Abstract
Objective: Patients with chronic obstructive pulmonary disease (COPD) often suffer from systemic co-morbidities, including anemia. However, anemia is related to multiple outcomes in COPD and in other chronic diseases, but it's impact is underestimated in COPD. The objective of the present study was to relate anemia in patients with COPD with disease-related outcomes, systemic inflammation and COPD related co-morbidities. Methods: Data of 321 patients with COPD admitted for pulmonary rehabilitation were analysed. Besides general characteristics, lung function, body composition, arterial gases and plasma haemoglobin concentration, disease-related outcomes (health-related quality of life by St. George's Respiratory Questionnaire, 6-minute walking distance, mMRC dyspnea scale, and BODE index), systemic inflammation (C-reactive protein (CRP)) and self-reported and objectified co-morbidities (low muscle mass, osteoporosis, renal failure, risk for undernutrition) were taken into account. Results: First, 20% of the patients were anemic, and 8% was polycythemic. Polycythemic patients had a lower proportion of men and a lower proportion of low muscle mass compared to the other groups. Anemic patients had higher plasma CRP levels and lower total body bone mineral density compared to the other groups. There was no difference in disease-related outcomes or other co-morbidities in the patients with and without anemia. Even after adjustment for confounders, anemia was an independent determinant for higher CRP levels and lower bone mineral density. Conclusion: Anemia is frequently present in patients with COPD and there is evidence that it is associated with lower whole body bone mineral density.
Introduction
Chronic obstructive pulmonary disease (COPD) is primary a respiratory disease caused by inhalation of noxious gases especially cigarette smoke (Citation1). Currently, COPD is projected to rank fifth in 2020 in the burden of disease worldwide (Citation2), and there is growing awareness that COPD is associated with other clinically relevant co-morbidities including cardio-vascular disease (Citation3), renal failure (Citation4) and osteoporosis (Citation5). There is growing interest in the interrelation between these co-morbidities and their effect on the outcome and prognosis in COPD (Citation6). As the prevalence of COPD and its co-morbidities rises with the older getting population, the contribution of co-morbidities will increase in the disease course in the coming years (Citation7).
As one of these co-morbidities, anemia is an easy to measure, treatable but underestimated co-morbidity in COPD (Citation8, 9). In theory, one would expect higher haemoglobin levels in patients with COPD as a compensatory mechanism for hypoxic episodes. Conflicting with this, research showed a prevalence of anemia of Citation13 to Citation20% in patients with COPD, depending on the applied definition of anemia and the population studied (Citation10–12). Furthermore, plasma haemoglobin concentration has been inversely associated with pro-inflammatory cytokines in COPD, which suggests that anemia is related to the chronic low-grade systemic inflammation in COPD (Citation11, Citation13). This is also described in other chronic inflammatory diseases, like renal failure, chronic heart disease and cancer (Citation12).
In COPD, anemia has been linked to health status (Citation14), exercise intolerance, degree of dyspnea (Citation15), hospital readmission rate (Citation16) and mortality (Citation17, 18). The presence of anemia was independent of other co-morbidities, but some studies showed lower body mass index in anemic patients (Citation19). Polycythemia on the other side does not seem to contribute to COPD related outcomes, and was only present in a small percentage of the patients (Citation15).
Recently, the contribution of anemia to COPD related co-morbidity has been hypothesized. As such, it has been suggested that anemia could be a result of the high prevalence of renal failure in COPD, as a consequence of lower synthesis of erythropoietin in the kidneys (Citation4). In addition, a relation between anemia and bone mineral density has been identified recently in postmenopausal Turkish women (Citation20). The contribution of anemia in the systemic manifestation of COPD is not investigated yet.
The aim of the present study was to investigate the clinical impact of anemia in patients with COPD admitted for a pulmonary rehabilitation. For this purpose, disease-related outcomes, systemic inflammation and objective measured co-morbidities were analysed and related to plasma haemoglobin concentration.
Methods
Data were collected in 321 subjects with COPD (182 men) who were screened for pulmonary rehabilitation at CIRO+, Centre of expertise for chronic organ failure, the Netherlands (Citation21). The inclusion criteria were: Caucasian race, aged 40 years or older and moderate to very severe COPD according to the ATS/ERS guidelines (Citation22). Because of the use of de-identified and pre-existing data, our retrospective study was institutional review board exempt.
