Abstract
In some patients with chronic asthma clinical and physiological similarities with COPD may exist, such as partial reversibility to bronchodilators and persistent expiratory airflow obstruction. However, pathological data comparing both diseases in patients of similar age and disease severity are scarce. We compared large and small airway dimensions in 12 younger (mean age 32 yrs) and 15 older (mean age 65 yrs) non-smoker adult fatal asthma patients with 14 chronic smokers with severe, fatal COPD (mean age 71 yrs). Using H&E, Movat pentachrome staining and image analysis, we quantified large airway basement membrane (BM) thickness (μm), submucosal gland area and large and small airway inner wall, smooth muscle and outer wall areas. Areas were normalized by BM perimeter (μm2/μm).
Younger adult fatal asthma patients had thicker BM, smooth muscle, and outer wall areas in both small and large airways when compared to COPD patients. In older asthmatics there was an overlap in BM thickness and airway structure in small airways. Inner wall layer in large and small airway level and submucosal gland areas were similar among groups. In conclusion, there are airway histological structural similarities between fatal asthma and fatal COPD. Older fatal asthmatics present overlapping airway structural features with younger adult fatal asthmatics and severe COPD patients. Our data contributes to a better understanding of asthma pathology in the elderly.
Keywords :
Introduction
In many circumstances, the distinction between asthma and COPD is not evident. COPD patients are usually older than asthmatics, but populations of older non-smoking asthmatics, usually with late onset disease, not infrequently share clinical and functional similarities with COPD patients (Citation1–4). Asthma and COPD are different chronic inflammatory diseases affecting the entire airway tree with extension to the parenchyma. Chronic reaction to injury and abnormal repair occur in a limited fashion in the lungs. It is possible that these two inflammatory diseases may, in some patients, lead to similar structural alterations of the airways.
There are several excellent reviews comparing the pathologic aspects of asthma and COPD, highlighting the differences and similarities in inflammation and remodeling patterns (Citation5,6). However, few studies compared structural alterations in asthmatics and COPD patients with similar age, disease severity and chronicity, or extended the observations to the entire airway tree (Citation7).
Since there are similar physiologic and clinical characteristics in older patients with asthma and COPD (Citation4), we hypothesized there may also be similar structural alterations in these groups. Therefore, we compared airway structure in autopsy tissue in three groups of patients: non-smoking adult fatal asthmatic patients, divided in two groups according to their age (younger and older) and patients with severe fatal COPD. Data were further compared to control patients, grouped according to age.
Methods
The Review Boards of Sao Paulo University Medical School and of Lakewood Regional Medical Center approved this study.
Patients
Fatal asthma (FA) tissue was obtained from autopsies performed at Sao Paulo University between 1996 and 2004. All patients had a previous history of only asthma, documented by interviews with the families. All were non-smokers and had no other lung diseases. Clinical data included age of onset and duration of asthma, treatment and previous hospitalization(s). Early onset asthma was defined when symptoms started before the age of 12 (Citation8). FA patients were divided in 2 subgroups: the Younger Fatal Asthma (YFA) group that included patients who died between ages 15–49 years. The Older Fatal Asthma (OFA) group referred to patients aged 56-78 years at death.
COPD lungs were obtained from fourteen subjects who had undergone autopsy at Lakewood Regional Medical Center, California between 1994 and 2002. All patients were ex-smokers, nonatopic, with no history of asthma. They had severe fixed expiratory airflow limitation and died either of COPD-related exacerbations or chronic respiratory failure. Last lung function was obtained 6 months prior to demise when patients were clinically stable. The post-bronchodilator response to 2 puffs albuterol (180 mcg) was < 200 ml and < 12% in all COPD patients.
Nineteen non-smoker subjects that died due to non-pulmonary causes, had no history of pulmonary disease (including-asthma) and had normal lungs at autopsy were included as controls. Controls were divided into two subgroups according to their age at death: Younger controls (YCTRL) that included patients who died between ages 44–55 years and older controls (OCTRL), with ages ranging from 57–79 years.
