzhou, China (Prof K-J Ying MD); First Affiliated Hospital of Dalian Medical University, Dalian, China (Prof Z-H Zhang MD); Second Affiliated Hospital of Dalian Medical University, Dalian, China (Prof Z-S Wang MD); First Affiliated Hospital of China Medical University, ShenYang, China (Prof J Kang MD); Shenyang PLA General Hospital, Shenyang, China (Prof P Chen MD); the First Affiliated Hospital of Harbin Medical University, Harbin, China (Prof J-M Huo MD); Beijing ChaoYang Hospital, Beijing, China (Prof Y-X Lin MD); Beijing Hospital, Beijing, China (Prof T-Y Sun MD); Peking University Third Hospital(Prof W-Z Yao MD); China-Japan Friendship Hospital, Beijing, China (Prof J-T Lin MD); Tianjin General Hospital, Tianjin Medical, China (Prof B-Y Chen MD); Henan Provincial People's Hospital, Zhengzhou, China (Prof L-J Ma MD); Qingdao Municipal Hospital, Qingdao, China (Prof H-P Tang MD); The First Affiliated Hospital of Lanzhou University, Lanzhou, China (Prof X-J Liu MD); Xiangya Hospital of Central-South Hospital, Changsha, China (Prof C-P Hu MD); Hainan Provincial People's Hospital, Haikou, China (Prof Y-J Huang MD); Tianjin First Central Hospital, Tianjin, China (Prof P Jiang MD).
References
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011. Available from: http://www.goldcopd.org/.
- Poole PJ, Black PN. Preventing exacerbations of chronic bronchitis and COPD: therapeutic potential of mucolytic agents. Am J Respir Med 2003; 2(5):367–370.
- Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med 1996; 153(5):1530–1535.
- Wedzicha JA, Donaldson GC. Exacerbations of chronic obstructive pulmonary disease. Respir Care 2003; 48(12):1204–1213.
- Zheng J-P, Kang J, Huang S-G, Chen P, Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): A randomized placebo-controlled study. Lancet 2008; 371:2013–2018.
- Blackwell TS, Blackwell TR, Holden EP, Christman BW, Christman JW. In vivo antioxidant treatment suppresses nuclear factor-kappa B activation and neutrophilic lung inflammation. J Immunol 1996; 157(4):1630–1637.
- Lappas M, Permezel M, Rice GE. N-Acetyl-cysteine inhibits phospholipid metabolism, proinflammatory cytokine release, protease activity, and nuclear factor-kappaB deoxyribonucleic acid-binding activity in human fetal membranes in vitro. J Clin Endocrinol Metab 2003; 88(4):1723–1729.
- van Overveld FJ, Demkow U, Górecka D, de Backer WA, Zielinski J. New developments in the treatment of COPD: comparing the effects of inhaled corticosteroids and N-acetylcysteine. J Physiol Pharmacol 2005; 56 Suppl 4:135–142.
- Ekberg-Jansson A, Larson M, MacNee W, Tunek A, Wahlgren L, Wouters EF, Larsson S. N-isobutyrylcysteine, a donor of systemic thiols, does not reduce the exacerbation rate in chronic bronchitis. Eur Respir J 1999; 13:829–834.
- Sadowska AM, Manuel-Y-Keenoy B, De Backer WA. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: Discordant in vitro and in vivo dose-effects: A review. Pulm Pharmacol Ther 2007; 20:9–22.
- Rubio ML, Martin-Mosquero MC, Ortega M, Peces-Barba G, González-Mangado N. Oral N-acetylcysteine attenuates elastase-induced pulmonary emphysema in rats. Chest 2004; 125(4):1500–1506.
- Urban T, Akerlund B, Jarstrand C, Lindeke B. Neutrophil function and glutathione-peroxidase (GSH-px) activity in healthy individuals after treatment with N-acetyl-L-cysteine. Biomed Pharmacother 1997; 51(9):388–390.
- Riise GC, Qvarfordt I, Larsson S, Eliasson V, Andersson BA. Inhibitory effect of N-acetylcysteine on adherence of Streptococcus pneumoniae and Haemophilus influenzae to human oropharyngeal epithelial cells in vitro. Respiration 2000; 67(5):552–558.
