Abstract
Background: Cognitive deficit is a common problem in patients with chronic obstructive pulmonary disease (COPD). The aim of this study was to prospectively evaluate if MRI can demonstrate microstructural volume loss and the diffusion anisotropic change in subjects with COPD, compared with cognitively normal (CN) subjects. Methods: Six subjects with severe COPD, 13 with moderate COPD, and 12 CN subjects underwent isotropic volumetric T1-weighted imaging and diffusion tensor imaging (DTI). Voxel-based statistical analyses among groups were performed on brain volumes, fractional anisotropy (FA) and trace. Cognitive function tests were performed in all subjects, and the Cognitive function tests (CFT) scores were compared among the three groups. Results: No significant regional difference in volume was found in both the severe and moderate COPD groups relative to the CN group. Comparing between severe COPD and CN, FA was reduced in both the cerebral cortices, and in frontoparietal periventricular white matter. The trace value of the severe COPD group was significantly higher in the cerebral cortices, and in frontoparietal periventricular white matter, than that of the CN group. The severe COPD group showed significantly lower scores in the language-related, visuospatial, and frontal executive functions compared to those of the CN and moderate COPD group. Conclusion: This study demonstrated that COPD could affect the axonal integrity in multiple brain regions, and change in DTI might be related with the severity of the COPD.
Introduction
Cognitive impairment has been documented as one of the important extrapulmonary problems and is common in patients with chronic obstructive pulmonary disease (COPD) (Citation1). Furthermore, impaired neuropsychologic performance may be associated with mortality and disability in certain COPD populations (Citation2). Specific treatment, such as oxygen therapy, pulmonary rehabilitation and surgery may prevent the deterioration of cognition impairment in COPD (Citation3–5). The diagnosis of cognition impairment remains on a clinical and neuropsychologic examination, and conventional structural brain imaging demonstrates no obvious brain lesions in COPD patients. Therefore, it is difficult to detect the cognitive impairment at subclinical stage.
More sensitive and quantitative MRI techniques can reveal the change of microstructure that assesses functional abnormality in a macroscopic normal brain. Recent development methods for the analysis of MRI data have enabled group differences in brain structure to be investigated on a voxel-by-voxel basis. Voxel-based morphometry (VBM) is useful to know the detailed anatomy of the brain and correlate the findings with neurologic manifestation. The results provide objective and bias-free information about the brain structure. Today, the voxel-based investigations of the brain structure are commonly applied neuropsychologic disorders, including cognitive deficit (Citation6–8).
Diffusion tensor imaging (DTI), one of the advanced MRI techniques, is an effective tool that evaluates the microstructural change of white matter in vivo. The analysis of fiber integrity using DTI has also been performed in a wide range of neuropsychologic disorders (Citation9). The two most common indices measured from DTI data are the fractional anisotrophy (FA) and the trace. FA can be used to measure the quantitative integrity of brain tissue, and the trace, a sum of the quantitative measure of the motion of water considered in all directions, is an index of water movement across cell membranes (Citation10–12).
We presumed that fiber integrity and structural volumetric MRI measures may allow the improvement of our understanding and quantification of the progression of the neuronal damage in COPD in vivo. We have found that some studies demonstrated microstructural alterations of the brain in obstructive sleep apnea (OSA), similar to COPD in term of chronic hypoxia, using VBM (Citation13) or DTI (Citation14). Therefore, if VBM or DTI can demonstrate the brain regions that are affected within the COPD group relative to the control population, these methods will be valuable in the field of research and or management of cognitive impairment of COPD patients.
The purpose of this study is to evaluate regional microstructural changes including brain volume loss and fiber integrity in COPD patients by comparing them with normal controls using VBM and DTI.
Methods
Subjects
Institutional review board approval and informed consent were obtained. Subjects were recruited through the database of pulmonary medicine of Kyung Hee University Hospital at Gangdong (C.W.C) based on following inclusion criteria. 1) Patients who were diagnosed as moderate (FEV1% predicted 50–80) and severe degree (under 50) of COPD. The diagnosis and classification of COPD were made according to the criteria provided by the American Thoracic Society (Citation15), 2) patients who received appropriate management by a subject's usual pulmonary physician prior to enrollment and had been clinically stable without an oxygen supply, 3) normal range in clinical laboratory evaluation (CBC, extended chemistry panel including albumin), and 4) the patients who have been receiving only a standardized daily therapy long-acting anticholinergic drug (Tiotropium, Spiriva 18 mcg). Using ventolin, prn inhalation was permitted for prevention of hypoxia attack. The last criterion was to avoid the compounding effect by medication.
