Abstract
Background: Exercise intolerance is a hallmark of chronic obstructive pulmonary disease (COPD) and forced expiratory volume in one second (FEV1) is the traditional metric used to define the severity of COPD. However, there is dissociation between FEV1 and exercise capacity in a large proportion of subjects with COPD. The aim of this study was to investigate whether other lung function parameters have an additive, predictive value for exercise capacity and whether this differs according to the COPD stage. Methods: Spirometry, body plethysmography and diffusing capacity for carbon monoxide (DLCO) were performed on 88 patients with COPD GOLD stages II-IV. Exercise capacity (EC) was determined in all subjects by symptom-limited, incremental cycle ergometer testing. Results: Significant relationships were found between EC and the majority of lung function parameters. DLCO, FEV1 and inspiratory capacity (IC) were found to be the best predictors of EC in a stepwise regression analysis explaining 72% of EC. These lung function parameters explained 76% of EC in GOLD II, 72% in GOLD III and 40% in GOLD IV. DLCO alone was the best predictor of exercise capacity in all GOLD stages. Conclusions: Diffusing capacity was the strongest predictor of exercise capacity in all subjects. In addition to FEV1, DLCO and IC provided a significantly higher predictive value regarding exercise capacity in COPD patients. This suggests that it is beneficial to add measurements of diffusing capacity and inspiratory capacity when clinically monitoring COPD patients.
Introduction
The burden of chronic obstructive lung disease (COPD) is predicted to increase excessively in the near future (Citation1). Impaired exercise capacity (EC) is a major component of COPD-related disability and exercise intolerance is a cardinal symptom of COPD, being ascribed to a complex interaction between ventilatory, cardiovascular and peripheral muscle abnormalities (Citation2, 3). The progression of respiratory impairment decreases the ability to carry out activities of daily living (ADL) and thus results in a declining quality of life (Citation4).
Although airflow obstruction, measured as decreased forced expired air volume during one second (FEV1), is a prominent pathophysiological feature of COPD and is the traditional lung function parameter used to define disease severity and disease progression (Citation5), it has been shown to be insufficient for predicting patients’ decreased physical capacity. Increasing evidence suggests that FEV1 explains only a part of the pulmonary limitation of the COPD patient's ability to carry out ADL or exercise (Citation6,7). In this context, other lung function parameters such as inspiratory capacity (IC) and static hyperinflation (IC/TLC) have been shown to be superior to traditional measures of airflow obstruction in predicting exercise impairment (Citation8).
Pulmonary gas exchange across the alveolar-capillary membrane, measured by carbon monoxide diffusion capacity (DLCO), provides quantitative and qualitative assessment of gas transfer in the lungs. Reduced DLCO in COPD reflects the alveolar-capillary membrane damage subsequent to emphysema (Citation9). Furthermore, low DLCO is shown to be an independent predictor of severe exacerbations in COPD (Citation10), and reduced DLCO along with air flow obstruction identifies a group of patients with significantly more symptoms (Citation11). In a retrospective analysis of 8,000 patients with pulmonary disease in 2001, low DLCO emerged as the strongest risk factor of oxygen desaturation during submaximal exercise tests (Citation12). Reduced DLCO appears to be associated with decreased EC in heavy smokers with no signs of fixed airflow obstruction (Citation13, 14), but this relationship has been scarcely studied in COPD patients (Citation15). Although impaired DLCO is well recognized in COPD patients and has been reported as a predictor of mortality (Citation16), no study, to our knowledge, has attempted to investigate the role of DLCO impairment on EC in relationship to disease severity.
The purpose of our study was to evaluate the relationship between exercise capacity, assessed by a standardized Incremental Cycling Test (ICT), and lung function parameters, obtained by spirometry, body plethysmography and DLCO, in patients with COPD in order to determine which lung function parameters have the best predictive value for exercise capacity. A secondary aim was to determine the predictive value of different lung function parameters for EC with regard to different stages of COPD severity.