Measurements
The following tests were performed as part of the assessment for entering pulmonary rehabilitation. Lung function parameters (post-bronchodilator forced expiratory volume in the first second, FEV1; forced vital capacity, FVC) were collected using standardized spirometry (Masterlab®, Viasys, Germany). Diffusion capacity of carbon monoxide (DLCO) was assessed by using single-breath method (Masterlab®, Jaeger, Germany). All values were expressed as percentages of the predicted value (Citation23). Arterial oxygen pressure (PaO2) and arterial carbon dioxide pressure (PaCO2) were determined in a radial artery blood sample while breathing room air. Modified Medical Research Council (mMRC) dyspnoea scale was used to assess dyspnoea perception, and cumulative smoking exposure was assessed as number of pack-years (PY). Use of long-term oxygen therapy (LTOT) was recorded. The 6-minute walking distance (6MWD), including a practice walk, was determined to assess functional exercise capacity. The test was performed twice and the highest distance was recorded for analysis (Citation24). In addition, blood saturation level was monitored before and during the 6MWD to record the lowest saturation levels. Disease specific health status was evaluated using the St.-George's Respiratory Questionnaire (SGRQ) score (Citation25).
A total body scan was performed by dual-energy x-ray absorptiometry (DEXA) using a Lunar Prodigy® system (GE Healthcare, Madison, WI, USA) for measuring the prevalence of osteoporosis and assessing body composition. Bone mineral density was measured at the hip and the lumbar spine (L1-L4). Diagnosis of osteoporosis was based on the lowest T-score of the hip and lumbar spine as defined according to the World Health Organization (WHO): osteoporosis: T-score ≤ –2.5; osteopenia: T-score between -1.0 and –2.5; and normal bone tissue: T-score ≥ –1.0 (Citation26). Body composition (BMI, fat free mass index ((FFMI) = fat free mass (lean mass + bone mineral density)/length2 was assessed from the total body DEXA scan. Low muscle mass was defined as FFMI <17 kg/m2 for men and FFMI <15 kg/m2 for women (Citation27). The BODE index was calculated by using the body mass index, airflow obstruction (FEV1), dyspnea (mMRC) and exercise capacity (6MWD)(Citation28). Risk for undernutrition was assessed by using the Short Nutritional Assessment questionnaire (SNAQ) (Citation29). Co-morbidity was assessed by a standard questionnaire.
Biochemical measurements
Serum C-reactive protein (CRP) was assessed in duplicate samples by high-sensitivity particle-enhanced immunoassay (COBAS Mira®, Radiometer, Copenhagen). Serum creatinine was assessed by the ABX Pentra 400® (Horiba Medical) for routine clinical tests, and plasma haemoglobin (Hb) and mean corpuscular volume (MCV) were analysed by the Cobas Micros® (Horiba Medical). To assess renal function, glomerular filtration rate (GFR) was estimated using the Cockcroft-Gault formula (Citation30). Renal function was classified as ‘normal’ if the GFR ≥ 60 ml/min, ‘concealed’ with normal serum creatinine concentration (<1.04 mg/dl for women and < 1.26 mg/dl for men) and ‘overt’ with increased creatinine concentration and GFR< 60 ml/min (Citation4). Anemia was defined according to the WHO: plasma Hb concentration <12 g/dl for women and Hb <13 g/dl for men (Citation31); and polycythemia is defined as plasma Hb concentration >15 g/dl for women and >17 g/dl for men (Citation15).
Statistical analysis
Data are described as mean ± standard deviation (SD), checked for normality and if necessary, log transformed (CRP). Comparison between groups was done by the analysis of variance test and the post-hoc LSD test, or the Chi2-test for categorical variables. Univariate and multivariate linear regression analysis was performed to test whether anemia or polycythemia are independent determinants for mMRC, CRP and total body BMD. Binary logistic regression analysis was performed to test whether anemia or polycythemia are independently associated with the prevalence of low muscle mass. Analyses were performed using Statistical Package for the Social Sciences (SPSS) version 20 for Windows®.
Results
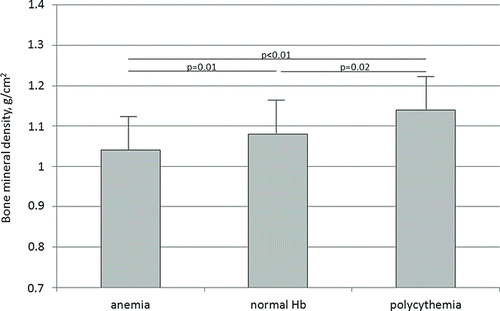
The study sample consisted of subjects with moderate-to-very-severe COPD, with normal blood gases and normal body composition but elevated plasma CRP levels (). First, 20% of the subjects were anemic, 8% of the subjects were polycythemic. There were no significant differences in age, lung function, arterial blood gases, number of pack-years, body composition and T-scores between the groups after stratification for plasma haemoglobin concentration. The group with polycythemic subjects included more women, while anemic subjects were more men. Moreover, total body bone mineral density was lower in the anemic group compared to the groups with normal and elevated Hb levels ().
