Abstract
Respiratory syncytial virus (RSV), although not typically considered an important pathogen in adults, may cause acute exacerbation of chronic obstructive pulmonary disease (COPD). It is unclear which COPD patients are at highest risk for developing serious RSV illness. Our objective was to identify risk factors for RSV illness among adult patients with COPD. We conducted a pooled analysis of data from COPD patients in 2 previously published longitudinal studies that examined RSV infection in high risk adults for ≤ 2 RSV seasons. Risk factors for RSV illness studied included age, sex, race, smoking status, exposure to children, home oxygen use, inhaled or oral steroid use, instrumental activities of daily living scores, and co-morbid conditions. Outcomes studied included symptomatic and medically attended RSV illness. Logistic regression was used to identify significant risk factors for RSV illness among older adults with COPD. Among 379 patients with COPD, the rate of symptomatic RSV illness was 11.1% (42/379); almost half (20/42) of whom required medical attention. In multivariable analyses, congestive heart failure (odds ratio [OR] = 4.18; 95% CI: 1.38, 12.69) and exposure to children (OR = 2.38; 95% CI: 1.03, 5.51) were risk factors for symptomatic RSV illness. Congestive heart failure (OR = 4.16; 95% CI: 1.02, 17.01) was the only significant risk factor for developing medically attended RSV illness. Exposure to children and congestive heart failure are risk factors for RSV illness among adult patients with COPD. Future prospective, well-designed studies are needed to corroborate these findings and examine other risk factors, including history of exacerbations.
Introduction
Respiratory syncytial virus (RSV) is an increasingly recognized cause of illness in adults, particularly among adults with chronic obstructive pulmonary disease (COPD) (Citation1–5). COPD is a progressive syndrome of expiratory airflow limitation caused by chronic inflammation of the airways and destruction of lung parenchyma (Citation6). Clinically, COPD is often characterized by chronic cough with or without sputum production, dyspnea on exertion, and wheezing on examination. COPD has a worldwide prevalence of 5% to 22% (Citation6–8), and is projected to be the fourth leading cause of death by 2030 (Citation9). In the United States, COPD affects more than 24 million adults and results in considerable morbidity and mortality (Citation10) and healthcare use, primarily due to hospitalizations during exacerbations (Citation11). Approximately half of COPD exacerbations can be attributed to viral infections, including RSV (Citation12).
Although RSV illness in healthy adults is typically self-limited and mild, illness in high-risk adults with chronic cardiopulmonary disease, including COPD or congestive heart failure (CHF), tends to be more severe and is more likely to require medical attention (Citation2). This association between underlying heart and lung disease and severe RSV illness is similar to the same well-established risk factors for severe disease in pediatric populations with premature birth, congenital heart disease, and chronic lung disease of prematurity (Citation13). Risk factors in premature infants for developing severe RSV illness include siblings in the household, attendance at day care, and low serum maternally-transmitted RSV-neutralizing antibody levels (Citation13,14). However, there are few data describing risk factors associated with serious RSV illness in adults, and none specifically in persons with COPD, who are at risk of developing severe disease when infected (Citation2).
The aim of this analysis was to determine the risk factors and co-morbid conditions that increase susceptibility to RSV illness and the development of medically attended respiratory illness among adults with COPD. Results of this analysis could be useful in identifying patient subsets that may benefit most from vaccination or other preventive medicines.
Methods
Study design and pooled patient population
We conducted a post-hoc analysis of 2 previously published longitudinal studies that examined RSV infection in high risk adults for ≤2 RSV seasons. Study 1 was conducted from 1999 to 2003; patients were recruited in late summer and early fall and monitored prospectively for a maximum of 2 winters, encompassing up to 2 RSV seasons (Citation2). The populations examined in this study included healthy elderly persons (≥65 years of age), high-risk adults (≥21 years of age with physician-diagnosed CHF or chronic pulmonary disease), and a hospitalized cohort of patients (all adults ≥65 years of age or with underlying cardiopulmonary illness admitted to the hospital with acute respiratory symptoms and diagnosed with pneumonia, upper respiratory infection, bronchitis, influenza, COPD, asthma, viral illness, or CHF) (Citation2).