Tissue processing and analyses
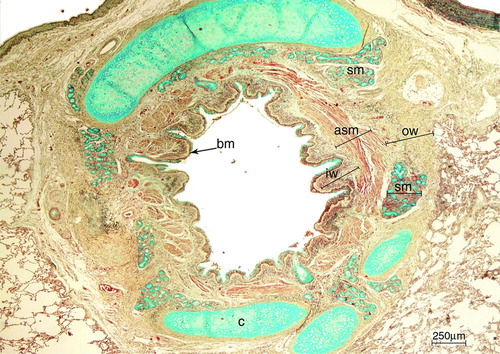
Specimens were fixed in 4% formaldehyde and paraffin-embedded. Five-μm-thick, H&E stained sections were used to measure basement membrane (BM) thickness. The Movat's pentachrome technique, as previously used by Hogg et al. (Citation9), was used for the quantification of airway dimensions ().
Figure 1. Large airway of an asthmatic patient stained with Movatエs pentachrome. With this staining, cartilage and mucous cells stain in blue, muscle in red, elastin in black/purple and connective tissue in yellow. The analyzed airway compartments were: inner wall (iw), airway smooth muscle (asm), submucosal glands area (sm) and outer wall (ow). Basement membrane = bm. Cartilage = c. Scale bar = 250 μm.
Quantifications were performed in 2 large and 3 small airways per patient, all transversally cut (Citation10). Small vs. large airway size was defined by an internal basement membrane (BM) perimeter < or ≥ 6 mm, respectively.
For BM thickness quantification, fifteen 400x fields were measured in large airways. In each field, the BM outer and inner borders were manually delineated over a certain length. Tangentially cut sections were avoided. The “Curve Thickness” tool of the software performed sequential measurements of distance between the delineated upper and lower borders of BM, presenting average distance as the output, expressed in μm.
The following airway compartments were assessed in large and small airways: lamina propria (inner wall), airway smooth muscle (ASM) and outer wall areas. In large airways, submucosal gland areas were also quantified.
The inner wall area was defined as the region comprising the epithelial BM and the inner border of the ASM. The outer wall area was the region comprising the outer border of the ASM to the outer border of the adventitia. ASM and gland areas were manually delineated. All airway circumferences were analyzed in magnifications ranging from 100· to 400·. Values were expressed as areas, corrected by BM perimeter.
A semi-quantitative evaluation of the cellular inflammation in H&E stained slides was performed in the inner and outer walls of large and small airways and scored from 0 to 3 (0 = no inflammation, 1 = mild inflammation, 2 = moderate inflammation, 3 = intense inflammation). This analysis was not performed in the ASM because there was little to absent cellular inflammation in this airway layer in all groups. In addition, mucus plugging in the small airways was evaluated as present or absent in the asthma and COPD groups.
The software Image-Pro® Plus 4.1 for Windows® (Media Cybernetics –Silver Spring, MD, USA), running on a microcomputer, connected to a digital camera (JVC TK-C1380 Color Video Camera, Victor Company of Japan Limited, Japan) coupled to an optical microscope (Leica DMR, Leica Microsystems, Wetzlar GmbH, Wetzlar, Germany) was used for quantifications (Citation11).
Statistical analysis
Data were expressed as mean ± SEM or median/IQR depending on data distribution. For comparison of the different disease groups with controls, Student's t-test or Mann–Whitney test were used. Comparison among the disease groups was performed with ANOVA or Kruskal–Wallis followed by the Bonferroni post-hoc test. Correlations were performed with the Pearson/Spearman tests. Categorical values were compared by means of the chi-square test. Regression analyses were performed with each quantified parameter using age as the dependent variable and different curve estimation models were generated. The curve that best fitted all the significant associations was selected. The level of significance was set at p ≤ 0.05. Statistical analysis was performed with the SPSS 15.0 software (SPSS, Chicago, Illinois, USA).
