Abstract
Background. Although occupational exposure is a known risk factor for Chronic Obstructive Pulmonary Disease (COPD), it is difficult to identify specific occupational contributors to COPD at the individual level to guide COPD prevention or for compensation. The aim of this study was to gain an understanding of how different expert clinicians attribute likely causation in COPD. Methods. Ten COPD experts and nine occupational lung disease experts assigned occupational contribution ratings to fifteen hypothetical cases of COPD with varying combinations of occupational and smoking exposures. Participants rated the cause of COPD as the percentage contribution to the overall attribution of disease for smoking, occupational exposures and other causes. Results. Increasing pack-years of tobacco smoking was associated with significantly decreased proportional occupational causation ratings. Increasing weighted occupational exposure was associated with increased occupational causation ratings by 0.28% per unit change. Expert background also contributed significantly to the proportion of occupational causation rated, with COPD experts rating on average a 9.4% greater proportion of occupational causation per case. Conclusion. Our findings support the notion that respiratory physicians are able to assign attribution to different sources of causation in COPD, taking into account both smoking and occupational histories. The recommendations on whether to continue to work in the same job also differ, the COPD experts being more likely to recommend change of work rather than change of work practice.
Keywords :
Introduction
COPD is a major cause of morbidity and mortality in the UK (Citation1) affecting up to 3.7 million people (Citation2). The World Health Organisation predicts that Chronic Obstructive Pulmonary Disease (COPD) will increase in the coming years and, by 2030, will become the third leading cause of death worldwide (Citation3). Traditionally, COPD has been thought of primarily as a smoking-related disease with limited scope for modifying the natural history of the disease, beyond smoking cessation, once it is established. Recently, these traditional assumptions have been challenged, as reflected in the UK Department of Health Outcomes Strategy for COPD (Citation4) and the related British Thoracic Society/National Institute for Clinical Excellence guidance (Citation1) and British Lung Foundation report (Citation2).
There has been an increasing recognition that factors other than smoking may also play a role in the causation of COPD, including potentially harmful inhaled occupational exposures, indoor and outdoor air pollution, and genetic factors (Citation5). Consistent estimates place 10–15% of the total burden of COPD as attributable to occupational exposures (Citation4,Citation6). Despite this general assessment of risk, it is extremely difficult to identify specific occupational contributors to COPD at the individual patient or worker level. Yet, decision regarding the contribution of the potential occupational agent(s) is important in COPD prevention, where occupational exposure reduction or elimination can mitigate adverse health effects.
Even in the arena of occupational asthma, a recent study identified considerable variation in diagnostic approaches among clinicians with an expertise in occupational lung disease (Citation7), and highlighted the need for a more unified diagnostic approach. The subsequent Standards of Care for occupational asthma reinforced these findings and emphasised greater diagnostic consistency in patients with suspected occupational asthma (Citation8). Indeed, there is growing interest in COPD developing as a result of longstanding asthma, although this has not been studied specifically in terms of occupational asthma overlapping with occupationally attributable COPD (Citation9).
The aim of this study, therefore, was to gain an understanding of how different expert clinicians attribute likely causation in COPD. Specifically, we wished to ascertain whether there were consistent differences in the pattern of attribution between COPD and occupational lung disease experts as two distinct groups of physician assessors.
Methods
Twelve COPD experts (COPD raters) and 12 occupational lung disease experts (OLD raters) were approached, the latter recruited from GORDS (the Group of Occupational Respiratory Disease Specialists (Citation10)).
Fifteen hypothetical cases of COPD were developed by four members of the study team (AD, CB, DF, PDB), intentionally including varying combinations of occupational and smoking exposures that might be differentially rated as causative in an individual COPD case. Consequently, based on the combination of tobacco and occupational contribution, cases could be assigned a priori into one of the 9 categories: tobacco smoking contribution was rated as low (0–14 pack-years), medium-moderate (15–49 pack-years) or very high (50 or more pack-years); and occupational exposure contribution was rated as low (0–10 years), medium-moderate (11–29 years) or very high (30 or more years). Occupational agents included in the study were those associated with increased risk of COPD based on current best evidence (Citation11,12,Citation13).
