Abstract
Knowledge about the pathogenesis and pathophysiology of chronic obstructive pulmonary disease (COPD) has advanced dramatically over the last 30 years. Unfortunately, this has had little impact in terms of new treatments. Over the same time frame, only one new class of medication for COPD has been introduced. Even worse, the rate at which new treatments are being developed is slowing. The development of new tools for the assessment of new treatments has not kept pace with understanding of the disease. In part, this is because drug development tools require a regulatory review, and no interested party has been in a position to undertake such a process. In order to facilitate the development of novel tools to assess new treatments, the Food and Drug Administration, in collaboration with the COPD Foundation, the National Heart Lung and Blood Institute and scientists from the pharmaceutical industry and academia conducted a workshop to survey the available information that could contribute to new tools. Based on this, a collaborative project, the COPD Biomarkers Qualification Consortium, was initiated. The Consortium in now actively preparing integrated data sets from existing resources that can address the problem of drug development tools for COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a major public health problem. It is currently the third-leading cause of death in the United States, is a major cause of morbidity and is a major driver of health care costs. Recent advances in understanding the pathogenetic mechanisms that underlie COPD have lead to the identification of many novel therapeutic targets. As a result, a large number of agents have been explored as potential treatments, both at the preclinical and clinical levels. However, the tools used to assess treatments for COPD have been limited and better tools are needed, as the number of new drugs (new molecular entities) being approved to treat COPD is declining and attrition rate is high.
As discussed in the FDA draft guidance for industry, drug development for COPD can be aimed at different aspects of the disease: improving airflow, providing symptom relief, modifying or preventing exacerbations, altering the disease process, or modifying lung structure or treating extra-pulmonary manifestations. While there has been some success with drug therapies for the first three of these disease aspects, it is in the most crucial areas of COPD treatment (i.e., altering the disease process, or modifying lung structure or treating extra-pulmonary manifestations) that effective therapies are lacking.
To date, the medications for the treatment of COPD have been FDA-approved primarily on the basis of improvements in lung function, i.e., FEV1. Although FEV1 may be adequate to evaluate treatments aimed at improving lung function and the airways obstruction that is associated with COPD, FEV1 (alone) may be inadequate to evaluate the efficacy of novel therapies targeting disease progression, lung structure or extrapulmonary manifestations of the disease. In the last four years, three U.S.-approved products: Advair ™250/50 (fluticasone propionate 250 mcg and salmeterol 50 mcg inhalation powder), Spiriva™ (tiotropium bromide inhalation powder) and Daliresp™ (roflumilast) have obtained the claim of reduction in COPD exacerbations. However, the initial approved indication for two of these three products (improvement in lung function for Advair, and maintenance treatment of bronchospasm for Spiriva) was based on FEV1.
The natural history of COPD is generally that of progressive deterioration of lung function and functional status, worsening quality of life, and, in many cases, demise. However, this progression is slow, occurring over years or decades, complicating interventional studies using these endpoints. In addition, COPD is extremely heterogeneous and while a number of attempts have been made to define subsets of COPD, efficient strategies to develop treatments targeting specific groups of COPD patients have yet to emerge. The extrapulmonary manifestations of COPD are becoming increasingly recognized as substantial causes of morbidity and mortality that need timely characterization and treatment. Biomarkers have the potential to reflect reliably the disease process and activity, and provide a better understanding of the COPD subtypes, especially the systemic or non-pulmonary manifestations.
Thus, biomarkers may allow for the development of therapies aimed at treating COPD using endpoints other than FEV1. A large number of biomarkers have been explored in this context. However, the use of biomarkers in drug development requires rigorous evaluation of their utility. Recognizing the importance of biomarkers to advance the development of new treatments, the Food and Drug Administration (FDA) in the United States initiated a Biomarker Qualification Process.
Biomarker qualification
The Biomarker Qualification Process is part of the Drug Development Tools Qualification program recently initiated at the FDA. The Biomarker Qualification process was established to support FDA's work with external scientists and clinicians in developing biomarkers to serve as tools in clinical trials. The importance of this effort is evidenced by the FDA Critical Path initiative, which identifies Biomarker qualification as one of the Critical Path Opportunities (Citation2). Furthermore, the importance of the Drug development Tools qualification programs (including biomarker qualification) is underscored by the recent (2010) publication of the draft guidance for industry onthe Qualification Process forDrug development Tools (Citation3), andby the identification of “Advancing Regulatory Science and Innovation” as one of the five cross-cuttingareas to serve as FDA strategic priorities over the 5-year period 2011–2015 (Citation4). The goal of the biomarker qualification process is to qualify biomarkers for specific contexts of use, which could impact drug development and may shorten the time necessary to develop a successful marketing application for regulatory review. A context of use defines how a biomarker, once qualified will be used in clinical or nonclinical decision-making. Some specific recognized contexts of use include but are not limited to: stratification of patient populations, use in dose ranging and as outcome (efficacy or safety) measures.
The reader is referred to the DDT guidance and the Agency's website of biomarker qualification located at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/ucm284076.htm for details on the biomarker qualification submission process.
Bethesda Workshop, 2010
A pathway for qualification of biomarkers as novel tools to aid drug development, therefore, has been defined. The process, necessarily, requires a substantial data set supported by a very careful analysis. Because of this, the process is likely to be expensive and time consuming. The resources required are likely to exceed those that could be justified for any single pharmaceutical company or that would be likely to merit high priority in competitive public funded research. Although a rich body of information about biomarkers is already available as a result of industry and government research funded, in 2009, it was unclear whether sufficient information was available to support qualification of selected biomarkers for drug development in COPD.