Results
Study population
Twenty-seven non-smoking FA patients (21 females) were included in the study; 18 were Caucasians, 8 were of African origin and 1 had Asian ancestry. There were 12 younger adult fatal asthma (YFA) cases and 15 older adult fatal asthma cases (OFA). Patients’ characteristics are shown in .
Table 1. Clinical characteristics of asthma and COPD patients
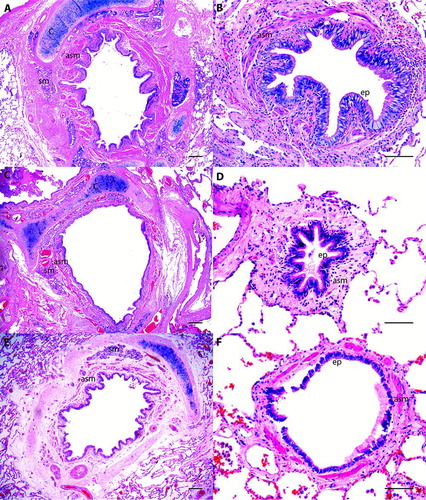
At autopsy there were histologic changes compatible with asthma such as hyperinflation, mucous hypersecretion, epithelial desquamation, thickening of the basement membrane, increased airway smooth muscle, mucosal inflammation with or without eosinophils and all deaths were ascribed to status asthmaticus by the pathologist (, A and B).
Figure 2. Histological examples of large and small airways of patients with asthma (A, B), with COPD (C, D) and a control patient (E, F). Observe the prominent airway smooth muscle layer (asm) in large and small airways of the asthmatic patient. Cartilage = c, airway smooth muscle = asm, submucosal gland = sm, ep = epithelium. H&E staining. Scale bar = 250 μm in A, C and E. Scale bar = 100 μm in B, D and F.
The COPD group consisted of 14 patients, 6 women, 12 Caucasians and 2 Hispanics, all ex-smokers. Clinical and functional characteristics of COPD patients appear in .
At autopsy, COPD patients had histological alterations showing extensive emphysema, bronchiolar wall distortion, goblet cell and squamous metaplasia of the bronchial epithelium, and mild to moderate lymphocytic and neutrophilic inflammation in the airways. There was no bronchopneumonia or secondary lung cancer in the samples (, C and D).
The control groups consisted of 19 individuals, 12 men, all never-smokers, 13 were Caucasians and 6 had African origin (, E and F and ).
Table 2. Characteristics of control patients
BM thickness
Large airways’ BM perimeter was measured in 27 (12YFA, 15OFA) patients with asthma, in 13 COPD patients and in 19 controls. Only one COPD patient did not have suitable large airways for analyses. The mean ± SEM perimeter of the large airways was 8771 ± 582 μm in YFA, 8914 ± 1020 μm in OFA and 11502 ± 800 μm in COPD, 9781 ± 871 μm in YCTRL and 10892 ± 1566 μm in OCTRL (p = 0.18).
The mean ± SEM length of BM analyzed per group was 4658 ± 90 μm in FA, 4429 ± 99 μm in OFA, 5066 ± 724 μm in COPD, 3750 ± 179 μm in YCTRL, 3921 ± 116 μm in OCTRL, p = 0.11.
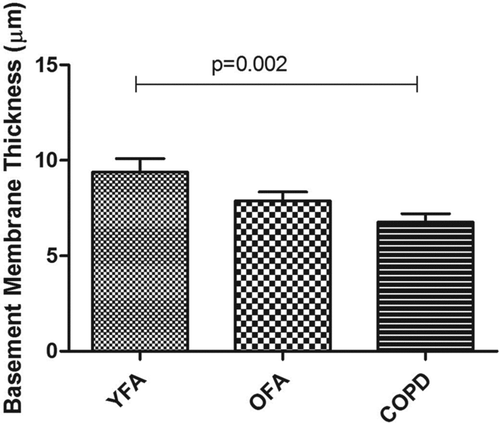
There were no differences in BM thickness between controls (p = 0.96). Younger FA patients had thicker BM than Younger Controls, p = 0.02. There were no differences in BM thickness when Older FA (p = 0.56) and COPD (p = 1.00) patients were compared to Older Controls ().