Methods and Materials
Participants
A total of 88 subjects with moderate to very severe COPD (Citation17) were consecutively invited to take part in the study when being referred for physical training to the Physiotherapy Unit of the Respiratory Department of the University Hospital in Uppsala, Sweden (Citation18). Patient demographics are described in . All participants met the following criteria: (a) FEV1 < 80% predicted and FEV1/FVC ratio < 0.7 after bronchodilatation; (b) capable of undergoing exercise testing to peak effort; (c) in a non-acute phase of their disease and receiving a stable drug regimen. Participants had no coexisting medical conditions that would interfere with physiological testing, exercise or the ability to comprehend written or oral instructions. Exclusion criteria included the presence of pre-existing cardiac disease, claudication limiting exercise capacity, musculoskeletal problems (joint or neurological disorders preventing the patient from performing exercise testing on an ergometer bike), significant ST-T depression or cardiac arrhythmia upon exercise testing.
Table 1. Demographic characteristics and indices of pulmonary function for the COPD patients evaluated
Pulmonary function tests
Spirometry was performed using a Masterlab Trans Spirometer (Erich Jaeger AG, Würzburg, Germany). Lung volumes were obtained with a Masterlab Body Plethysmograph (Erich Jaeger AG, Würzburg, Germany). The normal values according to Hedenström et al. (Citation19, 20) were used. The predicted values for IC were obtained by subtracting predicted value for FRC from predicted value for TLC in each individual patient. DLCO was measured by the single-breath technique using Masterlab Transfer (Erich Jaeger AG, Würzburg, Germany). The corrected values for actual hemoglobin values were used in the calculations. All lung function testing was performed by highly experienced technicians following the standards outlined by ATS/ERS (Citation21–23). Post-bronchodilatory values were used in the calculations except for DLCO where pre-bronchodilatory values were used.
The lung function parameters of interest were: FEV1, vital capacity (VC), forced vital capacity (FVC) and IC from spirometry, total lung capacity (TLC), residual volume (RV), functional residual capacity (FRC), inspiratory fraction (IC/TLC) and RV/TLC ratio from lung volumes, and diffusing capacity corrected for hemoglobin level (DLCO).
Exercise test
All patients performed an ICT on an ergometer cycle (Case 8000 Exercise Testing System, GE Medical Systems, Milwaukee, USA) with continuous ECG registration to a symptom-limited peak work capacity. All patients discontinued all their bronchodilator medications one day prior to examination. Patients started pedaling at 20W and the load was increased by 10W every minute. Peak EC was calculated with regard to the number of seconds the patient endured at the last working load:
Statistical analysis
All statistics were generated using computer software programs (STATA 8.2, StataCorp and StatView 5.0.1, SAS Institute Inc). Means ± standard deviations (SDs) were used to present the descriptive statistics.
The patients were divided in tertiles according to exercise capacity and a trend analysis of lung function was performed across these groups, with lung function parameters as outcome and exercise capacity as predictor ().
Table 2. Subjects’ lung function parameters divided upon their exercise capacity in tertiles
Simple linear regression was used to analyze the correlation between different single lung function parameters and EC. A stepwise regression model was used to determine the most important predictors of EC, when using data from spirometry alone or from a combination of spirometry, body plethysmography and DLCO. Finally, simple and multiple linear regression models when using best predictors of EC from the above model (FEV1, IC and DLCO) were used to predict EC in different COPD severity stages. A P-value < 0.05 was considered as statistically significant.
Ethics
The trial was approved by the Medical Ethics Committee of Uppsala University, number 01-159, and all patients gave informed consent.