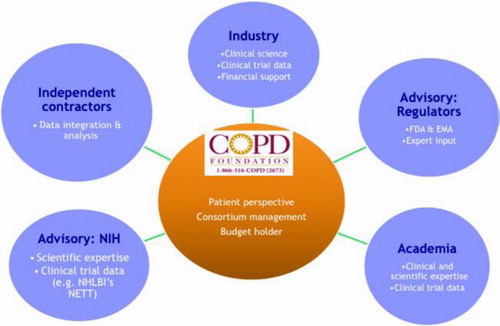
Building on the initial enthusiasm for the Biomarker Qualification process at the FDA and interest from pharmaceutical companies and academic research partners in sharing the scope of the data available, the “COPD Biomarkers Qualification Workshop” was organized in Bethesda, MD under the auspices of the COPD Foundation on January 10, 2010. The Foundation was able to provide a neutral ground where representatives of the FDA, European Medicines Agency (EMA), Industry, Academia and the NIH could meet and discuss available data in an open forum. The format for the workshop consisted of a series of sessions in which various biomarkers and classes of biomarkers were discussed with regard to several specific questions ().
Table 1. Questions relevant for each biomarker
Participants from government, academia and industry presented and led discussions () and were extremely open in sharing specific findings relevant to individual biomarkers from unpublished data sets. This was followed by straw votes on whether the various biomarkers were of interest and had sufficient data to merit consideration for qualification. Biomarkers in five general areas were felt to have a rich body of data available that might be sufficient to support qualification. In addition, several were felt to be of significant interest, but additional work was likely to be needed. One biomarker was noted to be in the process of review.
Table 2. Meeting agenda
COPD Exacerbations: Candidate Drug Development Tools
The EXACT-PRO (Exacerbations of Chronic Pulmonary Disease Tool –Patient-Reported Outcome) Initiative is a multi-sponsor project involving clinical, research, methodology, and regulatory experts in the development of a standardized PRO instrument to measure the frequency, severity, and duration of exacerbations in clinical trials of COPD. Phase I included focus groups and 1:1 interviews with 83 patients with COPD, and two expert panels to inform instrument content and structure. Phase II involved a two-group observational study of 222 patients with a clinician-confirmed exacerbation who completed the daily diary via personal digital assistant (PDA) on Days 1–28 of their exacerbation and Days 60-67.
A second group of 188 patients considered clinically stable completed the diary over seven days. The final 14 items comprising the EXACT include assessments of shortness of breath, chest congestion, cough, sputum, chest discomfort, feeling weak or tired, sleep disturbances, and concern or worry. Scores on the instrument exhibited strong evidence of reliability (internal consistency and reproducibility in stable patients) and validity, including responsiveness to change over time in acutely ill patients, correlation with the St. George's Respiratory Questionnaire (SGRQ), clinician and patient ratings of severity, and differentiation of responders and non-responders from Day 1 to 10 of the event.
These results suggested the instrument was ready for use in prospective clinical trials. Methods and results of the development and validation of the EXACT were summarized in a PRO Evidence Dossier submitted to the FDA to qualify the instrument for use in clinical trials testing the effect of treatment on frequency, severity and/or duration of exacerbations of COPD.
The discussion of the EXACT-PRO development process, which represented a collaboration among a number of pharmaceutical and academic collaborators with active participation of the FDA, was felt to be a successful paradigm for biomarker development.
Additional biomarkers for assessing COPD exacerbations were also discussed. Raised serum C-reactive protein (CRP) (Citation5) and plasma fibrinogen (Citation6) are associated with future risk of exacerbations and management strategies directed at normalizing sputum eosinophil counts have yielded reductions in exacerbation frequency (Citation7). However, the application of biomarkers to distinguish exacerbation and stable events in the same subjects has been disappointing. Although CRP in combination with a major symptom (Citation8) and serum amyloid A (Citation9) promise to identify exacerbations, their sensitivity and specificity was felt to be insufficient for widespread clinical utilization. Thus, no biochemical measures for exacerbations were considered ready for qualification.
Blood biomarkers
Blood biomarkers have attracted considerable interest and were discussed in detail. Several relatively novel biomarkers that may reflect lung specific activity were discussed. Blood levels of the lung specific biomarkers Clara Cell protein (CC)-16 (Citation10) and surfactant protein-D (Citation11) are significantly different in individuals with COPD when compared to smoker controls without airflow obstruction. As well as being associated with disease, serum surfactant protein-D is sensitive to the administration of both inhaled (Citation12) and oral corticosteroids (Citation11) in COPD. However, the clinical implication of raised or reduced levels of CC-16 and surfactant protein-D is uncertain and additional work is needed to determine their value in following disease progression and as intermediate endpoints in clinical studies.
The systemic component of COPD may also be reflected by raised mean values of blood fibrinogen, C-reactive protein (CRP) and IL-6. CRP and fibrinogen are associated with GOLD stage of disease and are elevated during COPD exacerbations of COPD. Elevated levels of CRP and fibrinogen have also been associated with increased risk for hospitalization and death from this disease (Citation13,14). CRP shows significant variability in COPD while fibrinogen is much more stable. Moreover blood fibrinogen is sensitive to intervention with inhibitors of p38 MAP kinase (dilmapimod [15] and losamapimod [16]) in COPD. Because of its stability, association with COPD-related outcomes (exacerbation, hospitalization and death) and sensitivity to intervention, fibrinogen was felt to be a very promising biomarker.
Health status
Health status/health-related quality of life measures are designed to provide a summative measure of the overall impact of COPD on health, regardless of phenotype. They are also designed to measure the overall benefit of treatment regardless of mechanism or site of action. The most widely used measure is the 50-item St. George's Respiratory Questionnaire (SGRQ). It has proven discriminative and evaluative properties and predictive validity. Much experience has been gained from its use in all pivotal trials in COPD over the last decade.
It uses empirical patient-derived weights to provide a valid overall measure of health impairment. This methodology has not found wide use because of its complexity and need for computerized scoring algorithms. Recent FDA guidance places great weight on deriving candidate PRO items directly from patients. A new instrument, the COPD Assessment Test (CAT) has done this and coupled it with modern mathematical techniques to ensure that it had very reliable measurement properties. This instrument only has 8 items and a simple 6-point scale. Studies in United States, Europe and China have shown a very strong correlation (> 0.8) with SGRQ. The strength of this association, between two instruments designed to measure the same construct but developed and structured in very different ways, provides powerful evidence that the SGRQ does measure impaired health in COPD. This, combined with the very rich data set available, led to a strong consensus that the SGRQ would be an excellent candidate biomarker for qualification.