Table 3. Airway dimensions in fatal asthma, severe fatal COPD and controls
BM thickness was significantly greater in YFA when compared to COPD (p = 0.002). The OFA group had a mean BM thickness intermediate to the YFA and COPD groups, which was not significantly different from either (p = 0.3) ( and ).
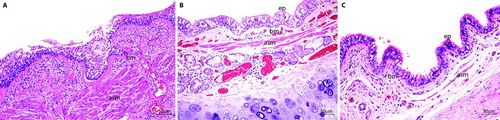
Figure 3. Longitudinal sections of a large airway of patients with asthma (A), COPD (B) and a control patient (C). The basement membrane (bm) in A is thicker than in B and C, and has an hyaline eosinophilic aspect. Observe the thick airway smooth muscle (asm) bundle in A. Submucosal gland = sm, cartilage = c. H&E staining. Scale bar = 50 μm.
Airway dimensions
Airways dimensions were measured in 21 patients (9 YFA, 12 OFA) with asthma, 14 patients with COPD and 19 controls. Six asthmatics patients were excluded from the dimension analysis because there were no extra adequate tissues or airways available for the Movat's pentachrome staining.
Younger vs. older controls
There were no differences between YCTRL and OCTRL regarding large and small airway dimensions, except for the gland area that was larger in older controls (p = 0.036).
There were no differences in gender regarding airway structure in both large and small airways. There was a positive correlation between gland area and age (r = 0.63, p = 0.005) in large airways. Data are shown in .
Controls vs. fatal asthma and controls vs. COPD, according to age groups
Younger controls (YCTR) vs. younger fatal asthma (YFA)
YFA had larger inner (p = 0.01), ASM (p < 0.001) and outer (p = 0.001) walls in large airways than younger controls. YFA had larger ASM (p = 0.02) in small airways than younger controls, no differences in other components were found ().
Older controls vs. older fatal asthma
OFA had larger inner wall areas in large airways (p = 0.007) than older controls, no differences in other components of large or small airways were found.
Older controls vs. COPD
COPD patients had larger inner wall areas in large airways (p = 0.01) than OCTRL. OCTRL had larger ASM (p = 0.003) and glands areas (p = 0.01) in large airways. In small airways, OCTRL had larger ASM (p = 0.007) than COPD patients.
Younger fatal asthma vs. older fatal asthma vs. COPD
When we compared fatal asthma cases grouped according to age, we observed that both YFA and OFA had increased ASM and outer wall areas when compared with COPD patients in large airways ( and ).
Figure 5. Large airway dimensions in younger adult fatal asthmatics (YFA), older adult fatal asthmatics (OFA) and in patients with severe Chronic Obstructive Pulmonary Disease (COPD). Areas were normalized by basement membrane perimeter (μm2/μm). Each bar indicates the median and the whiskers represent the interquartile range. IW = inner wall, ASM = airway smooth muscle, OW = outer wall, gland = submucosal glands area.
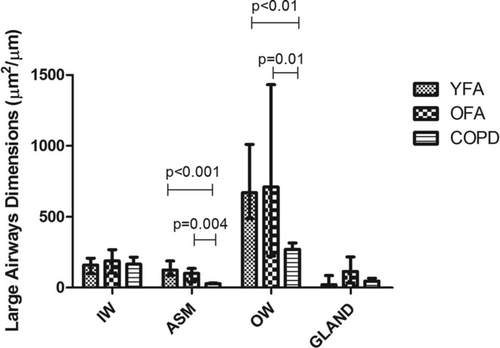
In this subset of 21 patients, large airway BM thickness yielded similar results to the larger group of patients (n = 27), i.e., YFA patients had significantly thicker BM than COPD patients (p = 0.009) with intermediate values in OFA (OFA vs. COPD, p = 0.24; YFA vs. OFA, p = 0.25).