Table 1. General characteristics of the total study group and after stratification for anemia
Furthermore, SGRQ scores, the 6MWD and mMRC scores were not different between groups. There was no difference in self-reported co-morbidity among the groups (). Concerning the biochemical parameters, plasma creatinine concentration was highest in the anemic patients but there was no difference in GFR between the groups. Plasma CRP concentration was significantly higher in the anemic subjects. MCV was not different between the groups. Of all subjects, 35% had low muscle mass, 31% had osteoporosis and 50% had osteopenia (). In addition, 25% of the total population had overt renal failure and 15% of the subject were at risk for developing undernutrition assessed by the SNAQ questionnaire. There were no differences among the prevalence of objectified co-morbidities, except for the prevalence of low muscle mass which was lowest in the polycythemic subjects but highest in the group with normal Hb concentration.
Table 2. Prevalence of objectively diagnosed co-morbidities and risk for undernutrition
In the univariate regression model, age, FEV1, BMI and plasma haemoglobin concentration were significantly associated with mMRC score, CRP and total body BMD. PaO2 is included in the model as we intended to investigate the effect of anaemia independent on hypoxia. In the multivariate regression analysis, anemia was an independent determinant for CRP and total body BMD, but not for mMRC (). In addition, there was a trend of an association of anemia with lower percentage of low muscle mass (p = 0.08, ).
Table 3. Association of anemia or polycythemia with mMRC, CRP and bone mineral density after adjustment for confounders
Table 4. Association of anemia or polycythemia with low muscle mass after adjusted for confounders
Discussion
The present study showed a prevalence of anemia in 20% of the COPD patients admitted for pulmonary rehabilitation, while the presence of polycythemia was 8%. Our data confirm other data that there is an inverse association between plasma haemoglobin concentration and plasma CRP, but the present study is the first that shows an independent inverse association between anemia and whole body bone mineral density. These data suggest that anemia in COPD needs further investigation as part of the multicomponent pathology in these patients.
There is no consistency in the definition for diagnosing anemia in literature. In the present study, we used the definition of the WHO to define anemia, and we found a prevalence of about 20%. This percentage is lower than previously reported in COPD by Cote et al. but higher cut-offs (Hb <13 g/dl) were used in that study (Citation15). Using the definition of the WHO, the percentage of anemia among elderly was about 10% in the NHANES III cohort (Citation32), and about 11% in U.S. people of 65 years and older (Citation33). Moreover, the prevalence of anemia was independent of the severity of COPD, although other studies reported a higher prevalence of anemia with increasing disease severity (Citation34). In addition, neither diffusion capacity nor the use of external oxygen therapy were different among the groups in the present study.
Remarkably, 20% of the subjects included in the present study had anemia, while only 3% reported to have anemia, indicating that this co-morbidity is largely underdiagnosed in COPD. Moreover, as most anemic patients had normal MCV levels, and this group had elevated CRP concentration in comparison to the subjects with normal or higher haemoglobin levels, we tend to conclude that the presence of anemia is largely related to the chronic systemic inflammatory disease state. Indeed, red blood cells tend to live less long due to systemic inflammation, and erythropoietin resistance occurs. Further, the bone marrow is not able to respond to the elevated demand of erythropoietin, and anemia develops (Citation35). However, in the presence of COPD, acute or chronic episodes of hypoxia may occur and, under normal conditions, result in elevated activity of erythropoietin, which is the reverse effect of systemic inflammation on plasma haemoglobin levels.
As no differences in arterial blood gases in rest or in saturation drop during the 6MWD were found between the groups in the present study, we hypothesize that the presence of systemic inflammation overlaps the potential effect of hypoxia on plasma haemoglobin level. On the other hand, hypogonadism have been shown in patients with COPD (Citation36), and this could also increase the risk for anemia (Citation37). Plasma testosterone concentration is however not measured in the present study. Although it can be hypothesised that polycythemia develops due to periods of hypoxia in COPD, polycythemia was only present in 8% of the subjects, and even less in men. The low prevalence is likely due to the fact that long-term oxygen therapy is more often prescribed to severe patients these days. The discrepancy between the percentage of patients with polycythemia in men and women could not be explained by the authors. Taken together, the present data imply that the percentage of subjects with COPD that are anemic is about double than the percentage in healthy elderly, and anemia in COPD is related with the systemic inflammatory state.