Lung mechanics
For over 150 years, spirometry has been the predominant tool used in COPD to assess the presence and severity of airways obstruction and measure the response to therapy. FEV1 or changes in FEV1, have been used to predict mortality, characterize patients as surgical candidates (e.g., lung cancer resectional surgery, lung volume reduction surgery), approve medications for clinical use and designate patients as legally handicapped or disabled from gainful employment. However, recent data show that FEV1 does not correlate well with patient reported outcomes such as quality of life, degree of breathlessness, extent of exercise performance or bronchodilator responsiveness in those most severely obstructed (i.e., GOLD Stages III–IV).
Conversely, measurements of lung hyperinflation (RV, RV/TLV, IC, IC/TLC, and EELV) have been found to be superior to FEV1 in assessing dyspnea, exercise capability, ability to wean from mechanical ventilation and even survival. Casanova demonstrated in patients with mild to severe COPD that an Inspiratory Capacity (IC) to Total Lung Capacity (TLC) ratio (IC/TLC) ≤ 0.25 was associated with a two-fold increase in mortality over a 4-year follow-up period compared to patients with an IC/TLC > 0.25.
Recent data from the ECLIPSE cohort in 1162 COPD subjects shows that hyperinflation is present in all stages and worsens with increasingly more severe airflow obstruction (RV% pred vs. FEV1% pred, r = –0.645, p < 0.001) (Citation17). Moreover, RV% predicted correlated directly with complaints of breathlessness (p < 0.001), more impaired overall health status (r = 0.492, p < 0.001) and inversely with 6 MWD (r = –0.211, p < 0.001). Physiological measurement of hyperinflation is an important marker of lung mechanics that is valuable in characterizing COPD patients and assessing their response to therapy. Based on the discussions and the potentially rich data set available, a measure of lung inflation, and in particular of hyperinflation, was felt to be a potential biomarker worth further consideration.
Lung imaging
Chest CT scans have become extremely valuable tools in the assessment of COPD, because, among other virtues, the degree of emphysema can be accurately and noninvasively quantified (Citation18). Both qualitative (radiologist scoring) and quantitative (computerized image analysis) approaches have been used to analyze the severity and distribution of emphysema. Quantitative emphysema assessments are often based on determining the percentage of lung voxels below a specific threshold, such as -910 or -950 HU (Citation19,20). Measurements of airway wall dimensions have been more challenging, and many different approaches have been used.
Hasegawa and colleagues reported an approach (curved multiplanar reconstruction) to obtain longitudinal airway reconstructions and accurate measurements of airway wall area with airways as small as 2 mm in lumenal diameter (Citation21). Of interest, they found stronger correlations of FEV1 with airway wall area measurements with smaller, rather than larger airways. This work indicates the potential value of airway wall measurements in dissecting the heterogeneity of COPD, as well as the importance of studying small airways in such analyses. Several studies have shown that quantitative CT measures of emphysema and airway disease are significant and independent predictors of airflow obstruction (Citation22).
Change in chest CT lung density has been assessed as an outcome for the effect of alpha-1 antitrypsin (AAT) augmentation therapy in two studies of AAT-deficient subjects (Citation23,24); in both cases, the CT density outcome was of borderline statistical significance. Applying quantitative chest CT assessments in clinical trials requires careful quality control. Understanding of the impact of CT scanner brand, model, and reconstruction kernel is required. Standardized approaches to measurements to be used in clinical trials would be beneficial. Chest CT scans can have been used effectively for subject stratification. For example, the National Emphysema Treatment Trial (NETT) identified a benefit from lung volume reduction surgery for subjects with upper lobe predominant emphysema (Citation25). CT scans engender a non-trivial radiation exposure; the appropriate radiation dosage to balance subject risk and quantitative imaging data quality remains to be determined.
Exercise
Several measures of exercise were considered. Peak oxygen uptake is a predictor of survival (Citation26,27) and constant or incremental work rate cardiopulmonary exercise testing provides rigorous assessment of performance, but both require specialized equipment and methods, making studies in large groups of subjects problematic. The 6-minute walk test (6MWT) is attractive because it has been performed in large cohorts and is predictive of long-term outcomes on its own (Citation26,Citation28) or as part of the widely used BODE index (Citation29).
There is great experience in the use of 6MWT, particularly in North America, and it is accepted for labeling claims in other conditions (e.g., pulmonary hypertension) by the FDA. The incremental shuttle walk test (ISWT), more popular in Europe, has the advantage of being externally paced and reproducible and captures improvements conferred by treatments of known efficacy such as pulmonary rehabilitation. Areas of concern for both tests include a ceiling effect so that they are less responsive in individuals with mild exercise intolerance. A practice walk increases precision and can reduce sample size. For the 6MWT, new analyses suggest the minimal clinically important difference (MCID) may be closer to 25 m rather than 54 m for a variety of disorders (Citation30–32) and this is being reassessed in COPD.
Quadriceps strength measurement has mostly been confined to small studies although values in a larger cohort have recently become available (Citation33) and it is known that weakness is associated with increased mortality (Citation34). Physical activity is an independent predictor of long-term outcome (Citation35); it can be assessed by questionnaire or electronic device (Citation36,37). A joint European Union-pharma funded initiative between the industry and academia in Europe (the PROactive project) has the goal of developing a patient reported outcome tool by combining physical activity monitoring with a questionnaire developed from qualitative research with patients. Activity monitoring technology is advancing rapidly, but standardization of measurement methodology crucial, and this is presently being established as part of PROactive.