Age-, and gender-matched healthy controls were enrolled in the study as the control group. For all individuals in the normal control group, a detailed medical history including pulmonary disease, dyspnea during rest and exercise, smoking, medication, etc. were reviewed by clinician's interview, and chest radiograph and oxygen saturation using pulse oxymeter were used to exclude the subject with potential pulmonary disease from normal control cohort.
Exclusion criteria for the study were as follows: 1) Patients having left heart failure or carotid stenosis over 50%; 2) presence of predicted memory or language deficit; 3) patients having taken any psychotropic drugs for at least 2 weeks prior to the study; 4) known neurologic disorders such as epilepsy, stroke, head injury, dementia, toxic chemical exposure, sleep disorder, etc.; 5) known concomitant severe or chronic medical illnesses such as hepatic failure, chronic renal failure, decompensate diabetes mellitus, etc.; 6) history of depression and other major psychiatric disorders (anxiety disorders, schizophrenia, substance or alcohol abuse/dependence) according to DSM-IV criteria (Citation16); 7) history of psychosurgery or any other neurosurgical procedure; 8) the usual contraindications for MRI, such as pacemakers, and a history of intraocular metal fragments, cochlear implants, or claustrophobia; 9) significant parenchymal lesion including territorial infarction, brain tumor, old ICH on structural brain MRI; and, 10) significant cerebral white matter lesion on MRI that was rated over 3 (the scoring of white matter lesion was used as the rating method that had been described by Wahlund et al. (Citation17)). The assessment of the last two criteria was performed after MRI acquisitions.
We assessed the demographics (age and sex), the known chronic diseases such as hypertension and diabetes mellitus, education age, and smoking history, smoking pack-year by the interview and the review of medical record in subjects and controls.
Neuro-psychological tests
All subjects were right-handed, had normal or corrected-to-normal vision, and normal hearing capacity. All patients and controls underwent a standard neuropsychologic test, which included the Mini-Mental State Examination for global cognitive ability (K-MMSE) and Barthel Activities of daily living (B-ADL), as well as a standardized neuropsychological test and battery of the Seoul Neuropsychological Screening Battery (Citation18). This battery contains tests for attention, language, praxis, four elements of Gerstmann syndrome, visuoconstructive function, verbal and visual memory, and frontal/executive function.
Among these, scorable tests included the digit span (forward and backward), the Korean version of the Boston Naming Test (KBNT), written calculations (three items each for addition, subtraction, multiplication and division; one point for each correct item), five items for ideomotor limb praxis (one point for each correct item), the Rey–Osterrieth Complex Figure test (RCFT: copying, immediate and 20 min delayed recall, and recognition), Seoul Verbal Learning Test (SVLT: three learning-free recall trials of 12 words, 20 min delayed recall trial for these12 items, and a recognition test), phonemic and semantic Controlled Oral Word Association Test (COWAT), and Stroop test (word and color reading of 112 items in 2 min). The scores of the items in each subject were recorded (H.Y.R).
MRI protocol
MR imaging was performed on a 3.0-T MR system (Achieva, Philips Medical system, The Netherlands) equipped with a neurovascular head and neck coil. For the volumetric analysis and image registration to a brain anatomy template, the isometric sagittal structural volumetric T1-weighted images (T1W) were acquired by magnetization-prepared rapid acquisition of gradient echo sequence. The imaging parameters were following: repetition time (TR)/echo time (TE) = 8 msec/4 msec; 8° flip angle; matrix = 236 × 480; voxel = 1 × 1 × 1 mm3. The T2-weighted and FLAIR images were also acquired. The imaging parameters of T2-weighted images were as follows: TR/TE = 3000/80; a 4.5 mm thickness; matrix = 232 × 240; 27 contiguous slices covering the entire brain. The imaging parameters of FLAIR images were as follows: TR/TE/TI = 11000/124/2800 ms; a 4.5 mm thickness; matrix = 232 × 336.