Each case scenario also contained information on demographics, medical history (including current symptoms and health care), health surveillance at work where applicable, detailed smoking history (including a pack-year summary estimate), previous medical history, relevant family history, medication use and allergies, and a detailed occupational history (stratified by job title, duration, and known exposures). Certain specific exposures thought to contribute to the risk of COPD development were included (e.g., silica exposure in Case 2). In addition, results of any relevant medical investigations were provided, including pulmonary function testing (FEV1 and FVC in all cases, and transfer factor, airway reactivity measures, serial peak flow recordings, SpO2, α1 anti-trypsin status (AAT), and radiology results, where appropriate). The full text of a case example (Case 11) is provided in Appendix 1.
Participants were asked to rate the cause of COPD in each case by assessing a percentage contribution to the overall attribution of disease in three categories: (i) smoking; (ii) occupational exposures; and (iii) other causes. Instructions were given that each category should be rated between 0–100%, such that each rated answer reflected the contribution that the factor in question had made to the presence and extent of currently diagnosed COPD. Participants were also provided the opportunity to make open-ended comments in relation to each case.
For a subset of cases (cases 11–15), longitudinal information was also supplied in the form of annual FEV1 values. Data supplied varied in each of these cases from between one to four previous values of FEV1. Two supplementary questions relating to annual decline were posed: (i) does the annual decline noted in FEV1 cause you concern? and (ii) given the FEV1 decline, what advice would you give the worker about continuing employment?
Data were analysed using SPSS v. 14.0.2 (SPSS Inc. 2006). Descriptive analyses included median rating and associated interquartile ranges (IQR) for the case attributions. The linear regression model was applied for predicting the rated occupational proportion in relation to the following explanatory (independent) variables: weighted occupational exposure, pack-years of smoking, and the type of rater (COPD expert compared to OLD expert).
The weighted occupational exposure was calculated as a product of years of employment in each recorded job multiplied by a factor for likely intensity of general vapour, gas, dusts, and fume (VDGF) exposures. The VGDF-associated factors used were developed and assigned by two of the study team (DF and PDB) and set at zero for no likely exposure, 1 for moderate exposure, and 2 for heavy exposure. Thus, for example, 5 years’ exposure at a job with no likely exposure and 15 years at a job with heavy exposure would yield a summary value of 30. Finally, the open-ended comments made by the raters were summarized and specific emergent themes were identified and documented. This study obtained ethics and local research and development office approval.
Results
Nine of the 12 occupational lung disease specialists approached participated in this study, as did 10 of the 12 COPD experts, representing an overall response of 79%.
The responses for the attribution of COPD causation for cases 1–15 are shown in . Overall, median ratings for each category varied widely. In general terms, the "Periodical" risk category option was rated only as an exception, as in the single case including information on alpha 1 anti-trypsin deficiency phenotype (PiMZ, case 6), where a median level of 65% causation to that genetic factor was attributed by the raters.
Table 1. Attribution of causation by Physician Case Raters
Smoking was identified as consistently the strongest contributor to COPD causation, this effect persisting even among cases with a relatively light smoking history and either a medium or low occupational exposure history. Only in the cases of light smoking and high risk and duration occupational exposures (cases 2 and 13) did the median ratings for occupational COPD contribution exceed those for smoking.
General comments relating to cases 1–10 were divided into those pertaining to (i) clinical assessment, (ii) exposure assessments, and (iii) assessing differential causation.
(i) Clinical assessment: These comments related to the need of further investigations to assist making a diagnosis, mostly relating to serial PEF values to exclude the possibility of asthma. Certain raters relied on CT findings (for example lack of emphysema on CT scan) to support an increased likelihood for an occupational causation.
(ii) Exposure assessments: These comments related to the need to better understand the effect of the occupational exposures, in particular specific exposures to silica, cadmium, and welding fume noted in selected case scenarios.