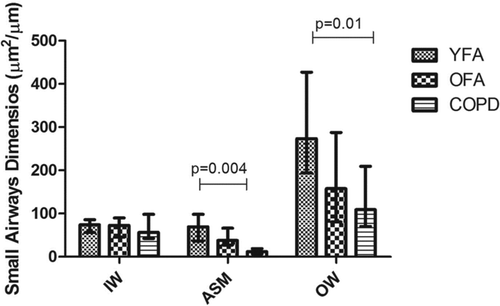
In small airways, we observed that OFA and COPD had similar airway compartment dimensions, with differences being observed only between YFA and COPD in the ASM layer (p = 0.004), and outer wall (p = 0.01) areas ( and ).
Figure 6. Small airway dimensions in younger adult fatal asthmatics (YFA), older adult fatal asthmatics (OFA) and in patients with severe Chronic Obstructive Pulmonary Disease (COPD). Areas were normalized by basement membrane perimeter (μm2/μm). Each bar indicates the median and the whiskers represent the interquartile range. IW = inner wall, ASM = airway smooth muscle, OW = outer wall.
There were no significant differences in BM thickness or airway dimensions between asthmatics that used corticosteroids regularly or not.
Asthmatic patients that had early onset asthma (n = 7) had larger ASM areas in larger airways (early onset = 182.78/153.48, late onset = 97.67/57.67, p = 0.035) and larger outer wall areas in the small airways (early onset = 338.81/179.54, late onset = 119.27/201.33, p = 0.019).
Curve estimation regression models
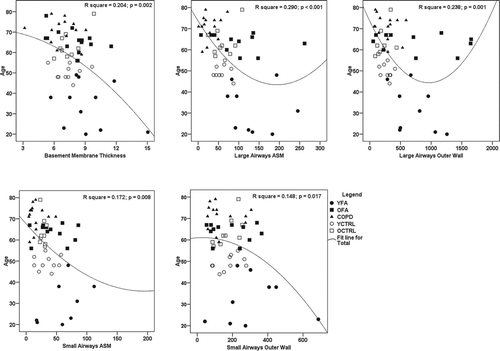
Five variables from the eight analyzed (large airway BM thickness and submucosal gland area and large and small airway inner wall, ASM and outer wall areas) remained significant in curve estimation regression models using age as the dependent variable. Quadratic models generated the curves that best fitted each parameter significantly associated with age. These curves are shown in .
Figure 7. Curve estimation plots of parameters associated with age in regression analyses. Fit lines represent quadratic curves. ASM, airway smooth muscle; COPD, chronic obstructive pulmonary disease; OCTRL, older controls; OFA, older fatal asthma; YCTRL, younger controls; YFA, younger fatal asthma. Age presented in years, basement membrane thickness presented in μm, ASM and outer wall areas presented in μm2/μm.
Airway inflammation
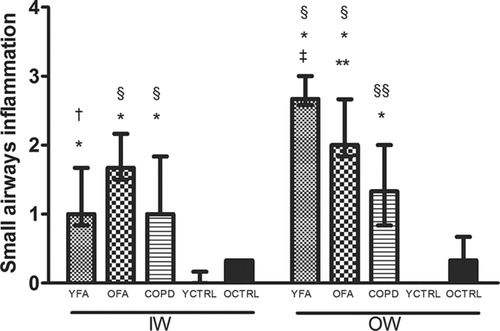
Data regarding inflammation scores in the airways are shown in and . Small airway mucus plugging was present in 33.3% of YFA, 33.3% of OFA and 28.6% of COPD patients (p > 0.05).