For DTI data, a single-shot spin-echo echo-planar imaging (EPI) sequence was used with the following imaging parameters: TR/TE = 5700/78 ms; SENSE factor, 2.5; matrix = 112 × 109; voxel = 2.2 × 2.2 × 2.2 mm3; 52 contiguous slices that were each 2.2-mm thick; and b = 0 and 800 sec/mm2 applied along 32 diffusion-encoding directions. Additional spin-echo EPI scans without diffusion gradients (b = 0 image) were also acquired for mapping diffusion indices and normalizing diffusion measurements into the volumetric T1W image.
Following acquisition and image reconstruction, the data were transferred to an off-line personal computer for image processing and analysis. All subsequent image processing was performed by one of the authors (M.J.K).
Image processing and image analysis
Visual examination
The individual brain images of all subjects were evaluated before the imaging process for visible brain lesions conformed to exclusion criteria and the rating of subcortical white matter hyperintense lesions on FLAIR MRI. One subject was in the moderate COPD group was found to have a major parenchymal infarction, and this case was excluded from subjects.
DTI and volumetric data
For each subject, all following processing steps, were performed using the Statistical Parametric Mapping program version 5 (SPM5, Wellcome Department of Cognitive Neurology, London, UK).
First, the DTI studio software (Johns Hopkins University, Baltimore, MD) was used to create FA and trace maps from DTI the data. The DTI image without a diffusion gradient (DT_b0) and corresponding FA and trace maps were co-registered to the T2-weighted image using a normalized mutual information algorithm. Then, the T2-weighted image and all of the maps were further co-registered to the anatomical 3 dimensional (3D) T1W image. Before the spatial normalization step, a specific brain template was generated using the volumetric T1W image because the Montreal Neurological Institute (MNI) template was generated with young normal subjects. This template was created using 43 senior Korean subjects (mean age, 64.9 years; standard deviation (SD), 7.60 years) with normal cognition, who had larger ranges in age than those of the MNI template used in SPM5.
After creating the specific template, individual 3D T1W images were spatially normalized to the specific template using a nonlinear transformation. To obtain the brain tissue maps, the 3D T1W image was segmented into gray matter, white matter, and cerebrospinal fluid. All normalized 3D T1W image data were multiplied by the binary GM mask data in order to predominantly remove white matter, cerebrospinal fluid, and non-brain voxels. In order to spatially normalize all DTI indices for each subject, transport information of the volumetric T1W image for each subject into the standard space was applied to the DTI indices. Finally, masked 3D T1W images were smoothed with a 5-mm isotropic full-width-at half-maximum (FWHM) Gaussian kernel according to the optimized VBM processing algorithm. We also smoothed the Gaussian kernel of 8 × 8 × 10 mm FWHM for the FA and trace maps, which was about 4 times of the original voxel sizes.
Statistical analysis
The subjects were classified in three groups: moderate COPD groups, severe COPD groups and normal control. To compare the demographics and clinical characteristics among three groups, we used one-way ANOVA or Kruskal–Wallis test. The Kruskal–Wallis with Bonferroni-corrected post hoc adjustment was used to compare scores of the neuropsychologic test among the three groups (p < 0.05). Statistical tests of MRI were performed using the SPM5. The voxel-wise analysis of covariance (one-way ANOVA) was performed for the GM volumes, FA, and trace images between patients and controls co-varying for subject age. We used multiple comparisons using a false discovery rate (FDR) of 5% with a threshold looking for clusters with at least 10 contiguous voxels for GM volumes and a FDR of 5% with a threshold looking for clusters with at least 10 contiguous voxels for FA and Trace diffusivity.
Results
Six subjects with severe COPD (6 men, mean age, 69.80 years), 13 with moderate COPD (12 men, 1 women; mean age, 65.23 years), and 12 healthy control (normal cognitive) subjects (12 men, mean age, 63.92 years) were enrolled in this study. There was no significant difference in the clinical characteristics among the groups () except smoking history. All COPD patients had the smoking history, while 66.7% of the control group had smoking history (p = 0.035, Kruskal–Wallis test) ().