Because of the wealth of experience available, its current use by the FDA and acceptance in the clinical community, the 6MWT was felt to be the activity measure with the best available data set for qualification. Other measures including physical activity and quadriceps strength were considered of interest for further research and are currently being evaluated by a U.K. government-funded consortium, COPD MAP.
Other biomarkers
As noted above, a number of other biomarkers were discussed that were not felt to be likely candidates for qualification based on existing data sets. Two, however, were felt to be of considerable promise and were the subject of active ongoing research programs.
Desmosine
Elastin degradation products can be effective biomarkers in COPD (Citation38,39). Lung elastin undergoes degradation in COPD related to smoke exposure or genetically determined alpha-1 antitrypsin deficiency (AATD) (Citation40,41). Elastin degradation is detectable by measurements of specific amino acids, desmosine and isodesmosine (DI), which occur only in elastin. Recent methods have improved accuracy and sensitivity of measurements of DI in all body fluids (Citation42,43), including sputum. Accumulating data suggest that desmosine reflects disease severity, may reflect disease activity and may be sensitive to change, thus giving it great potential a biomarker.
Plasma levels of DI are increased in COPD (Citation44), are higher in AATD (Citation44) and increase with progression of disease (Citation45). Measurements of DI in urine correlate with COPD severity (Citation38). Healthy non-smokers have the lowest levels of DI excretion, which are progressively increased in smokers with normal lung function, in patients with stable COPD, and in patients with COPD during exacerbations. The highest levels have been observed in. Measurements of DI in urine and plasma in patients from the Swedish Twin Registry in 115 subjects with COPD demonstrated correlations with all lung function measurements after adjustments for age, gender, height, body mass index (BMI) and smoking.
Plasma desmosine values were uniformly correlated with FEV1 and DLCO (Citation46). Urinary desmosine values were significantly correlated with all lung function measurements after adjustments for age, gender, height, body mass index (BMI) and smoking. Finally, plasma and sputum levels of DI were lowered following treatment with tiotropium in although whether this is related to long term progression of emphysema is not known (Citation47). Desmosine, therefore, has great potential as a biomarker as it directly relates to disease pathogenesis.
Sputum measures
The collection of biological specimens from the airways involves either spontaneous or (more recently) induced sputum that provides a means to directly sample the lung, and is very appealing as a means to access potential biomarkers. These samples contain a multitude of inflammatory mediators that are associated with the disease process in COPD patients. However, sample processing and analyte measurements in a non-plasma matrix require considerable validation including spike recovery, which has not always been done. In addition, issues relating to the effect of variable dilution during the collection process and the presence or absence of spontaneous sputum, bacterial colonization, smoking habit and therapy need to be considered. As these have not generally been performed, sputum measures were not felt to be ready, at the present time, for qualification.
Exhaled breath biomarkers have the potential to sample the lung in a non-invasive manner. Sampling methodologies are well-tolerated, allowing repeated assessments of airway lining fluid in the clinic or at home using commercially available devices. There is a significant body of literature that supports the application of exhaled breath condensate (EBC) and exhaled breath volatiles in COPD drug development (Citation48, 49). However, there are currently several key issues that limit the potential for any exhaled breath biomarker for qualification (Citation50,51). Markers that have been explored included CO2 pH, NO, CO, metabolites, leukotrienes, prostanoids, hydrogen peroxide, cytokines, and aldehydes. Issues that remain to be resolved for these biomarkers include: (i) variability in sample collection methods, (ii) lack of standard assay methodology leading to variability in reported levels of biomarkers, (iii) lack of reproducibility data, (iv) dilution of EBC limits assay accuracy with currently available methodologies, (v) inconsistent associations with clinical measures based on data obtained from small numbers of subjects and (vi) difficulty in publishing negative studies.
Follow-up
At the conclusion of the Bethesda workshop, there was considerable enthusiasm on three fronts. First, it was felt that for several biomarkers sufficient data may exist in the form of completed studies to support a dossier for qualification. Second, it was clear that there was willingness of many parties, including pharmaceutical companies, to collaborate in an effort to prepare dossiers for qualification of biomarkers, including sharing unpublished proprietary data. Finally, the COPD Foundation seemed an ideal organization to provide a “neutral” platform that could facilitate a collaborative effort based on pooled existing data.
With this background, the COPD Foundation embarked on the creation of the COPD Biomarkers Qualification Consortium (CBQC). The Consortium would be supported by member pharmaceutical companies, each of which would contribute financial support, data and expertise. The consortium would also include experts from academia with the FDA, EMA and NHLBI in an advisory role.
Concurrent with the organizational efforts, five ad hoc working groups, reflecting the biomarkers that had the highest enthusiasm at the Bethesda workshop, met informally on a number of occasions during 2010. These groups conducted more detailed discussions on the merits and limitations of candidate biomarkers and conducted a more detailed survey of available data.
Based on the discussions of these groups, it was felt that three biomarkers would merit highest consideration for qualification: fibrinogen for stratification of subjects at risk for hospitalization and mortality, 6-minute walk distance for stratification of subjects at risk for mortality and SGRQ for subject stratification and as an outcome measure. Imaging was felt to be a very promising biomarker. However, a parallel effort for qualification of CT Scanning was currently in progress through the Society of Thoracic Radiology, so action on this biomarker was deferred. Measurement of lung volumes as a biomarker was felt to be promising, but a number of issues and concerns were raised which required further evaluation of available data and review of methods of quality control used on various studies.
The COPD Biomarkers Qualification Consortium
An organizational structure was created and arrangements made (), and the CBQC was officially launched on October 4th, 2010. Founding members included GlaxoSmithKline, BoehringerIngelheim, AstraZeneca and Pfizer. Novartis joined in October, 2011. The consortium is supported by restricted funds contributed by participating pharmaceutical companies. All intellectual property resulting from the CBQC efforts will be owned by the COPD Foundation and will be made freely available to all, thus facilitating the development of new treatments. The consortium is governed by a Steering committee with elected representatives for each partner as well as specific working groups (e.g., exercise, biochemical biomarkers, PROs, lung function, and two expert support groups: Regulatory and Data Management).