The present study is the first that showed an association between plasma haemoglobin concentration and whole body bone mineral density in patients with COPD. Low bone mineral density can result in osteoporosis, which is a frequent co-morbidity in COPD and result in higher fracture risk and worsening lung function (Citation38). Therefore, identifying risk factors in the development of alterations in the skeletal mass is relevant. Recently, a study including postmenopausal women also found an association between bone mineral density and plasma haemoglobin levels. In addition, an association between plasma haemoglobin concentration and vertebral fractures was found in healthy multi-ethnic elderly women (Citation39), and between plasma haemoglobin and bone measurements by peripheral quantitative computerized tomography in the INCHIANTI cohort (Citation40).
The effect of anemia on bone metabolism is not entirely clear yet, but it is thought from animal research that hypoxia induced by anemia could play a role (Citation41). However, we showed an association between haemoglobin and bone mineral density independent of arterial oxygen pressure. Moreover, previous studies concluded that the effect of anemia on bone metabolism was particularly present in the cortical bones (Citation40), but we found no association with the femoral T score, which largely represent cortical bones. Furthermore, we were not able to show higher presence of osteoporosis in the anemic patients and future studies are necessary to confirm our result.
There was no difference in disease-related outcomes among the patients with and without anemia. Although it is previously reported that anemic patients have higher dyspnea scores (Citation15), plasma haemoglobin concentration was no independent determinant for the mMRC scores after correction for other variables in the present study. There is one study showing an effect of anemia on the physical score of the SF-36 (Citation14), a marker for generic quality of life. In the present study, we used the SGRQ to measure disease-specific health status and no differences were found between anemic and non-anemic subjects. In addition, the 6MWD and the BODE score were not different among the patients with and without anemia. This finding is in conflict with a study by Cote et al., who showed lower 6MWD in anemic patients (Citation15). One explanation may be that most of the patients of the present study did not have ‘severe’ anemia as in only 8% of the patients haemoglobin levels were <12 g/dl, while the anemic patients of the study by Cote et al. had all haemoglobin levels <12 g/dl except three of them.
Of our total group, 25% of the patients had renal failure, all of them overt. This implies that renal failure is frequently present in patients with COPD and should not be neglected. A comparable percentage was reported recently by Incalzi et al. (Citation4). In that study, it was hypothesized that anemia could be the result of worse kidney function in patients with COPD, as in renal failure, erythropoietin synthesis in the kidney is affected. In our population, we could not find a difference in the prevalence of renal failure nor in the GFR among patients with or without anemia. In line, there were no differences in body mass index among the groups, but there was a trend for a lower percentage of patients with low muscle mass in the anemic subjects. We do not have an explanation for this finding, particularly since anemia is currently included in the definition of cachexia (Citation42), as it is hypothesized that anemia contributes to weight loss. However, our study nor other studies could show a relationship between anemia and risk for undernutrition in patients with COPD (Citation11).
The present study contributes to the understanding of the interrelation of the systemic co-morbidity in COPD. However, several points need to be addressed. First, due to the observational study design, it is not possible to make conclusions about causality. Secondly, consistent cut-off points are present for anemia nor polycythemia. Therefore, its prevalence can be different among studies. Third, there was an independent association between anemia and bone mineral density, but future studies need to take additional confounders into account like vitamin D concentration, lifestyle, and medication use. However, from the present data, we can argue that anemia develops as a consequence of the low-grade systemic inflammation present in COPD. As anemic patients may develop systemic hypoxia, this might have an effect on bone mineral density. Additional research, however, has to prove this theory.
In conclusion, we state that about one fifth of the subjects with COPD have anemia which is independently associated with CRP and total body bone mineral density. Clinicians should be aware of anemia among their patients and the diagnosis is easily made. Whether it is possible to normalize plasma haemoglobin concentration, and whether this results in better outcome need to be explored.