Study 2 comprised patients ≥40 years of age with physician-diagnosed COPD who were past or active smokers (Citation1). Patients were enrolled between July and October 2004 and were monitored prospectively for 12 months, encompassing one RSV season. Of these, 97% were under the care of a pulmonary specialist and 58% had pulmonary function test results available with a mean forced expiratory volume at one second of 44%±19% of predicted. For both studies, patient demographics, medical history, functional status, medication use, and contact with children were collected at baseline.
Study protocols and patient informed consent were approved by the respective institutional review boards for each study. The present analytic sample is limited to the patients from both studies who were diagnosed with COPD.
RSV Illness evaluation and definition of outcome measures
In study 1, respiratory illness was determined by patient self-report from November 15 to April 15 of each year and was based on the development of respiratory symptoms consistent with RSV illness including nasal congestion, sore throat, hoarseness, new or worsening cough, sputum production, and dyspnea, with or without fever. Nasal swabs and blood samples for diagnosis of RSV were collected during home visits.
In study 2, patients were routinely examined for illness every 2 months and after self-reported respiratory symptoms, the same constellation of symptoms noted above. Patients testing positive for RSV had respiratory samples collected weekly and sputum samples collected daily until no virus was detectable for 2 weeks. RSV testing included serum serology, viral culture, and reverse transcriptase polymerase chain reaction (RT-PCR) assay. For both studies, patients were followed throughout their illnesses and use of health care was recorded. All patients were visited 1 month after RSV diagnosis to review illness details.
Symptomatic RSV illness was defined as any respiratory illness associated with a positive RSV RT-PCR assay, positive RSV viral culture, or a ≥4-fold rise in RSV antibody. All testing was performed using previously described methods (Citation15,16). Medically attended RSV illness was any symptomatic RSV illness that also required a visit to a doctor's office or emergency department (ED) or hospitalization.
Risk factors
Factors that were evaluated for an association with RSV illness included age, sex, race, smoking status, exposure to children, home oxygen use, inhaled or oral steroid use, instrumental activities of daily living (IADL) scores, and co-morbid conditions. Exposure to children was measured as the number of days per month that patients had children in their household and defined as the presence of children in the household; the number or age of children was not indicated. Smoking status was classified as active, past, or never. Any home oxygen use >21% (normal room air) was classified as a positive response. Steroid use (inhaled or oral) was classified as routine use or not. Functional status was assessed using the IADL Scale (Citation17). The IADL score (0 = independent, 1 = some help, 2 = dependent) measured the level of independence for six activities including food preparation, housework, shopping, using money, using the telephone, and transportation (complete independence = 0 and total dependence = 12). Cardiopulmonary function scores were measured using a simplification of the New York Heart Association (NYHA) Functional Classification of Heart Failure as 0 = no limitations, shortness of breath with: 1 = a flight of stairs, 2 = walking around level house, 3 = dressing, 4 = at rest (Citation18). Co-morbid conditions included on case report forms included cerebrovascular disease, CHF, coronary artery disease, diabetes, hypertension, hypothyroidism, and peripheral vascular disease.
Statistical analyses
Descriptive statistics were used to describe variables and logistic regression was used to identify the significant risk factors. Adjusted odds ratios (OR) for the risk factors for RSV illness were identified using univariate and multivariable logistic regression. SAS v9.1 (SAS Institute, Inc., Cary, NC, USA) was used for all analyses. A significant difference was indicated if the 95% CI did not include 1. Because both studies used similar case report forms and methodologies, pooling data across both studies was straightforward.
Results
The original studies examined 2648 total patients (study 1, n = 2536; study 2, n = 112), as described in . By limiting the analytic sample from the previous studies to only patients with COPD, our final sample was 379 patients (study 1, n = 267; study 2, n = 112; and ). The mean (SD) age of the pooled study population was 69.9 (9.6) years of age; the majority of patients were white (n = 362 [95.5%]), had exposure to children (n = 256 [67.5%]), and used inhaled steroids (n = 246 [64.9%]). Patients generally did not require assistance with daily living tasks (IADL score mean [SD], 0.24 [0.4]) and had moderate baseline shortness of breath (cardiopulmonary function score mean [SD], 1.7 [1.1]). The most frequent co-morbid condition was hypertension (n = 138 [36.4%]).