A data analysis structure was created, and, after soliciting bids, a contract was awarded to INC Research who will assemble a database pooling data provided by collaborators from industry, government and academic sources. This structure allowed sharing of anonymized clinical trials data in a pre-competitive setting, a key component for Public-Private-Partnerships.
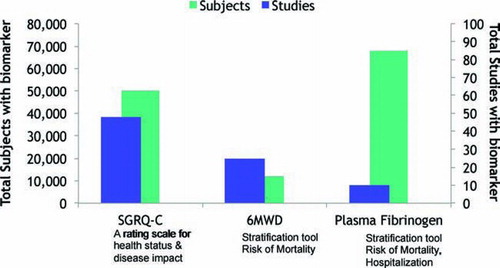
Formal working groups for fibrinogen, SGRQ, 6-minute walk distance and lung volumes were constituted. Because it was given a lower priority, work on lung volumes was deferred. The other working groups then conducted a series of assessments confirming the viability of these biomarkers and collecting a set of data from specific studies that could support a dossier (). Based on these reviews, letters of intent to submit a dossier for qualification of each these biomarkers were then submitted to the FDA. This led to a meeting with the FDA to discuss the overall process. The FDA expressed enthusiasm for the development of biomarkers to aid as tools in drug development. Specific comments were also made regarding the three letters of intent. The letter of intent for fibrinogen was accepted and the Biomarker Qualification Review Team (BQRT) has been created.
Figure 2. Number of studies and subjects that will be included in the analysis of the initial biomarker targets by the Consortium.
The response to the SGRQ and the 6-minute walk measures, however, was different. The FDA clarified that measures such as the SGRQ and the 6-minute walk were not considered as biomarkers, but rather as clinical assessments. The FDAalso indicatedthat the SGRQwas already used in clinical trials for COPD and did not need to go through thequalification process. The FDA also explained that the 6-minute walk could be used in clinical trials for stratification via direct interaction with the review Division without qualification. However, the Consortium was also encouraged by the FDA to consider the 6-minute walk as an outcome for which the Agency would be willing to engage in the process of qualification. Although FDA currently accepts the use of the SGRQ in clinical trials and will accept the 6-minute walk as a stratification tool for patient enrollment, careful review of these measures is still necessary for rigorous study design.
In particular, if a measure is used during a drug development program, there must be confidence that the measure itself will be acceptable to regulators. The analyses provided by the Consortium will provide clear delineation of the cut points used for stratification and evidence based estimates of sample size needed for clinical trials. General acceptance of these aspects of these measures is essential to facilitate drug development. To these ends, assembly and analysis of the data equivalent to that required for a dossier remain necessary, even if FDA review would not be required. The results from these efforts should be prepared as white papers which would be available for public comment. The working groups for the SGRQ and 6-minute walk distance were charged to meet these goals.
Output
A key part of the FDA review process is publication of the data submitted in the dossier. In this context, “publication” means make public. This is essential both for there to be public review and comment and for the data and analyses to be widely usable. It is the intent of the CBQC to publish the analyses and summaries of the combined data sets as white papers. It is anticipated that these will be online publications in the Journal of COPD, which is the official journal of the COPD Foundation.
At present, three such publications are expected, one each for fibrinogen, SGRQ and 6-minute walk distance. It is also anticipated that, as these data and analyses are completed, that they will contribute to future guidance prepared by the FDA relating to drug development in COPD. Of the three biomarkers that are being reviewed, it is currently anticipated that a formal regulatory application for fibrinogen, supporting a subject stratification context of use, will be submitted. SGRQ and 6-minute walk distance, which have already been used in applications approved by the FDA, will be supported by White Papers that will define their measurement characteristics, as well as their use in clinical trials as stratification tools and outcome measures. Finally, it is hoped that the process pioneered by these efforts will represent a paradigm for the development of additional biomarkers that can serve as tools to facilitate the development of new treatments for COPD.
Declaration of Interest Statement
SR received honoraria for lectures from AARC, Almirall, Am Col Osteopathic Physicians, Asan Medical Center, American Thoracic Society, California Society of Allergy, CME Incite, COPD Foundation, Creative Educational Concepts, Dey, Duke University, Forest, France Foundation, HSC Medical Education, Information TV, Lung Association, Novartis (Horsham, Nycomed, Otsuka, PeerVoice, Pfizer, Shaw Science, University of Washington, University of Alabama Birmingham, VA Sioux Falls.
SR received honorarium for consulting with the following: ABIM, Able Associates, Adelphi Research, Align2Acton, Almirall/Prescott, APT Pharma/Britnall, Astra-Zeneca, American Thoracic Society Beilenson, Boehringer Ingelheim, Boehringer Ingelheim (ACCP), BoomCom, Britnall and Nicolini, Capital Research, Chiesi, Clarus Acuity, CommonHealth, Complete Medical Group, Consult Complete, COPDForum, DataMonitor, Decision Resources, Dunn Group, Easton Associates, Equinox, Forest, Frankel Group, Fulcrum, Gerson Lehman, Globe Life Sciences, Guidepoint, Health Advanced, Hoffman LaRoche, Informed, Insyght, KOL Connection, Leerink Swan, M. Pankove, McKinsey, MDRxFinancial, Medimmune, Merck, Novartis, Nycomed, Oriel, Osterman, Peal, Penn Technology, Pennside, Pfizer, PharmaVentures, Pharmaxis, Prescott, Price Waterhouse, Propagate, Pulmonary Reviews, Pulmatrix, Reckner Associates, Recruiting Resource, Roche, Sankyo, Schering, Schlesinger Medical, Scimed, Smith Research, Sudler and Hennessey, Summer Street Research, Talecris, Think Equity, UBC, Uptake Medical, Vantage Point Management.
RT-S is an employee and shareholder of GlaxoSmithKline.