(iii) Assessing differential causation: These comments related to the difficulties in applying relative weightings for causation in the face of an uncertain evidence base, in particular, some raters approaching effects of smoking and occupation as additive and others as multiplicatively interactive.
For cases 11-15, the annual decline data represented in each case is shown below, and a summary of associated responses and comments are shown for each case. A summary of responses is also shown in .
Table 2. Annual decline in lung function (FEV1), rated concern level, and workplace advice for cases 11—15
Case 11
The FEV1 decline shown in this case represented 380 ml loss over 4 years (95 ml/y), in a 50-year-old male, smoking, asymptomatic, wood-exposed worker. Seventeen of 19 raters expressed concern over this level of decline, yet one rater (OLD) felt this was acceptable and within normal limits for decline. One rater (OLD) did not respond to this question. Comments related to prevention included the need for serial PEF measurements to exclude occupational asthma, the role of health surveillance, and the priorities for controlling workplace exposures using standard hierarchy of control measures (e.g., local exhaust ventilation being preferred to efficiently maintained personal protective equipment).
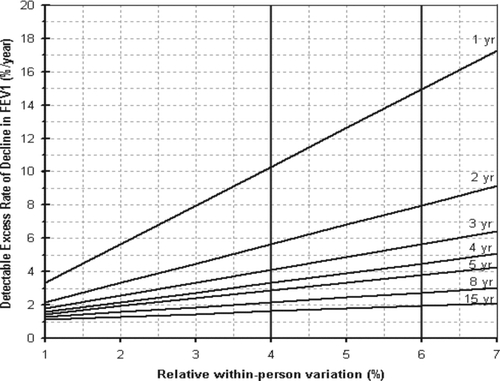
In terms of advice to the worker, responses varied from smoking cessation alone (although with “further (lung function) loss may need to stop work”) through to “not to continue without appropriate workplace protection.” This case represents a decline in FEV1 of 4.13% of baseline per year. Generally, it is assumed that the expected within-person variation is approximately 4% when the spirometry data in a monitoring program is of good quality (Citation14). denotes the detectable excess rate of decline in FEV1 as a percentage of the baseline per year, in relation to the expected with-person variation in a monitoring program, as derived from previous work (Citation15). The corresponding minimum detectable relative decline per year over 4 years is 3.5%, which indicated that this case met the threshold for a significant excess decline.
Figure 1. Detectable excess rate of decline in FEV1 (%/year) by relative within-person variation and duration of follow-up (1–15 years). Indicated are two recommended limits (two vertical lines) based on monitoring programs with good data quality (within-person variation of 4%) and by the ACOEM (within person variation of 6%). FEV1 = forced expiratory volume in 1 second).
Case 12
The FEV1 decline shown in this case represented 500 ml loss over 3 years (167 ml/y), in a 42-year-old, male, smoking collier. Of 19 raters, 18 expressed concern at this level of FEV1 decline (the lone outlier was a COPD rater). One rater suggested further investigation for asthma, as this could have been the explanation for discrepancy in the rate of decline of FEV1 between first 2 years. Once again, the requirement for health surveillance, possible redeployment within the workplace to a lesser-exposed environment, and the need to control inhaled workplace exposures were mentioned by the raters.
In terms of advice to the worker, 3 raters (1 OLD and 2 COPD) suggested stopping work, with 2 noting that, as the worker had just stopped smoking, continued spirometry to assess future FEV1 decline was warranted. This case represents a decline in FEV1 of 4.9% of baseline per year. shows a corresponding detectable relative decline per year over 3 years of 4%, assuming within-person variation of 4%, which indicated that this case met the threshold for a significant excess decline.
Case 13
The FEV1 decline shown in this case represented 400 ml loss over 1 year, in a 50-year-old, male, smoking fettler (tasks involving manual grinding of metal casts to create a smooth finish using a rapidly rotating abrasive disc) with radiographic evidence of small opacities. There were 17 raters who expressed concern relating to this level of decline, one OLD rater undecided (citing the rationale that only one previous estimate of FEV1 was available) and one COPD rater did not respond to this question. Open-ended comments expressed the need for additional FEV1 measures, smoking cessation, and the priority of adequately reducing workplace inhaled exposures.