Figure 1. Pooled analysis population. CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; RSV = respiratory syncytial virus.
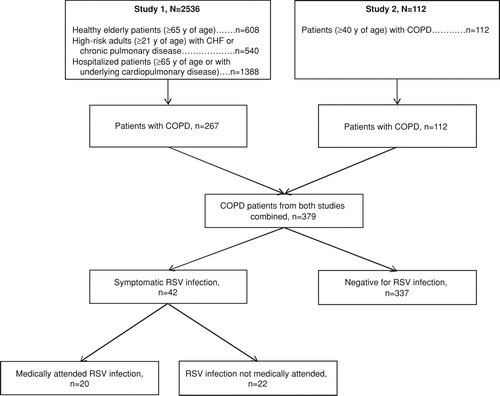
Table 1. Demographic and clinical characteristics at baseline
Symptomatic respiratory syncytial virus illness
The proportion of COPD patients with a symptomatic RSV illness was 11.1% (study 1, n = 32; study 2, n = 10). Illnesses were moderately severe; 20 (48%) subjects sought medical attention and 7 (17%) were hospitalized. Most subjects experienced illnesses consistent with acute exacerbation of COPD with 83% complaining of cough, 62% increased sputum production and dyspnea, and 48% wheezing. Only 10% had upper respiratory symptoms alone. During this period an additional 7 subjects had asymptomatic RSV infections diagnosed by a significant rise in RSV antibody during the winter season or RSV RNA detected during a stable visit.
In bivariate analysis, patients with COPD were more likely to develop symptomatic RSV illness if they had CHF (P = 0.01, Fisher exact test; ) and were exposed to children (χ2 = 3.87; P = 0.05; ). No other risk factors were significant.
Figure 2. A. Proportion of patients with symptomatic RSV illness by patient characteristic. B. Proportion of patients with symptomatic RSV illness by patient co-morbidity. CAD = coronary artery disease; CHF = congestive heart failure; CVD = cerebrovascular disease; No = did not have the characteristic or co-morbidity in question; PVD = peripheral vascular disease; RSV = respiratory syncytial virus; sRSV = symptomatic RSV. Yes = had the characteristic or co-morbidity in question. aIADL = instrumental activities of daily living (0 = independent, 1 = some help, 2 = dependent). bCardiopulmonary Function Score (0 = no limitations; shortness of breath with: 1 = a flight of stairs, 2 = walking around level house, 3 = dressing, 4 = at rest). *P = 0.05, chi-square test. †P = 0.01, Fisher exact test.
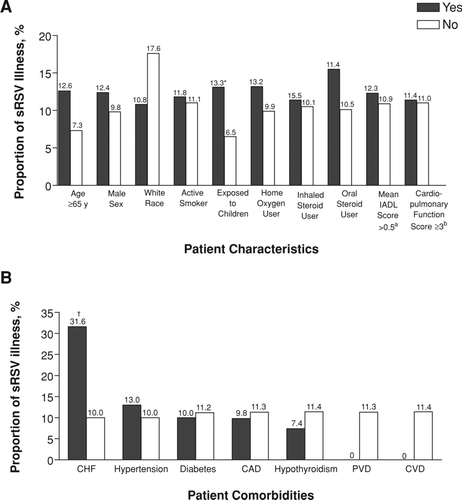
Univariate logistic regression analyses identified CHF as a statistically significant risk factor for symptomatic RSV illness (OR = 4.16; 95% CI: 1.49, 11.60; ). A positive but not statistically significant association was seen for age and exposure to children in patients with COPD (). In multivariable analyses, CHF (OR = 4.18; 95% CI: 1.38, 12.69) and exposure to children (OR = 2.38; 95% CI: 1.03, 5.51) were significant risk factors for symptomatic RSV illness. Age (OR = 1.04; 95% CI: 1.00, 1.08) was observed to have a positive but not statistically significant association with symptomatic RSV illness in patients with COPD ().