- Mata M, Morcillo E, Gimeno C, Cortijo J. N-acetyl-L-cysteine (NAC) inhibit mucin synthesis and pro-inflammatory mediators in alveolar type II epithelial cells infected with influenza virus A and B and with respiratory syncytial virus (RSV). Biochem Pharmacol 2011; 82(5):548–555.
- Drost EM, Skwarski KM, Sauleda J, Soler N, Roca J, Agusti A, MacNee W. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax 2005; 60(4):293–300.
- Decramer M, Rutten-van Molken M, Dekhuijzen PNR, Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): A randomised placebo-controlled trial. Lancet 2005; 365:1552–1560.
- Dekhuijzen PNR. Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease. Eur Respir J 2004; 23:629–636.
- National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. London: National Clinical Guideline Centre. Available from: http://guidance.nice.org.uk/CG101/Guidance/pdf/English, 2010.
- Gerrits CMJM, Herings RMC, Laufkens HGM, N-Acetylcysteine reduces the risk of re-hospitalisation among patients with chronic obstructive pulmonary disease. Eur Respir J 2003; 21:795–798.
- Zuin R, Palamidese A, Negrin R, High dose N-Acetylcysteine in Patients with Exacerbations of Chronic Obstructive Pulmonary Diseases. Clin Drug Invest 2005; 25(6):401–408.
- Moher D, Schulz KF, Altman DG, The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001; 357:1191–1194.
- Declaration of Helsinki World Medical Association. Available from: http://www.wma.net/e/ethicsunit/helsinki.htm.
- International Conference on Harmonisation (ICH)—Good Clinical Practice (GCP). ICH GCP (E6): Adopted by CPMP, July 1996, issued as CPMP/ICH/135/95/Step5, Explanatory Note and Comments to the above, issued as CPMP/768/97.
- Anthonisen NR, Manfreda J, Warren CPW, Hershfield ES, Harding GKM, Nelson NA, Manitoba W. Antibiotic therapy in exacerbation of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106:196–204.
- Jones PW. Health status measurement in chronic obstructive pulmonary disease. Thorax 2001; 56:880–887.
- Crapo RO, Hankinson JL, Irvin C, American Thoracic Society. Standardization of spirometry—1994 update. Am J Respir Crit Care Med 1995; 152: 1107–1136.
- Quanjer PH. Standardized lung function testing. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993; 16:5–40.
- Zheng JP, Zhong NS. Normative values for pulmonary function testing in Chinese adults. Chin Med J 2002; 115:50–54.
- Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, Fabbri LM, Goldin JG, Jones PW, Macnee W, Make BJ, Rabe KF, Rennard SI, Sciurba FC, Silverman EK, Vestbo J, Washko GR, Wouters EF, Martinez FJ. Chronic obstructive pulmonary disease phenotypes: the future of COPD.Am J Respir Crit Care Med 2010; 182(5):598–604.
- Burge PS, Calverley PMA, Jones PW, Spencer S, Anderson JA, Maslem TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000; 320:1297–1303.
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M, for the UPLIFT Study Investigators. A 4-year trial of tiotropium in Chronic Obstructive Pulmonary Disease. N Engl J Med 2008; 359:1543–1554
- Zheng JP, Zhong NS, Jiang M, Carbocisteine for acute exacerbations of COPD — Authors’ reply. Lancet 2008; 372:1631–1632.
- Vestbo J, Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE). ERJ 2008; 31:869–873.
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, Calverley P, Rennard S, Wouters EF, Wedzicha JA; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363:1128–1138.
- Liu S, Zhou Y, Wang X, Wang D, Zheng J, Zhong N, Ran P, Biomass fuels are the probable risk factor for chronic obstructive pulmonary disease in rural South China. Thorax 2007; 62:889–897.
- Tashkin DP, Celli B, Decramer M, Liu D, Burkhart D, Cassino C, Kesten S. Bronchodilator responsiveness in patients with COPD. Eur Respir J 2008; 31: 742–750.
- Zhang F-Q, Zheng J-P, Wang J-H, Lu W-B, Wu R-X, Li X-S, Xian Q-Z, Gao H, Jiang M. Comparison of lung volume response with airflow response to bronchodilator in patients with chronic obstructive pulmonary disease. Zhong Hua Jie He Hu Xi Za Zhi (Chin J Tuberc Respir Dis) 2010; 33(2):109–113.