One rater specifically noted that silica exposure should be reduced or avoided altogether, going on to state that if further lung function decline was manifested, redeployment to a lower exposed task would be essential. Other raters also alluded to silica exposure cessation or stopping present employment altogether. Monitoring further lung function decline and introducing respiratory protective equipment were also cited. This case represents a decline in FEV1 of 16% of baseline over the year. notes a corresponding minimum statistically significant detectable relative decline per year over one year of 10%, assuming program within-person variation of 4%, which indicated that this case met the threshold for a significant excess decline
Case 14
The FEV1 decline shown in this case represented 420 ml loss over 4 years (105 ml/y), in a 57 year-old male, currently smoking, welder and metal plater. He was also noted to have ischaemic heart disease and a CT demonstrating a single calcified pleural plaque. There were 14 raters who expressed concern at this level of decline, yet 4 did not feel this was of concern (three COPD, one OLD rater) and one non-responder (a COPD rater). Specific comments referred to smoking cessation, usage of personal respiratory protection, and either changing employment, stopping employment, or remaining at work with appropriate health surveillance. One rater specifically noted that the decline was within the 95% confidence interval of normality, sanctioning continued exposure if under careful surveillance. This case represents a decline in FEV1 of 3.4% of baseline per year. notes a corresponding minimum detectable relative excess decline per year over 4 year of 3.5%, assuming program's within-person variation of 4%, which indicated that this case did not meet the threshold for a statistically significant excess decline.
Case 15
The FEV1 decline shown in this case represented 300 ml loss over 2 years (150 ml/y), in a 38 year-old male, currently smoking baker with ongoing flour exposure and radiological evidence of hyperinflation. Of 19 raters, 16 expressed concern with this level of decline while 3 (OLD raters) did not feel this was of concern. Specific comments included smoking cessation, the use of respiratory protective equipment, and continuing but more vigilant health surveillance. One rater felt that, as the worker had no current symptoms, it was “reasonable to discuss continued work with smoking cessation and (health) surveillance.” One rater noted that the FEV1 decline was well within the 95% confidence interval, and therefore no action was required. Two COPD raters suggested stopping work, with one OLD rater admitted to being unsure as to the correct advice concerning future employment. This case represents a decline in FEV1 of 5% from baseline per year. shows a corresponding minimum detectable relative excess decline per year over 2 years of 6%, which indicated that this case did not meet the threshold for a statistically significant excess decline.
Results of the linear regression analysis () show that increasing pack-years of tobacco smoking was associated with significantly decreased proportional occupational causation ratings (–0.52% attribution per pack year, a fall in 10.4% with 20 pack years, occupational exposure held constant). Increasing weighted occupational exposure was associated with increased occupational causation ratings by 0.28% per unit change (that is, a rise in proportional attribution of 11.2% for 20 years exposure at the highest COPD likelihood job, smoking exposure held constant). Expert background (COPD in comparison to OLD) also contributed significantly to the proportion of occupational causation rated, with COPD experts rating on average a 9.4% greater proportion of occupational causation per case than the OLD experts, smoking and occupational factors taken into account.
Table 3. Estimates from the linear regression analysis for predicting the proportion of occupational causation rated (%) in each case
Discussion
This study has, for the first time, assessed attribution of causation in individual cases of COPD based on clinical case scenarios designed a priori to assess this. Whilst there was variability in the relative attributions assigned, raters appeared to be assessing the relative occupational contribution, in part, as an inverse function of the contribution of smoking. Nonetheless, the intensity and duration of likely harmful occupational exposures was also taken into account. Indeed, the overall weighting of 20 pack-years of smoking compared to 20 years of high-risk VGDF exposure was remarkably similar. Of particular note, the background expertise of the rater (COPD versus OLD) also affected the size of the occupational contribution rated. Contrary to what might be expected, the COPD experts assigned higher work-related attributions, on average 9% greater than the occupational exposures.