MP discloses receiving personally fees for lecturing or consultancy from Portaero, Broncus, GSK, AZ, Chiesi, & Novartis. His institutions have held or hold funding on his behalf from AZ, GSK, Lilly, the MRC, the EU, the Wellcome Trust and the Technology Strategy Board. His contribution to this project was funded by the NIHR Respiratory Biomedical research unit at the Royal Brompton and Harefield Foundation NHS Trust and Imperial College who part fund his salary.
The authors alone are responsible for the content and writing of the paper.
Acknowledgements
CBQC Steering Committee: Cerasoli, Frank, Pfizer; Compton, Christopher, Novartis; Disse, Bernard, Boehringer-Ingelheim; Goldmann, Mitchell, AstraZeneca; Lomas, David, Cambridge University; Martin, Ubaldo, AstraZeneca; Merrill, Debora, COPD Foundation; Tal-Singer, Ruth, GlaxoSmithKline (co-chair); Rennard, Stephen, University of Nebraska, (co-chair); Tetzlaff, Kay, Boehringer-Ingelheim; Thomashaw, Byron, Columbia University; Walsh, John, Alpha-1 Foundation and COPD Foundation; Liaison –NHLBI.
CBQC Working groups: Fibrinogen: Barr, Graham, Columbia University; Compton, Christopher, Novartis; Goldmann, Mitchell, AstraZeneca; Lomas, David, Cambridge University; Lowings, Michael, Glaxo-SmithKline; Mannino, David, University of Kentucky (co-chair); Miller, Bruce, GlaxoSmithKline (co-chair); Merrill, Debora, COPD Foundation; Rennard, Stephen, University of Nebraska; Snyder, Jeffrey, Boehringer-Ingelheim; Tal-Singer, Ruth, GlaxoSmithKline; Tetzlaff, Kay, Boehringer-Ingelheim; Vestbo, Jorgen, University of Manchester; SGRQ: Glendenning, Alastair, Novartis; Goldmann, Mitchell, AstraZeneca; Jones, Paul, University of London (co-chair); Karlsson, Niklas, AstraZeneca; Menjoge, Shailendra, Boehringer-Ingelheim; Merrill, Debora, COPD Foundation; Muellerova, Hana, GlaxoSmithKline; Rudell, Katja, Pfizer; Rennard, Stephen, University of Nebraska; Tabberer, Margaret, GlaxoSmithKline (co-chair); 6-minute Walk: Celli, Bartolome, Harvard University (co-chair); Casaburi, Richard, LA BioMed; Cerasoli, Franklin Jr., Pfizer; Compton, Christopher, Novartis; Criner, Gerard, Temple University; Croxton, Thomas, NHLBI; Goldmann, Mitchell, AstraZeneca; Locantore, Nicholas, GlaxoSmithKline; McLoughlin, Katrina, GlaxoSmithKline; Merrill, Debora, COPD Foundation; Polkey, Michael, Royal Brompton; Rennard, Stephen, University of Nebraska; Sciurba, Frank, University of Pittsburgh; Tal-Singer, Ruth, GlaxoSmithKline; Tetzlaff, Kay, Boehringer-Ingelheim (co-chair); Weisman, Idelle, Novartis; Lung Volumes: Casaburi, Richard (Co-Chair), LA BioMed; Cerasoli, Franklin Jr. (Co-Chair) Pfizer;, Goldman, Mitchell, AstraZeneca; Lazaar, Aili, GlaxoSmithKline; Kesten, Steven, Boehringer-Ingelheim; Tetzlaff, Kay, Boehringer-Ingelheim, Rennard, Stephen, University of Nebraska, Gomes, Joe, Roche; Mattes, William, COPD Foundation; Walsh, John, Alpha-1 Foundation and COPD Foundation; CBQC Data committee: Locantore, Nicholas, Glaxo-SmithKline; Menjoge, Shailendra, Boehringer-Ingelheim; Peppe, Dennis, Boehringer-Ingelheim; Sutradhar, Santosh, Pfizer; Walsh, Simon, Novartis; CBQC Regulatory Committee: Aprile, Peter, Pfizer; Lowings, Michael, GlaxoSmithKline; McLoughlin, Katrina, GlaxoSmithKline; Snyder, Jeff, Boehringer-Ingelheim; Staff: Jamie Lamson Sullivan, COPD Foundation; and the COPD Foundation Biomarkers Workshop chairs: Gilbert McClain, Lydia, FDA; Kiley, James, NHLBI; Presenters: Brightling, Christopher, Leicester University; Burke, Laurie, FDA; Casaburi, Richard, LA BioMed; Celli, Bartolome, Harvard University; Criner, Gerald, Temple University; Croxton, Thomas, NHLBI; Fabbri, Leo, University of Modena & Reggio Emilia; Gaw, Alastair, AstraZeneca; Gilbert McClain, Lydia, FDA; Goodsaid, Frederico, FDA; Jones, Paul, University of London; Kilty, Ian, Pfizer; Leidy, Nancy, United BioSource Corporation; Kiley, James, NHLBI; Lomas, David, Cambridge University; O'Donnell, Dennis, Queen's University; Polkey, Michael, Royal Brompton Hospital; Rennard, Stephen, University of Nebraska; Sciurba, Frank, University of Pittsburgh; Silverman, Edwin, Harvard University; Stockley, Robert, University Hospital Birmingham, NHS Foundation Trust; Tal-Singer, Ruth, GlaxoSmithKline; Vestbo, Jorgen, University of Manchester; and Participants: Anderson, Wayne, GlaxoSmithKline; Aprile, Peter, Pfizer; Bao, Warren, Pfizer; Baribaud, Fred, Johnson & Johnson; Barnathan, Elliot, Johnson & Johnson; Barr, Graham, Columbia University; Bode, Fred, Sepracor; Boshoff, Chris, NIH; Brightling, Christopher, Leicester University; Burke, Laurie, FDA; Casaburi, Richard, UCLA; Celli, Bartolome, Harvard University; Cerasoli, Franklin, Pfizer; Chowdhury, Badrul, FDA; Compton, Christopher, Novartis; Coyle, Anthony, Medimmune; Crapo, James, National