Figure 8. Large airways inflammation in the inner wall (IW) and outer wall (OW) in younger adult fatal asthmatics (YFA), older adult fatal asthmatics (OFA), patients with severe Chronic Obstructive Pulmonary Disease (COPD), younger controls (YCTRL) and older controls (OCTRL) based on a semi-quantitative score (0 = no inflammation, 1 = mild inflammation, 2 = moderate inflammation, 3 = intense inflammation). Data presented as median/IQR. *p ≤ 0.01 vs. COPD. †p < 0.001 vs. YCTRL and OCTRL. §p < 0.01 vs. YCTRL and OCTRL.
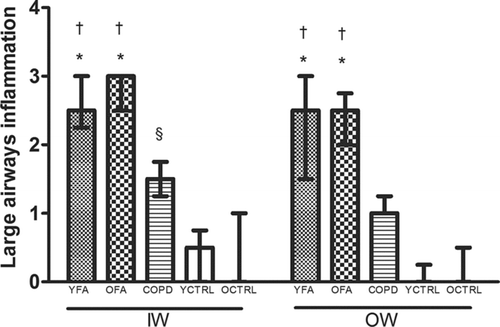
Figure 9. Small airways inflammation in the inner wall (IW) and outer wall (OW) in younger adult fatal asthmatics (YFA), older adult fatal asthmatics (OFA), patients with severe Chronic Obstructive Pulmonary Disease (COPD), younger controls (YCTRL) and older controls (OCTRL) based on a semi-quantitative score (0 = no inflammation, 1 = mild inflammation, 2 = moderate inflammation, 3 = intense inflammation). Data presented as median/IQR. *p ≤ 0.001 vs. YCTRL. †p = 0.002 vs. OCTRL. §p ≤ 0.001 vs. OCTRL. ‡p < 0.001 vs. COPD. **p = 0.04 vs. COPD. §§p = 0.004 vs.OCTRL.
Discussion
In this study, we compared airway dimensions in fatal asthmatics, severe fatal COPD and control adult patients. Our data show that older asthmatics have overlapping structural characteristics with severe COPD patients, at BM thickness and small airway level. To our knowledge, this is the first article to show that similarities occur between older asthmatics and COPD patients also at the tissue level.
Except for increased submucosal gland areas in large airways in older controls, there were no major airway dimension changes between younger and older adult subjects that died due to non-pulmonary diseases. In addition, although significant, regression analyses showed modest associations when morphometrical data were plotted against age. Taken together, it is likely that the differences observed between asthmatics and COPD patients are probably not solely reflective of aging.
Our group of younger controls was not perfectly age-matched with the younger asthmatics, which is an important limitation of this study. However Bai et al. could not find differences in airway structure in control patients with a similar age range of our controls and younger asthmatics (Citation12). When compared to their controls, younger fatal asthmatics had larger airway dimensions than controls, but fewer differences were found between older fatal asthmatics and their respective controls. These nuances could be related to varying phenotypes of asthma in both age groups.
Interestingly, older patients dying of asthma and patients with severe COPD did not have evidence of increased airway dimensions other than increased larger inner wall areas. In addition, COPD patients had less ASM mass in both large and small airways. These results seem to conflict with the assumption that COPD is always associated with airway thickening. A recent study by McDonough et al. suggests that bronchioles may obliterate and disappear in patients with extensive emphysema and GOLD IV COPD. Our results certainly give support to these assumptions (Citation13). Nagai et al. found a negative relation between ASM area in large airways and the extent of emphysema (Citation14). In addition, the expression of genes related to matrix deposition growth factors, fibronectin and collagen III declined with FEV1 progression (Citation15).
No previous study has compared large and small airway structures in fatal asthma and fatal, severe COPD in patients of similar age and disease severity. Here, we demonstrated that older asthmatics have intermediate BM thickness when compared to younger adult FA and severe COPD patients. Thickening of the lamina reticularis due to ECM deposition is considered a pathognomonic feature of asthma, whereas little is known about BM composition in COPD (Citation16). Most studies have reported a thicker BM layer in asthmatics compared to COPD patients. Our results confirm that YFA patients have thicker BM than COPD patients, but an overlap exists in the OFA group. This is despite similar use of inhaled and oral corticosteroids among asthmatics.