Results
Patients’ characteristics and lung function parameters are presented in . Lung function parameters are also presented in after dividing subjects in tertiles according to their EC. A significant trend of increasing DLCO, FEV1, IC, FVC and VC with increasing EC was found, yet a significant trend of decreasing TLC, RV, FRC and Raw with increasing EC was seen. The strongest correlation to EC was found with DLCO, followed by FEV1 and IC. The majority of the other lung function parameters were significantly associated with EC () with the exception of FRC and TLC.
Figure 1. Explanatory value (R2 values) from simple linear regression models of each of the lung function parameters (absolute values) for the patient's exercise capacity.
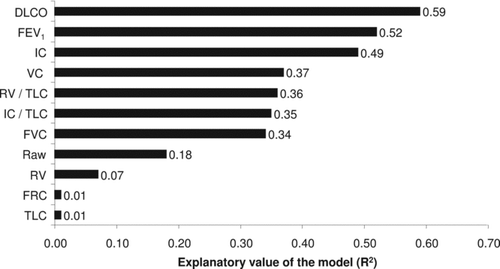
In a stepwise regression analysis where only data from spirometry was used, FEV1 and IC were the main determinants of EC with an explanatory value of 58% (). By adding DLCO data into the model, FEV1, IC and DLCO were the main determinants of EC with an explanatory value of 72%. None of the lung volumes obtained by body plethysmography contributed to the explanatory value of the model.
Table 3. Stepwise regression models of determinants of exercise capacity when using data from spirometry alone or from spirometry and diffusion capacity
The predictive value of FEV1 for EC is presented along with the additive information of IC and DLCO in a sub-analysis according to the GOLD stages (). In , the predictive value of each of the lung function parameters (FEV1, IC and DLCO) is presented for each GOLD stage as well as the relative contributions to predict EC in a multiple linear regression model. Overall, DLCO was the most consistent predictor of EC in each individual GOLD stage (, ). The additive information of IC and FEV1 in GOLD stages II and IV was minor.
Figure 2. The explanatory value of FEV1, FEV1+ IC, FEV1+ IC + DLCO for exercise capacity in all subjects, with subjects stratified according to GOLD class. Values presented as R2 values from the linear regression model.
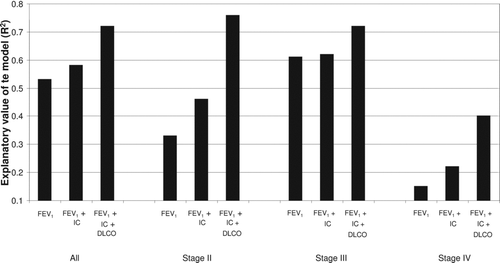
Figure 3. Correlation between FEV1 (left panel), IC(middle panel), DLCO (right panel) and exercise capacity in all study subjects (open circles GOLD 2, closed squares GOLD 3, open triangles GOLD 4),
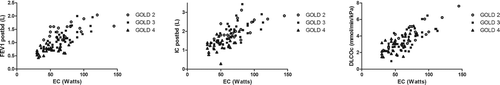
Table 4. Predictive value of FEV1, IC and DLCO for exercise capacity in each COPD GOLD stage in a simple regression model (left panel, R2 values) and multiple linear regression models (right panel)
Discussion
The major finding of this study was that DLCO was the strongest predictor of EC in COPD patients, followed by FEV1 and IC. Almost 80% of the variance of EC could be explained by combining information from all three lung function parameters. The explanatory value of DLCO for EC in COPD patients was additive to the explanatory value of spirometry measurements, and this value was most prominent in the moderate and very severe stages of the disease. The body plethysmography measurements had a low predictive value for the EC and did not offer any additional information.