Advice relating to the annual decline in FEV1 appeared to differ among experts. Although most of the physician raters deemed declines excessive (3 cases actually demonstrated statistically significant excess decline (Cases 11–13) and 2 were non-significant (Cases 14 and 15), and were concerned about prevention of occupational exposure, rater type did appear to influence the advice given relating to work. The reasons for these differences are difficult to identify from this study design, although presumably certain experts will estimate “significance” based on clinical empirical criteria, whereas others might adopt a mathematical approach driven wholly by statistical significance.
There are limitations to this study. This was a small, convenience sample and, although the response rate of those approached was acceptable, the highly specialised nature of their backgrounds (either COPD or occupational respiratory hospital based experts) makes the generalisation of these findings more difficult to wider populations of health care professionals dealing with COPD patients. Even within each specialty group, there is significant variation of practice type. Nevertheless, it was appropriate to investigate the views among a clinical group closest to the issues studied, since it is to these or similar sub-specialist that COPD cases with a potential occupational contribution are most likely to be referred for consultation.
The use of narrative, clinical case scenarios also introduces potential difficulties. For example, open-ended comments made by the raters related specifically to the difficulty posed by interpreting the relatively limited information provided and the lack of a substantial enough body of evidence to support any conclusion. In a sense, this was the specific reasoning behind carrying this process out, since the use of carefully constructed cases allowed assessment over of a range of differentially weighted causation likelihood combining smoking and occupational factors.
The use of perhaps should be recommended more generally to identify the critical rates of decline in percentage of the baseline FEV1 per year, over the first 7 years of follow-up. For example, when spirometry is known to be of good quality, an average within-person variation of 4% can be assumed, then critical rates of decline per year are, respectively, ≈10%, 6%, 4%, 3.5%, and 3%, for a follow-up of between 1 to 5 years. When spirometry quality is known to be varied, a limit based on a relative within-person variation of 6%, as recommended by the American College of Occupational and Environmental Medicine (Citation16, 17) is more suitable, corresponding to critical rates of decline/year of respectively ≈15%, 8%, 6%, 4.5%, and 4%. The rate of decline and its statistical significance can provide additional information to be considered in conjunction with the level of lung function in decision-making for tertiary prevention, although it is unlikely to play a role in causal attribution. Absolute levels of decline can also be used to identify excess annual FEV1 losses, although not the focus of the ACOEM recommendations.
The routine use of SPIROLA (Spirometry Longitudinal Data Analysis) software, or similar statistical methods of interpreting serial changes in FEV1, should reduce the misinterpretation of serial measurements of lung function often carried out in different places and on different spirometers over time. SPIROLA is a software package with a visual and quantitative tool intended to assist the health care provider in monitoring and interpreting computerised longitudinal spirometry data for individuals, as well as for a group of individuals (Citation18).
Our findings support the notion that respiratory physicians are able to assign attribution to different sources of causation in COPD, taking into account both smoking and occupational histories. The recommendations on whether to continue to work in the same job also differ between rater type, the COPD experts being more likely recommending change of work rather than change of work practice.
How these findings relate to the routine clinical practice of such sub specialists is not known, nor how this might play into referral patterns by generalists. Further work is needed to develop useful working definitions of occupational COPD that can apply in diagnostic and preventative activities.
Declaration of Interest Statement
This study was funded by the Health and Safety Executive (HSE).
There are no competing interests declared by; DF, JS, CB, RB, RAL, PC, SB, TP, CBo, NH, PB, EH, RN, AD and CW. PR undertakes medico legal work. KP has undertaken expert witness work in this field for 30 years. JH has received lecture fees and sponsorship for conference attendance from Boehringer-Ingelheim.
The authors are responsible for the content and the writing of this paper.
Acknowledgments
This work was made possible by the kind support of the Kirkwood Memorial Trust. The authors also would like to thank Professor Jon Ayres for his invaluable input to this work. NSH was supported by the NIHR Respiratory Biomedical Research Unit at Royal Brompton and Harefield NHS Foundation Trust and Imperial College London.