Table 2. Bivariate and multivariable analyses of factors associated with symptomatic and medically attended RSV illness
Medically attended respiratory syncytial virus illness
In this population of COPD patients, concomitant CHF was the only statistically significant risk factor for medically attended RSV illness identified through bivariate (OR = 3.78; 95% CI: 1.01, 14.24) and multivariable analyses (OR = 4.16; 95% CI: 1.02, 17.01; ). Almost half (n = 20 of 42; study 1, n = 14; study 2, n = 6) of COPD patients with a symptomatic RSV illness required medical attention, including hospitalization (n = 8), or an ED visit (n = 4).
Discussion
Respiratory syncytial virus infection can result in serious illness in adults, especially those with underlying cardiorespiratory conditions. Previous research has shown that adults with COPD are at increased risk for complications from RSV illness (Citation19). By defining specific subgroups among patients with COPD who are at higher risk for RSV illness and at highest risk of complications, prophylactic strategies and treatments may be targeted to the most susceptible groups.
In this study, we found that exposure to children and the presence of CHF were associated with an increased risk of illness with RSV among patients with COPD. The association of exposure to children and risk of acquiring RSV illness is not surprising because respiratory secretions of infants with primary infections contain high viral titers of RSV for relatively long periods and infectious fomites contaminating environmental surfaces may remain viable for extended periods (Citation20–22). Contact with RSV-infected grandchildren, for example, might be expected in persons with COPD and we found that 68% of our study participants had regular exposure to children. The association of CHF and increased risk of illness may reflect increased exposure to hospital environments where nososcomial transmission has been documented to occur (Citation23,24).
Individuals with impaired pulmonary function have an elevated risk of cardiovascular disease (Citation25–28). As such, in addition to environmental factors, it is plausible that CHF and COPD may affect immunoregulatory functions that would predispose a person to viral infection. For example, interferon-γ plays a key role in the immune response to viral pathogens and is responsible for recruiting cytotoxic T cells to the site of viral infection (Citation29). Interferon-γ can be decreased in patients with CHF after viral exposure compared with healthy adults (Citation30). Because patients with COPD have defects in cell-mediated immunity that can be restored with interferon-γ (Citation31), interferon-γ signaling may also be deficient in this population. Moreover, interferon-γ has been suggested as a potential treatment for the immune defects in COPD (Citation32). Given the role of interferon-γ in viral clearance and the potential defects in interferon-γ signaling in CHF and COPD, patients with both CHF and COPD may have an immunologic predisposition for increased severity of RSV illness.
The association of increased disease severity with RSV illness among patients with concomitant COPD and CHF was not surprising. It is well accepted that the combined presence of heart and lung disease increases the chance of dying from pneumonia during influenza epidemics compared with the presence of either condition alone (Citation33). The risk of death increased from 240 per 100,000 in persons older than 45 years with lung disease to 870 per 100,000 in patients with chronic heart and lung disease. The association between cardiopulmonary diseases and increased severity of respiratory disease is likely multifactorial and includes changes in immune function, stress of hypoxia and fever, and possible prothrombotic changes induced by inflammatory disease (Citation34,35). Finally, we also noticed a trend with increasing RSV risk with advancing age. Due to sample size limitations our study may not have had enough power to detect a difference.
Certain limitations should be considered when evaluating the results of this analysis. As in all studies with a narrow patient population, extrapolating results to a larger population may not always be valid. Additionally, among patients classified with COPD, a uniform measurement of COPD disease severity (eg, criteria described by the Global Initiative for Chronic Obstructive Lung Disease) with spirometry was not always available and represents the major limitation of our study (Citation6). Another limitation of the current analysis is that both studies differed in the period of data collection and our analyses did not account for those differences. Whereas study 1 recruited patients and followed them for 1 RSV season, study 1 recruited patients over 4 consecutive seasons and followed each participant for 2 seasons. A further limitation is that diagnosis of CHF was determined by local physicians; the study protocols did not provide uniform criteria for the diagnosis.