The finding that the DLCO was the strongest predictor of exercise capacity independent of disease severity might reflect the fact that emphysema is a frequent finding in patients with COPD (Citation24). Shaker et al. reported that almost 90% of patients with COPD showed signs of emphysema in their CT scans regardless of disease severity (Citation25). Moreover, emphysema can precede the airway obstruction (Citation26). The extent of emphysema assessed by quantitative CT was poorly correlated to the decline of FEV1 and physiological parameters could only partly predict the extent of the emphysema. These findings suggest an underestimation of the magnitude of diminished diffusion surface in COPD patients. The impact of DLCO on maximal exercise has been investigated in a limited way in previous studies (Citation27,28). Tzani et al. (Citation27) studied heavy smokers with normal lung function and reported that DLCO could explain approximately 40% of the variance of their exercise capacity. In a recently published study, DLCO was also found to be an important predictor of 6-minute walking distance (6MWD) in COPD patients, explaining almost 25% of 6MWD (Citation15).
In our study a significant association was found between FEV1 and exercise capacity. Decreased FEV1 levels mirrored exercise intolerance in different stages of COPD and FEV1 could explain about 50% of the variance of EC in our material. These results are in accordance with those of the National Emphysema Treatment Trial where Brown et al reported that FEV1 could explain 66% of the variance of maximum EC (Citation29). However, a wide range of maximum exercise capacity was observed for any given FEV1, which illustrates the limited predictive value of FEV1 for EC in the individual patient.
Our results suggest that FEV1 alone is insufficient in assessing COPD patients, as differences in FEV1 in patients with moderate and very severe COPD had a relatively low explanatory value for exercise capacity. It could be argued that the narrow range of values of FEV1 in different stages of COPD limited to some extent the strength of the correlations between FEV1 and EC. However, the differences in the explanatory value of FEV1 and EC between different COPD stages should not have been affected as a similar spread of FEV1 values was seen among different COPD stages.
IC was the third important predictor of exercise capacity with an explanatory value of about 50%. Including IC in addition to FEV1 increased the explanatory value of the model by about 10% and this effect was more prominent in moderate COPD. Surprisingly, adding IC to FEV1 had no significant impact on predicting EC in patients with severe COPD. Previous studies have also reported correlations between inspiratory capacity and inspiratory fraction and EC in COPD patients (Citation8). Diaz et al. reported that IC was the major predictor of exercise capacity in individuals with expiratory flow limitation (Citation30). Dynamic hyperinflation has not been assessed in the present material. However, a recent study (Citation31) suggests that acute hyperinflation has little effect on exercise capacity and therefore chronic hyperinflation is a more important parameter in order to understand exercise limitation.
An important finding of our study was that almost 80% of the variance of exercise capacity could be explained by combining an airflow obstruction parameter (FEV1), a static hyperinflation parameter (IC) and a lung diffusion parameter (DLCO). In our study, the explanatory value of the investigated lung function parameters towards peak work rate was significantly higher in patients in stages II and III than in patients in stage IV. As far as we know, this relationship has not been reported in other studies. We believe that these results mirror the complexity of COPD in advanced stages. Patients with a heavier disease burden are more likely to exhibit systemic effects (Citation32) such as systemic inflammation, pulmonary hypertension, right ventricular failure, skeletal muscle dysfunction, weight loss and depression, all of which could have a high impact on exercise tolerance.
No significant relationship was found between lung volumes obtained by body plethysmography and exercise capacity. This reflects the results of some other studies. Owens et al reported that FEV1 and DLCO were significantly lower in patients with desaturation during exercise whereas FVC, FRC, TLC and RV were not significantly related to exercise-induced desaturation (Citation33). IC was one of the important predictors of exercise capacity in our study, but this parameter can also be measured by standard spirometry. Surprisingly, IC/TLC, a measure of hyperinflation previously reported to relate well to EC in COPD patients (Citation34), related less strongly than IC to exercise capacity in our material. However there were differences concerning patient demographics in our study compared to the study of Vassaux et al. (Citation34), with a predominance of female patients and less severe disease in our study.