The authors would also like to acknowledge the assistance of Professor Moira Whyte, Professor Bill MacNee, Dr. David Singh and Dr. Chris Stenton.
References
- National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease. Management of chronic obstructive pulmonary disease in adults in primary and secondary care. http://www.nice.org.uk/nicemedia/live/13029/49397/49397.pdf (last accessed 29.11.2012).
- As written already plus; British Lung Foundation 2007. Invisible Lives. COPD - Finding the missing Millions British Lung Foundation http://www.lunguk.org/Resources/British%20Lung%20Foundation/Migrated%20Resources/Documents/I/Invisible%20Lives%20report.pdf.
- Global Surveillance, Prevention and Control of Chronic respiratory Diseases. A Comprehensive Approach. WHO 2007. COPD http://www.who.int/respiratory/copd/en/
- American Thoracic Society. American Thoracic Society statement: Occupational contribution to the burden of airway disease. Am J Respir Crit Care Med 2003; 67:787–797.
- Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, Romieu I, Silverman EK, Balmes JR; Committee on Nonsmoking COPD, Environmental and Occupational Health Assembly. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010 Sep 1;182(5):693–718.
- Trupin L, Earnest G, San Pedro M, Balmes JR, Eisner MD, Yelin E, Katz PP, Blanc PD. The occupational burden of chronic obstructive pulmonary disease. Eur Resp J 2003; 22:462–469.
- Fishwick D, Bradshaw L, Henson M, Occupational asthma: an assessment of diagnostic agreement between physicians. Occup Environ Med 2007; 64:185–190.
- Fishwick D, Barber CM, Bradshaw LM, British Thoracic Society Standards of Care Subcommittee Guidelines on Occupational Asthma. Standards of care for occupational asthma. Thorax 2008; 3:240–250.
- Kim SR, Rhee YK. Overlap between asthma and COPD: where the two diseases converge. Allergy Asthma Immunol Res. 2010; 2:209–214.
- Group of Occupational Respiratory Disease Specialists (GORDS) website; http://www.hsl.gov.uk/centres-of-excellence/centre-for-workplace-health/gords.aspx last accessed July 2011.
- Blanc PD, Menezes AM, Plana E, Mannino DM, Hallal PC, Toren K, Eisner MD, Zock JP. Occupational exposures and COPD: an ecological analysis of international data. Eur Respir J 2009; 33(2):298–304. Epub 2008 Nov 14.
- Blanc PD, Iribarren C, Trupin L, Earnest G, Katz PP, Balmes J, Sidney S, Eisner MD. Occupational exposures and the risk of COPD: dusty trades revisited. Thorax 2009; 64(1):6–12. Epub 2008 Aug 4.
- Bang KM, Syamlal G, Mazurek JM. Prevalence of chronic obstructive pulmonary disease in the U.S. working population: an analysis of data from the 1997–2004 National Health Interview Survey. COPD 2009; 6(5):380–387.
- Hnizdo E, Hakobyan A, Fleming J, Beeckman-Wagner L. Spirometry-based medical monitoring: spirometry quality vs. data precision. Journal of Occupational and Environmental Medicine 2011; 53:1205–1209.
- Hnizdo E, Glindmeyer HW, Petsonk EL. Workplace spirometry monitoring for respiratory disease prevention: a methods review. Int J Tuberc Lung Dis 2010; 14(7):796–805.
- American College of Occupational and Environmental Medicine, Occupational and Environmental Lung Disorder Committee. ACOEM Position Statement: spirometry in the occupational health setting—2010 update. Evaluating results over time. Elk Grove Village, IL, USA: ACOEM, 2010. www.acoem.org/guidelines/article.aspx?ID = 756 Accessed July 2011.
- Townsend MC. Evaluating pulmonary function change over time in the occupational setting. ACOEM evidence-based statement. J Occup Environ Med 2005; 47:1307–1316.
- SPIROLA. Spirometry Longitudinal Data Analysis. http://www.acdc.gov/niosh/topics/spirometry/spirola-software.html.