Furthermore, a number of potentially important risk factors were not captured in this analysis, including differences between RSV seasons, frequency of COPD exacerbations, household crowding, socioeconomic status, and serum RSV neutralizing antibody titers. Assessing a broader number of risk factors would produce a more complete understanding of the potential risks leading to RSV illness and disease severity among patients with COPD. In addition, certain variables such as exposure to children did not specify age of the children and more precise defintions may allow for better risk estimation. Bacterial complications of RSV were not specifically sought and therefore, risk factors for co-infection may be somewhat different than for symptomatic RSV alone. An additional concern might be that detection of RSV RNA has been noted in stable COPD by several groups raising a question of causality with illness (Citation36,37). However, subjects in the second cohort were monitored regularly for one year and RSV RNA was infrequently detected in the stable state. Finally, despite pooling, the sample size of this study was small, which could lead to a beta error for ascertainment of independent risk factors. However, a strength of the analysis was that all cases of RSV were laboratory confirmed.
Current treatments for adult patients with RSV illness are supportive, with specific antiviral therapy used mainly in patients with immunocompromised status or severe respiratory failure (Citation38). It would be useful to have therapies to treat RSV illness in adults who are at highest risk for severe disease and even more so to be able to prevent RSV illness entirely through immunization.
In conclusion, adult patients with COPD are at high risk for developing symptomatic RSV illness, especially if they are exposed to children or have CHF. Those with CHF are also more likely to experience more severe illness and subsequently use more health care resources. Further well-designed studies to define additional risk factors of illness and disease severity in patients are needed so that future trials of RSV vaccines and preventative strategies can be targeted to those at highest risk.
Declaration of Interest Statement
This study was sponsored by MedImmune, LLC. Original studies from which this analysis was derived were supported through grant NIH NIAID RO1 AI 45969. J. Mehta was an employee of MedImmune at the time of this analysis; P.J. Mahadevia is currently an employee of MedImmune. E.E. Walsh and A.R. Falsey have served as consultants for MedImmune.
Acknowledgments
The authors wish to thank Maryanne Formica, Gloria Andolina, and Susan Romansky for technical support and Patricia Hennessey and Mary Criddle for patient evaluation and Evgeniya Antonova for her comments. Editorial support was provided by Susan E. DeRocco, PhD, John E. Fincke, PhD, and Gerard P. Johnson, PhD of Complete Healthcare Communications, Inc. (Chadds Ford, PA) funded by MedImmune.
References
- Falsey AR, Formica MA, Hennessey PA, Criddle MM, Sullender WM, Walsh EE. Detection of respiratory syncytial virus in adults with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 173(6):639–643.
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352(17):1749–1759.
- De Serres G, Lampron N, La Forge J, Importance of viral and bacterial infections in chronic obstructive pulmonary disease exacerbations. J Clin Virol 2009; 46(2):129–133.
- Camargo CA, Jr., Ginde AA, Clark S, Cartwright CP, Falsey AR, Niewoehner DE. Viral pathogens in acute exacerbations of chronic obstructive pulmonary disease. Intern Emerg Med 2008; 3(4):355–359.
- Ramaswamy M, Groskreutz DJ, Look DC. Recognizing the importance of respiratory syncytial virus in chronic obstructive pulmonary disease. COPD 2009; 6(1):64–75.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Available at: http://www.goldcopd.org/uploads/users/files/GOLDReport_April112011.pdf. Accessed July 1, 2011.
- Buist AS, Vollmer WM, McBurnie MA. Worldwide burden of COPD in high- and low-income countries. Part I. The burden of obstructive lung disease (BOLD) initiative. Int J Tuberc Lung Dis 2008; 12(7):703–708.
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370(9589):765–773.
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006; 3(11):e442.
- Make B, Belfer MH. Primary care perspective on chronic obstructive pulmonary disease management. Postgrad Med 2011; 123(2):145–152.
- Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest 2000; 117(2 suppl):5S–9S.
- Kherad O, Kaiser L, Bridevaux PO, Upper-respiratory viral infection, biomarkers, and COPD exacerbations. Chest 2010; 138(4):896–904.
- Langley GF, Anderson LJ. Epidemiology and prevention of respiratory syncytial virus infections among infants and young children. Pediatr Infect Dis J 2011; 30(6):510–517.
- Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr 1981; 98(5):708–715.
- Falsey AR, Cunningham CK, Barker WH, Respiratory syncytial virus and influenza A infections in the hospitalized elderly. J Infect Dis 1995; 172(2):389–394.
- Falsey AR, Formica MA, Walsh EE. Diagnosis of respiratory syncytial virus infection: comparison of reverse transcription-PCR to viral culture and serology in adults with respiratory illness. J Clin Microbiol 2002; 40(3):817–820.
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The Index of Adl: A standardized measure of biological and psychosocial function. JAMA 1963; 185:914_919.
- The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston, MA: Little, Brown & Co; 1994.
- Walsh EE, Peterson DR, Falsey AR. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis 2004; 189(2):233–238.
- Hall CB, Douglas RG, Jr., Geiman JM. Respiratory syncytial virus infections in infants: quantitation and duration of shedding. J Pediatr 1976; 89(1):11–15.
- Hall CB, Geiman JM, Douglas RG, Jr., Meagher MP. Control of nosocomial respiratory syncytial viral infections. Pediatrics 1978; 62(5):728–732.
- Hall CB, Kopelman AE, Douglas RG, Jr, Geiman JM, Meagher MP. Neonatal respiratory syncytial virus infection. N Engl J Med 1979; 300(8):393–396.
- Hall CB. Nosocomial respiratory syncytial virus infections: the “Cold War” has not ended. Clin Infect Dis. 2000; 31(2):590–596.
- Mazzulli T, Peret TC, McGeer A, Molecular characterization of a nosocomial outbreak of human respiratory syncytial virus on an adult leukemia/lymphoma ward. J Infect Dis. 1999; 180(5):1686–1689.
- Agusti AG, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J 2003; 21(2):347–360.
- Baillard C, Boussarsar M, Fosse JP, Cardiac troponin I in patients with severe exacerbation of chronic obstructive pulmonary disease. Intensive Care Med 2003; 29(4):584–589.
- Curkendall SM, DeLuise C, Jones JK, Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol 2006; 16(1):63–70.
- Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003; 107(11):1514–1519.
- Baumgarth N, Kelso A. In vivo blockade of gamma interferon affects the influenza virus-induced humoral and the local cellular immune response in lung tissue. J Virol 1996; 70(7):4411–4418.
- McElhaney JE, Herre JM, Lawson ML, Cole SK, Burke BL, Hooton JW. Effect of congestive heart failure on humoral and ex vivo cellular immune responses to influenza vaccination in older adults. Vaccine 2004; 22(5-6):681–688.
- Reyes E, Prieto A, de la Hera A, Treatment with AM3 restores defective T-cell function in COPD patients. Chest 2006; 129(3):527–535.
- Todisco T, Vecchiarelli A, Dottorini M, Interferon-gamma (r-IFN-gamma) induced activation of alveolar macrophages (AM) from anergic patients with chronic obstructive pulmonary disease (COPD). J Biol Regul Homeost Agents. 1992; 6(3):87–92.
- Barker WH, Mullooly JP. Pneumonia and influenza deaths during epidemics: implications for prevention. Arch Intern Med 1982; 142(1):85–89.
- Kaba NK, Francis CW, Hall WJ, Falsey AR, Smith BH. Protein S declines during winter respiratory infections. J Thromb Haemost 2003; 1(4):729–734.
- Meier CR, Jick SS, Derby LE, Vasilakis C, Jick H. Acute respiratory-tract infections and risk of first-time acute myocardial infarction. Lancet 1998; 351(9114):1467–1471.
- Seemungal T, Harper-Owen R, Bhowmik A, Respiratory Viruses, Symptoms, and Inflammatory Markers in Acute Exacerbations and Stable COPD. Am J Respir Crit Care Med 2001; 164:1618–1632
- Borg I, Rohde G, Loseke S, Evaluation of a quantitative real-time PCR for the detection of respiratory syncytial virus in pulmonary diseases. Eur Respir J 2003; 21(6):944–951.
- Walsh EE. Respiratory syncytial virus infection in adults. Semin Respir Crit Care Med 2011; 32(4):423–432.