Jewish Health; Criner, Gerald, Temple University; Croxton, Thomas, NHLBI; Cruz, Cristina, Talecris; Disse, Bernard, Boehringer-Ingelheim; Donohue, Christine, CSL Behring; Dorney Koppel, Grace Ann; Drone, C. Alison, NIH; Fabbri, Leo, Unimore. it; Fan, Ying, FDA; Fernandes, Peter, Novartis; Finn, Symma, NIH; Forsman-Semb, Kristina, AstraZeneca; Gaw, Alasdair, AstraZeneca; Gervais, Francois, Merck; Glendenning, Alastair, Novartis; Goldmann, Mitchell, AstraZeneca; Goodsaid, Federico, FDA; Han, MeiLan K., University of Michigan; Herrie, Myra, Novartis; Hitchcock, Chris, Pfizer; Jhingran, Priti M., GlaxoSmithKline; Ji, Ping, FDA; Jones, Paul, University of London; Karlsson, Niklas, AstraZeneca; Kesten, Steven, Boehringer-Ingelheim; Kilty, Iain, Pfizer; Kramer, Benjamin, Novartis; Lamson, Jamie, COPD Foundation; Lipson, David A., GlaxoSmithKline; Locantore, Nichloas, GlaxoSmithKline; Lomas, David, Cambridge University; Lowings, Mike G., GlaxoSmithKline; Loza, Matt, Johnson & Johnson; Lyons, David, Irish Medicines Board; Ma, Shuren, Columbia University; MacMillan, Christine, CSL Behring; Mannino, David, University of Kentucky; Martin, Ubaldo, AstraZeneca; Martinez, Fernando, University of Michigan; Mascelli, MaryAnn, Medimmune; McLoughlin, Katrina, GlaxoSmithKline; Shailendra, Menjoge, Boehringer-Ingelheim; Merrill, Debora, COPD Foundation; Michele, Theresa, FDA; Miller, Bruce E., GlaxoSmithKline; Miller, Douglas, Wyeth/Pfizer; Molfino, Nestor, Medimmune; Mullerova, Hana, GlaxoSmithKline; Mummaneni, Padmaja, FDA; Leidy, Nancy, United BioSource Corporation; Newbold, Paul, AstraZeneca; Nocka, Karl, Pfizer; Noone, Marianne, FDA; O'Donnell, Denis, Quenn's University; Pariser, Anne, FDA; Peppe, Dennis, Boehringer-Ingelheim; Pillaj, Sreekumar, Roche; Polkey, Michael, Royal Brompton & Harefield NHS; Punturieri, Antonello, NHLBI; Rennard, Stephen, University of Nebraska; Rudell, Katja, Pfizer; Sahajwalla, Chandrahas, FDA; Sandhaus, Robert, Alpha-1 Foundation; Sciurba, Frank, UPMC; Seymour, Sally, FDA; Silberstein, David, AstraZeneca; Silverman, Edwin, Harvard; Snyder, Jeffrey, Boehringer-Ingeelheim; Stockley, Robert, BHAM; Stringer, Clive, AstraZeneca; Sutradhar, Santosh, Pfizer; Swensen, Andrine, Novartis; Tabberer, Margaret, GlaxoSmithKline; Tal-Singer, Ruth, GlaxoSmithKline; Tan, Keith, Pfizer; Tetzlaff, Kay, Boehringer-Ingelheim; Thomashaw, Byron, Columbia University; Turino, Gerard, Columbia University; Vestbo, Jorgen, Manchester; Walsh, John, COPD Foundation; Walsh, Simon, Novartis; Walton, Marc, FDA; Waltz, David, Novartis; Ward, Christine, Medimmune; Weinmann, Gail, NIH; Weisman, Idelle, Novartis; Whittaker, Paul, Novartis; Wire, Patrick D., GlaxoSmithKline; Wolf-Rodda, Julie, NIH; Wolford, Eric, Talecris; Woodruff, Prescott G., University of California, San Francisco; Yao, Yihong, Medimmune; Yates, Julie C., GlaxoSmithKline; Zeldin, Robert, Novartis; Zhang, Jie, Novartis.
References
- Kaitin KI, DiMasi JA. Pharmaceutical innovation in the 21st century: new drug approvals in the first decade, 2000–2009. Clin Pharmacol Therapeut 2011; 89:183–188.
- Woodcock J, Woosley R. The FDA critical path initiative and its influence on new drug development. Annu Rev Med 2008; 59:1–12.
- FDA. Guidance for Industry Qualification Process for Drug Development Tools. 2010.
- FDA. Strategic Priorities: Cross-Cutting Strategic Priorities. http://www.fda.gov/aboutFDA/Reportsmanualsforms/Reports/ucm246737.htm
- Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 175:250–255.
- Dahl M, Tybjaerg-Hansen A, Vestbo J, Lange P, Nordestgaard BG. Elevated plasma fibrinogen associated with reduced pulmonary function and increased risk of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164:1008–1111.
- Siva R, Green RH, Brightling CE, Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J 2007;29:906–13.
- Hurst JR, Donaldson GC, Perera WR, Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 174:867–74.
- Bozinovski S, Hutchinson A, Thompson M, Serum amyloid a is a biomarker of acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 177:269–78.
- Lomas DA, Silverman EK, Edwards LD, Miller BE, Coxson HO, Tal-Singer R. Evaluation of serum CC-16 as a biomarker for COPD in the ECLIPSE cohort. Thorax 2008; 63:1058–63.
- Lomas DA, Silverman EK, Edwards LD, Serum surfactant protein D is steroid sensitive and associated with exacerbations of COPD. Eur Respir J 2009; 34:95–102.
- Sin DD, Man SF, Marciniuk DD, The effects of fluticasone with or without salmeterol on systemic biomarkers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 177:1207–1214.
- Sin DD, Vestbo J. Biomarkers in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2009; 6:543–545.