Declaration of Interest Statement
There is no conflict of interest to declare for the present manuscript. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
This work was performed at CIRO +,Centre of Expertise for Chronic Organ Failure in Horn, The Netherlands. We are grateful to the lab technicians Annelies Derks, Kitty Coenen, Els Joosten, Mariette Logtenberg, Ala Shaheen, Petra van Tuel, Gonny Vervuurt, Peggy de Wilde who took care after the collection of the metabolic material data; the technicians Koen Stakenborg, Marco Akkermans, Jos Peeters, Linda Op ‘t Veld, Tim Ubachs, Martijn Cuijpers, Trineke Hofstra and Ans Suntjens who took care of the clinical data collection, and Miriam Groenen who contributed to acquisition of data.
References
- Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 2006; 27(2):397–412.
- Rabe KF, Beghe B, Luppi F, Fabbri LM. Update in chronic obstructive pulmonary disease 2006. Am J Respir Crit Care Med 2007; 175(12):1222–1232.
- Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003; 107(11):1514–1519.
- Incalzi RA, Corsonello A, Pedone C, Battaglia S, Paglino G, Bellia V. Chronic renal failure: a neglected comorbidity of COPD. Chest 2010;137(4):831–837. Epub 2009/11/12.
- Graat-Verboom L, Wouters EF, Smeenk FW, van den Borne BE, Lunde R, Spruit MA. Current status of research on osteoporosis in COPD: a systematic review. Eur Respir J 2009; 34(1):209–218. Epub 2009/07/02.
- Rabinovich RA, MacNee W. Chronic obstructive pulmonary disease and its comorbidities. Br J Hosp Med (Lond) 2011; 72(3):137–145. Epub 2011/04/09.
- Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto-Plata V, Comorbidities and risk of mortality in patients with COPD. Am J Respir Crit Care Med 2012; Epub 2012/05/09.
- Yohannes AM, Ershler WB. Anemia in COPD: a systematic review of the prevalence, quality of life, and mortality. Respir Care 2011; 56(5):644–652. Epub 2011/02/01.
- Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis 2011; 6:199–208. Epub 2011/06/11.
- Chambellan A, Chailleux E, Similowski T. Prognostic value of the hematocrit in patients with severe COPD receiving long-term oxygen therapy. Chest 2005; 128(3):1201–1208. Epub 2005/09/16.
- John M, Hoernig S, Doehner W, Okonko DD, Witt C, Anker SD. Anemia and inflammation in COPD. Chest 2005; 127(3):825–829. Epub 2005/03/15.
- John M, Lange A, Hoernig S, Witt C, Anker SD. Prevalence of anemia in chronic obstructive pulmonary disease: comparison to other chronic diseases. Int J Cardiol 2006; 111(3):365–370. Epub 2005/10/26.
- Boutou AK, Pitsiou GG, Stanopoulos I, Kontakiotis T, Kyriazis G, Argyropoulou P. Levels of inflammatory mediators in chronic obstructive pulmonary disease patients with anemia of chronic disease: a case-control study. QJM: J Asso Phys 2012; Epub 2012/02/23.
- Krishnan G, Grant BJ, Muti PC, Mishra A, Ochs-Balcom HM, Freudenheim JL, Association between anemia and quality of life in a population sample of individuals with chronic obstructive pulmonary disease. BMC Pulm Med 2006; 6:23. Epub 2006/09/07.
- Cote C, Zilberberg MD, Mody SH, Dordelly LJ, Celli B. Haemoglobin level and its clinical impact in a cohort of patients with COPD. Eur Respir J 2007; 29(5):923–929. Epub 2007/01/26.
- Barba R, Casasola GG, Marco J, Emilio Losa J, Plaza S, Canora J, Anemia in chronic obstructive pulmonary disease: a readmission prognosis factor. Curr Med Res Opin 2012; 28(4):617–622. Epub 2012/03/14.
- Halpern MT, Zilberberg MD, Schmier JK, Lau EC, Shorr AF. Anemia, costs and mortality in chronic obstructive pulmonary disease. Cost effectiveness and resource allocation: C/E. 2006; 4:17. Epub 2006/10/18.
- Martinez-Rivera C, Portillo K, Munoz-Ferrer A, Martinez-Ortiz ML, Molins E, Serra P, Anemia is a mortality predictor in hospitalized patients for COPD exacerbation. COPD. 2012. Epub 2012/03/01.
- Boutou AK, Stanopoulos I, Pitsiou GG, Kontakiotis T, Kyriazis G, Sichletidis L, Anemia of chronic disease in chronic obstructive pulmonary disease: a case-control study of cardiopulmonary exercise responses. Respiration 2011; 82(3):237–245. Epub 2011/05/18.