We could not find differences in inner wall areas in large and small airways between fatal asthmatics and patients with COPD. These similarities could be due to a similar extent of extracellular matrix deposition, increased vascularity or a combination of these parameters. A previous study by Bourdin et al. (Citation17) reported that non-specialized pathologists were unable to differentiate asthma vs. COPD when analyzing endobronchial biopsies, a procedure that samples largely the BM and inner wall in large airways.
In the current study, our results suggest that inflammation contributes to differences in airway dimensions mainly in the outer walls of the airways. Importantly, increases in the muscle layer area seem to be mainly due to hyperplasia/hypertrophy of ASM cells (Citation18).
Increased ASM mass is an important feature of asthma and a determinant of airway hyperresponsiveness in asthma and COPD (Citation19). Previous studies have demonstrated increased ASM mass in mild COPD patients when compared to controls, but not to asthmatics (Citation17). Our data show that the amount of ASM mass in large airways is a feature that distinguishes asthma from COPD irrespective of age.
The duration of disease was unlikely to account for the similarities observed between older asthmatics and COPD patients, since both asthma groups had similar disease duration. On the other hand, there were differences related to the age of asthma onset, with most of the older asthmatics having late onset disease. Age of asthma onset seem to be related to different clinical phenotypes. Late onset asthmatics have less atopy, increased expiratory airflow limitation, and distinct patterns of neutrophilic mediated inflammation in bronchial biopsies (Citation20). Our data suggest that the phenotype of early onset asthma is related to more prominent structural alterations in both large and small airways in fatal asthmatics than late onset asthma.
We are aware that fatal asthma subjects may represent a distinct phenotype of the disease, therefore the extent to which our findings could be transposed to non-fatal asthmatic patients remains unclear. Our fatal asthma population is characterized by low use of inhaled steroids and lack of regular medical care, all known risk factors for asthma complications and death, and lung functions studies were absent in the majority of patients (Citation21). An important confounding factor is that all COPD patients were using inhaled and low dose oral corticosteroid, whereas a smaller number of the asthmatics used them regularly. There is little data about the effects of corticosteroids on airway structure in asthma and COPD and evidence suggests that steroids may not reverse bronchial collagen deposition in asthma (Citation22). In this study, when comparing the use of corticosteroids within the asthmatics group we could not find significant differences in airway structure. Despite this, we cannot exclude that some of the encountered structural differences among groups might have been influenced by the more regular use of corticosteroids in COPD patients. In addition, it is possible that ethnic, environmental and social differences between asthmatics and COPD influenced our results.
Conclusions
In conclusion, adult younger fatal asthma patients have thicker large airway BM and greater airway smooth muscle mass and outer wall thicknesses compared to fatal older COPD patients. Older fatal asthmatics, despite similar duration of asthma as younger adult FA patients, share similar large airway BM thickness and small airway wall structural abnormalities as fatal severe COPD patients. These results do not seem to be a sole reflection of aging and may be influenced by the age of asthma onset. Asthma in the elderly is frequently underecognized and undertreated, with relatively higher mortality rates (Citation23). It is essential therefore to better understand asthma pathogenesis and its relation to COPD in this subset of older individuals (Citation24).
Declaration of Interest Statement
This work was funded by the “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (CNPq). The authors declare they have no other conflicts of interest to disclose.
Acknowledgments
The authors wish to thank Drs. J. Hogg, R. Green and J. Kollin for performing the autopsies and analyzing the lungs of patients with COPD. We also thank Drs. M. Saieg and F. Bernardi for helping with tissue collections. We are indebted to all autopsy assistants and pathologists of the SVOC-USP that helped us gathering the fatal asthma cases.