- Danesh J, Lewington S, Thompson SG, Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA 2005; 294:1799–1809.
- Barnes NC, Pavord ID, Maden C, Gomez E, Keene O, Tal-Singer R. Evaluation of an oral p38 mitogen activated protein kinase (MAPK inhibitor) SB-681323 in COPD. Eru Resp J 2009; 34(sup):544s.
- Lomas DA, Lipson DA, Miller BE, An oral inhibitor of p38 MAP kinase reduces plasma fibrinogen in patients with chronic obstructive pulmonary disease. J Clin Pharmacol. 2012; 52:416–424.
- Agusti A, Calverley PM, Celli B, Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010; 11:122.
- Bergin C, Muller N, Nichols DM, The diagnosis of emphysema. A computed tomographic-pathologic correlation. Am Rev Respir Dis 1986; 133:541–546.
- Muller NL, Staples CA, Miller RR, Abboud RT. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest 1988; 94:782–787.
- Gevenois PA, De Vuyst P, Sy M, Pulmonary emphysema: quantitative CT during expiration. Radiology 1996; 199:825–829.
- Hasegawa M, Nasuhara Y, Onodera Y, Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 173:1309–1315.
- Patel BD, Coxson HO, Pillai SG, Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 178:500–505.
- Dirksen A, Piitulainen E, Parr DG, Exploring the role of CT densitometry: A randomised study of augmentation therapy in alpha-1 antitrypsin deficiency. Euro Respir J 2009; 33:1345–1353.
- Dirksen A, Dijkman JH, Madsen F, A randomized clinical trial of alpha(1)-antitrypsin augmentation therapy. Am J Respir Crit Care Med 1999; 160:1468–1472.
- Fishman A, Martinez F, Naunheim K, A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003; 348:2059–2073.
- Cote CG, Pinto-Plata V, Kasprzyk K, Dordelly LJ, Celli BR. The 6-min walk distance, peak oxygen uptake, and mortality in COPD. Chest 2007; 132:1778–1785.
- Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am J Respir Crit Care Med 2003; 167:544–549.
- Spruit MA, Polkey MI, Celli B, Predicting outcomes from 6-minute walk distance in chronic obstructive pulmonary disease. J Am Med Dir Assoc 2012; 13:291–297.
- Celli BR, Cote CG, Marin JM, The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350:1005–1012.
- Gremeaux V, Troisgros O, Benaim S, Determining the minimal clinically important difference for the six-minute walk test and the 200-meter fast-walk test during cardiac rehabilitation program in coronary artery disease patients after acute coronary syndrome. Arch Phys Med Rehabil 2011; 92:611–619.
- du Bois RM, Weycker D, Albera C, Six-minute-walk test in idiopathic pulmonary fibrosis: test validation and minimal clinically important difference. Am J Respir Crit Care Med 2011; 183:1231–1237.
- Gabler NB, French B, Strom BL, Validation of 6-minute walk distance as a surrogate end point in pulmonary arterial hypertension trials. Circulation 2012; 126:349–356.
- Seymour JM, Spruit MA, Hopkinson NS, et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J 2010; 36:81–88.
- Waschki B, Kirsten A, Holz O, Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest 2011; 140:331–342.
- Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171:972–977.
- Waschki B, Spruit MA, Watz H, Physical activity monitoring in COPD: Compliance and associations with clinical characteristics in a multicenter study. Respir Med 2012; 106:522–530.
- Luisetti M, Ma S, Iadarola P, Desmosine as a biomarker of elastin degradation in COPD: current status and future directions. Eur Respir J 2008; 32:1146–1157.
- Turino GM, Ma S, Lin YY, Cantor JO, Luisetti M. Matrix elastin: a promising biomarker for chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2011; 184:637–641.
- Chrzanowski P, Keller S, Cerreta J. Elastin content of normal and emphysematous lung parenchyma. Am J Med 1988; 69:351–359.
- Wright RR. Elastic tissue of normal and emphysematous lungs: a tridimensional histological study. Am J Pathol 1961; 39:355–363.
- Ma S, Lieberman S, Turino GM, Lin YY. The detection and quantitation of free desmosine and isodesmosine in human urine and their peptide-bound forms in sputum. Proc Natl Acad Sci USA 2003; 100:12941–12943.
- Ma S, Turino GM, Lin YY. Quantitation of desmosine and isodesmosine in urine, plasma, and sputum by LC-MS/MS as biomarkers for elastin degradation. J Chromatogr B Analyt Technol Biomed Life Sci 2011; 879:1893–1898.
- Ma S, Lin YY, Turino GM. Measurements of desmosine and isodesmosine by mass spectrometry in COPD. Chest 2007; 131:1363–1371.
- Fregonese L, Ferrari F, Funagalli M, Luisetti M, Stolk J, Iadarola P. Long-term variability of desmosine/isodesmosine as biomarker in alpha-1-antitrypsin deficiency-related COPD. J COPD 2011; 3:229–333.
- Lindberg CA, Engstrom G, de Verdier MG, Total desmosines in plasma and urine correlate with lung function. Eur Respir J 2012; 39:839–845.
- Ma S, Lin YY, Tartell L, Turino GM. The effect of tiotropium therapy on markers of elastin degradation in COPD. Respir Res 2009; 10:12.
- Montuschi P. Analysis of exhaled breath condensate in respiratory medicine: methodological aspects and potential clinical applications. Ther Adv Respir Dis 2007; 1:5–23.
- Borrill ZL, Roy K, Singh D. Exhaled breath condensate biomarkers in COPD. Eur Respir J 2008; 32:472–486.
- Effros RM, Casaburi R, Su J, The effects of volatile salivary acids and bases on exhaled breath condensate pH. Am J Respir Crit Care Med 2006; 173:386–392.
- Effros RM, Casaburi R, Porszasz J, Rehan V. Why conventional exhaled breath condensate pH studies cannot provide reliable estimates of airway acidification. Chest 2011; 140:1099.