- Korkmaz U, Korkmaz N, Yazici S, Erkan M, Baki AE, Yazici M, Anemia as a risk factor for low bone mineral density in postmenopausal Turkish women. Eur J Intern Med 2012; 23(2):154–158. Epub 2012/01/31.
- Spruit MA, Vanderhoven-Augustin I, Janssen PP, Wouters EF. Integration of pulmonary rehabilitation in COPD. Lancet 2008; 371(9606):12–13.
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23(6):932–946. Epub 2004/06/29.
- Clausen JL, Coates AL, Quanjer PH. Measurement of lung volumes in humans: review and recommendations from an ATS/ERS workshop. Eur Respir J 1997; 10(6):1205–1206.
- Hernandes NA, Wouters EF, Meijer K, Annegarn J, Pitta F, Spruit MA. Reproducibility of 6-minute walking test in patients with COPD. Eur Respir J 2011; 38(2):261–267. Epub 2010/12/24.
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992; 145(6):1321–1327. Epub 1992/06/01.
- Osteoporosis. WSGotPaMo. Prevention and Management of Osteoporosis; report of a WHO scientific group. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2003.
- Rutten EPA, Spruit MA, Wouters EFM. Critical view on diagnosing muscle wasting by single-frequency bio-eletrical impedance in COPD. Respir Med 2010; 104(1):91–98. Epub 2009/08/04.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350(10):1005–1012. Epub 2004/03/05.
- Wijnhoven HA, Schilp J, van Bokhorst-de van der Schueren MA, de Vet HC, Kruizenga HM, Deeg DJ, Development and validation of criteria for determining undernutrition in community-dwelling older men and women: The Short Nutritional Assessment Questionnaire 65+. Clin Nutr 2012; 31:351–358. Epub 2011/11/29.
- Robertshaw M, Lai KN, Swaminathan R. Prediction of creatinine clearance from plasma creatinine: comparison of five formulae. Br J Clin Pharmacol 1989; 28(3):275–280. Epub 1989/09/01.
- Nutritional anaemias. Report of a WHO scientific group. World Health Org Tech Rept Ser; 1968; 405: 5–37. Epub 1968/01/01.
- Guralnik JM, Ershler WB, Schrier SL, Picozzi VJ. Anemia in the elderly: a public health crisis in hematology. Hematology/the Education Program of the American Society of Hematology. Amer Soc Hematol Educ Prog 2005: 528–532. Epub 2005/11/24.
- Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood 2004; 104(8):2263–2268. Epub 2004/07/09.
- Watz H, Waschki B, Boehme C, Claussen M, Meyer T, Magnussen H. Extrapulmonary effects of chronic obstructive pulmonary disease on physical activity: a cross-sectional study. Am J Respir Crit Care Med 2008; 177(7):743–751.
- Markoulaki D, Kostikas K, Papatheodorou G, Koutsokera A, Alchanatis M, Bakakos P, Hemoglobin, erythropoietin and systemic inflammation in exacerbations of chronic obstructive pulmonary disease. Eur J Intern Med. 2011; 22(1):103–107. Epub 2011/01/18.
- Van Vliet M, Spruit MA, Verleden G, Kasran A, Van Herck E, Pitta F, Hypogonadism, quadriceps weakness, and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172(9):1105–1111.
- Ferrucci L, Maggio M, Bandinelli S, Basaria S, Lauretani F, Ble A, Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med 2006; 166(13):1380–1388. Epub 2006/07/13.
- Ionescu AA, Schoon E. Osteoporosis in chronic obstructive pulmonary disease. Eur Respir J Suppl 2003; 46:64s–75s.
- Chen Z, Thomson CA, Aickin M, Nicholas JS, Van Wyck D, Lewis CE, The relationship between incidence of fractures and anemia in older multiethnic women. J Am Geriatr Soc 2010; 58(12):2337–2344. Epub 2010/12/15.
- Cesari M, Pahor M, Lauretani F, Penninx BW, Bartali B, Russo R, Bone density and hemoglobin levels in older persons: results from the InCHIANTI study. Osteoporos Int 2005; 16(6):691–699. Epub 2004/09/30.
- Fujimoto H, Fujimoto K, Ueda A, Ohata M. Hypoxemia is a risk factor for bone mass loss. J Bone Miner Metab 1999; 17(3):211–216. Epub 2000/04/11.
- Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Cachexia: A new definition. Clin Nutr 2008; 27(6):793–799.