A limitation of our study is the fact that we used the resting lung function parameters to predict EC, as it is suggested that increasing lung hyperinflation during exercise (dynamic hyperinflation) is more closely related to exercise intolerance and clinical dyspnoea than airflow limitation and static hyperinflation. The strength of our study is the standardized methodology with all the lung function and exercise testing parameters gathered by the same skilled research assistants in a clinical physiology laboratory setting. A selection bias cannot be excluded with regard to the dominance of women in our material. This reflects probably that women are referred for physical training to a greater extent than men. This preponderance of female subjects in our material adds more knowledge on peak EC in women with COPD as many previous studies concerning exercise ability in COPD patients have included mostly men (Citation35).
Conclusion
In conclusion, our findings indicate that the inclusion of DLCO and IC measurements along with the standard evaluation of FEV1 gives a more comprehensive assessment of patients with COPD. The DLCO measurements were shown to be the single best predictor of exercise capacity. These measurements are still performed in physiological laboratories and are not so easily accessible for the primary health care professional, but this might change with the appearance of newer, portable devices (Citation36). The routine use of this equipment and its cost efficiency remain to be investigated. On the other hand, IC, which also showed an additive value in predicting EC, is easily measured by a simple spirometry device (Citation37). The high predictive ability of these three pulmonary function parameters suggests that combining spirometry and DLCO measurements might be a good work tool in clinical practice to estimate exercise capacity in patients with moderate-to-severe COPD.
Declaration of Interest Statement
None of the authors has any conflict of interest to disclose. The authors are responsible for the content and the writing of this paper.
Acknowledgment
This study was supported financially by the Swedish Heart and Lung Foundation.
References
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370(9589):765–773.
- O'Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 158(5 Pt 1):1557–1565.
- Serres I, Gautier V, Varray A, Prefaut C. Impaired skeletal muscle endurance related to physical inactivity and altered lung function in COPD patients. Chest 1998; 113(4):900–905.
- Celli BR. Standards for the optimal management of COPD: a summary. Chest 1998; 113(4 Suppl):283S–287S.
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977; 1(6077):1645–1648.
- Cooper CB. Airflow obstruction and exercise. Respir Med 2009; 103(3):325–334.
- O'Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164(5):770–777.
- Albuquerque AL, Nery LE, Villaca DS, Inspiratory fraction and exercise impairment in COPD patients GOLD stages II-III. Eur Respir J 2006; 28(5):939–944.
- Morrison NJ, Abboud RT, Ramadan F, Comparison of single breath carbon monoxide diffusing capacity and pressure-volume curves in detecting emphysema. Am Rev Respir Dis 1989; 139(5):1179–1187.
- Balcells E, Anto JM, Gea J, Characteristics of patients admitted for the first time for COPD exacerbation. Respir Med 2009; 103(9):1293–1302.
- Garcia-Aymerich J, Serra I, Gomez FP, Physical activity and clinical and functional status in COPD. Chest 2009; 136(1):62–70.
- Hadeli KO, Siegel EM, Sherrill DL, Beck KC, Enright PL. Predictors of oxygen desaturation during submaximal exercise in 8,000 patients. Chest 2001; 120(1):88–92.
- Teixeira PJ, Costa CC, Berton DC, Versa G, Bertoletti O, Canterle DB. [Six-minute walk work is not correlated to the degree of airflow obstruction in patients with Chronic Obstructive Pulmonary Disease (COPD)]. Rev Port Pneumol 2006; 12(3):241–254.
- van Wetering CR, van Nooten FE, Mol SJ, Hoogendoorn M, Rutten-Van Molken MP, Schols AM. Systemic impairment in relation to disease burden in patients with moderate COPD eligible for a lifestyle program. Findings from the INTERCOM trial. Int J Chron Obstruct Pulmon Dis 2008; 3(3):443–451.
- Fujimoto H, Asai K, Watanabe T, Kanazawa H, Hirata K. Association of six-minute walk distance (6MWD) with resting pulmonary function in patients with chronic obstructive pulmonary disease (COPD). Osaka City Med J 2011; 57(1):21–29.