References
- Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA) 2010. Available from: http://www.ginasthma.org/.
- Global strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstrutive Lung Disease (GOLD) 2010. Available from: http://www.goldcopd.org/.
- Mauad T, Dolhnikoff M. Pathologic similarities and differences between asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med 2008 Jan; 14(1):31–38.
- Gelb AF, Zamel N, Krishnan A. Physiologic similarities and differences between asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med 2008 Jan; 14(1):24–30.
- Jeffery PK. Remodelling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med 2001 Nov 15; 164(10 Pt 2):S28–38.
- Jeffery PK. Remodeling and Inflammation of bronchi in asthma and Chronic Obstructive Pulmonary Disease. Proc Am Thorac Soc 2004; 1(3):176–183.
- Kuwano K, Bosken CH, Pare PD, Small airways dimensions in asthma and in chronic obstructive pulmonary disease. Am Rev Respir Dis 1993 Nov; 148 (5):1220–1225.
- Miranda C, Busacker A, Balzar S, Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol 2004 Jan; 113(1):101–108.
- Hogg JC, Chu F, Utokaparch S, The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004 Jun 24; 350(26):2645–2653.
- Mauad T, Silva LF, Santos MA, Abnormal alveolar attachments with decreased elastic fiber content in distal lung in fatal asthma. Am J Respir Crit Care Med 2004 Oct 15; 170(8):857–862.
- Image-Pro Plus reference guide for windows, Version 4.1, Media Cybernetics, L.P. Silver Spring, MD, 1999. p. 2–391.
- Bai T, Cooper J, Koelmeyer T, The effect of age and duration of disease on airway structure in fatal asthma. Am J Respir Crit Care Med 2000 Aug; 162(2):663–669.
- McDonough JE, Yuan R, Suzuki M, Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 2011 Oct 27; 365(17):1567–75.
- Nagai A, West WW, Paul JL, The National Institutes of Health Intermittent Positive-Pressure Breathing trial: pathology studies. I. Interrelationship between morphologic lesions. Am Rev Respir Dis 1985 Nov; 132(5):937–945.
- Hogg JC, McDonough JE, Gosselink JV, What drives the peripheral lung-remodeling process in chronic obstructive pulmonary disease? Proc Am Thorac Soc 2009 Dec; 6(8):668–672.
- Liesker JJ, Ten Hacken NH, Zeinstra-Smith M, Reticular basement membrane in asthma and COPD: similar thickness, yet different composition. Int J Chron Obstruct Pulmon Dis 2009; 4:127–135.
- Bourdin A, Serre I, Flamme H, Can endobronchial biopsy analysis be recommended to discriminate between asthma and COPD in routine practice? Thorax 2004 Jun; 59(6):488–493.
- James AL, Elliot JG, Jones RL, Airway smooth muscle hypertrophy and hyperplasia in asthma. Am J Respir Crit Care Med 2012 May 15; 185(10):1058–1064.
- Lambert RK, Wiggs BR, Kuwano K, Functional significance of increased airway smooth muscle in asthma and COPD. J Appl Physiol 1993 Jun; 74(6):2771–2781.
- Moore WC, Meyers DA, Wenzel SE, Identification of asthma phenotypes using cluster analysis in the severe Asthma Research Program. Am J Respir Crit Care Med 2010 Feb 15; 181(4):315–323.
- Mauad T, Ferreira DS, Costa MB, Characterization of autopsy-proven fatal asthma patients in São Paulo, Brazil. Rev Panam Salud Publica 2008 Jun; 23(6):418–423.
- Chakir J, Shannon J, Molet S, Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol 2003 Jun; 111(6):1293–1298.
- Diette GB, Krishnan JA, Dominici F, Asthma in older patients: factors associated with hospitalization. Arch Intern Med 2002 May 27; 162(10):1123–1132.
- Gibson PG, McDonald VM, Marks GB. Asthma in older adults. Lancet 2010 Sep 4; 376(9743):803–813.