- Martinez FJ, Foster G, Curtis JL, Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 2006; 173(12):1326–1334.
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23(6):932–946.
- Arnardottir RH, Boman G, Larsson K, Hedenstrom H, Emtner M. Interval training compared with continuous training in patients with COPD. Respir Med 2007; 101(6):1196–1204.
- Hedenstrom H, Malmberg P, Agarwal K. Reference values for lung function tests in females. Regression equations with smoking variables. Bull Eur Physiopathol Respir 1985; 21(6):551–557.
- Hedenstrom H, Malmberg P, Fridriksson HV. Reference values for lung function tests in men: regression equations with smoking variables. Ups J Med Sci 1986; 91(3):299–310.
- Macintyre N, Crapo RO, Viegi G, Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005; 26(4):720–735.
- Miller MR, Hankinson J, Brusasco V, Standardisation of spirometry. Eur Respir J 2005; 26(2):319–338.
- Wanger J, Clausen JL, Coates A, Standardisation of the measurement of lung volumes. Eur Respir J 2005; 26(3):511–522.
- Saetta M, Kim WD, Izquierdo JL, Ghezzo H, Cosio MG. Extent of centrilobular and panacinar emphysema in smokers’ lungs: pathological and mechanical implications. Eur Respir J 1994; 7(4):664–671.
- Shaker SB, Stavngaard T, Hestad M, Bach KS, Tonnesen P, Dirksen A. The extent of emphysema in patients with COPD. Clin Respir J 2009; 3(1):15–21.
- Klein JS, Gamsu G, Webb WR, Golden JA, Muller NL. High-resolution CT diagnosis of emphysema in symptomatic patients with normal chest radiographs and isolated low diffusing capacity. Radiology 1992; 182(3):817–821.
- Tzani P, Aiello M, Colella M, Lung diffusion capacity can predict maximal exercise in apparently healthy heavy smokers. J Sport Sci Med 2008; 7(2):229–234.
- Nagelmann A, Tonnov A, Laks T, Sepper R, Prikk K. Lung dysfunction of chronic smokers with no signs of COPD. COPD 2011; 8(3):189–195.
- Brown CD, Benditt JO, Sciurba FC, Exercise testing in severe emphysema: association with quality of life and lung function. COPD 2008; 5(2):117–124.
- Diaz O, Villafranca C, Ghezzo H, Role of inspiratory capacity on exercise tolerance in COPD patients with and without tidal expiratory flow limitation at rest. Eur Respir J. 2000; 16(2):269–275.
- Guenette JA, Webb KA, O'Donnell DE. Does dynamic hyperinflation contribute to dyspnoea during exercise in patients with COPD? Eur Respir J 2012; 40(2):322–329.
- Agusti A, Soriano JB. COPD as a systemic disease. COPD 2008; 5(2):133–138.
- Owens GR, Rogers RM, Pennock BE, Levin D. The diffusing capacity as a predictor of arterial oxygen desaturation during exercise in patients with chronic obstructive pulmonary disease. N Engl J Med 1984; 310(19):1218–1221.
- Vassaux C, Torre-Bouscoulet L, Zeineldine S, Effects of hyperinflation on the oxygen pulse as a marker of cardiac performance in COPD. Eur Respir J 2008; 32(5):1275–1282.
- Carter R, Holiday DB, Nwasuruba C, Stocks J, Grothues C, Tiep B. 6-minute walk work for assessment of functional capacity in patients with COPD. Chest 2003; 123(5):1408–1415.
- Buess C, Lehnigk B, Magnussen H. Comparison of two systems for measuring the carbon monoxide diffusing capacity of the lung. Am J Respir Crit Care Med 2010; 181(1_MeetingAbstracts):A6489.
- Sterner JB, Morris MJ, Sill JM, Hayes JA. Inspiratory flow-volume curve evaluation for detecting upper airway disease. Respir Care 2009; 54(4):461–466.