Table 1. Demographic factors and neuropsychological test of normal control, moderate, and severe COPD patients
Neuro-psychological Tests
The Kruskal–Wallis test demonstrated a significant difference among groups for language-related function (total calculation score), visuospatial function (Rey CFT copy time) and frontal executive function (COWAT phonemic score, Stroop Test Color reading correct). These scores, confirmed by a Mann–Whitney post hoc test, were significantly lower in the severe COPD group than both the control and moderate COPD groups. The results were shown in .
VBM of volumetric MR imaging
VBM did not present any significant regional difference in brain volume in both severe and moderate COPD groups compared with the normal control group, and there were no significant regional differences between the moderate and severe COPD groups with respect to regional brain volume.
VBM of diffusion tensor imaging
FA
( and ) The voxel-based analysis of FA difference between the control and moderate COPD groups showed that FA was reduced in both the superior and middle frontal gyri and white matter, and right occipital subcortical white matter in the moderate COPD group. Comparison between moderate and severe COPD demonstrated the reduction of FA in bilateral frontal subcortical white matter, right temporal white matters, and pons in severe COPD. The comparison between severe COPD and control subjects showed significantly lower FA values in the gray matter of brain (including bilateral frontal, left parietal, right occipital lobes) as well as white matter (frontal, parietal, temporal and occipital subcortical white matter, occipitofrontal fasciculus, and corticospinal tract). No area showed significantly higher FA values in COPD subjects compared with controls, and severe COPD subjects compared with moderate COPD. The differences in the FA values in white matter were distributed relatively more extensively in severe COPD than moderate COPD, compared to control subjects.
Figure 1. Results of the voxel-wise comparisons of FA maps among the three subject groups of cognitive normal, moderate chronic obstructive pulmonary disease (COPD), and severe COPD. Maps are superimposed on a three-dimensional surface rendering of a template brain (upper left: anterior view; upper right: posterior view, middle: lateral views, lower left: inferior view, lower right: superior view). The red color indicates a statistically significant difference in FA (false discovery rate lower than 5%, one-way ANOVA). A. Results from the comparison between the moderate COPD patient and the control group (Normal > Moderate COPD). B. Results from the comparison between the severe COPD patient and the control group (Normal > Severe COPD). C. Results from the comparison between the severe and moderate COPD patient group (Moderate COPD > Severe COPD).
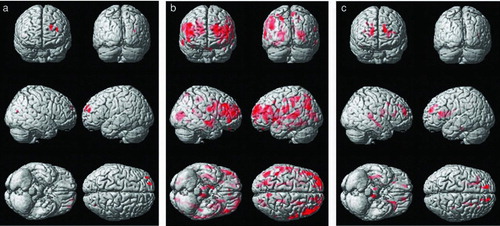
Table 2. Results of Voxel-Wise comparisons of FA maps among three subject groups of cognitive normal, moderate, and severe COPD (Note: areas with z-score more than 3.8 were listed in these tables)
Trace
( and ): A VBM analysis of the trace value was conducted and the moderate COPD group was significantly higher in bilateral occipital gray and white matter, the left precentral gyrus and left temporal white matter was higher than the control group. Compared to moderate COPD, the trace value of the severe COPD group was significantly higher in regions including right frontal subcortical white matter, and anterior limb of internal capsule. The comparison between control and severe COPD showed significantly lower trace value in the frontal, temporal and occipital cortices, frontal subcortical white matter, occipitofrontal fasciculus, and the corticospinal tract. Many regions with increased trace in severe COPD groups were matched with the regions with decreased FA. No area showed lower trace values in COPD subjects compared with controls, and in severe COPD compared with moderate COPD.
Figure 2. Results of the voxel-wise comparisons of trace maps among the three subject groups, which include the cognitive normal, moderate chronic obstructive pulmonary disease (COPD), and severe COPD. Maps are superimposed on a three-dimensional surface rendering of a template brain (upper left: anterior view; upper right: posterior view, middle: lateral views, lower left: inferior view, lower right: superior view). The red color indicates a statistically significant difference in trace (false discovery rate lower than 5%, one-way ANOVA). A. Results from the comparison between the moderate COPD patient and the control group (Normal < Moderate COPD). B. Results from the comparison between the severe COPD patient and the control group (Normal < Severe COPD). C. Results from the comparison between the severe and moderate COPD patient group (Moderate COPD < Severe COPD).
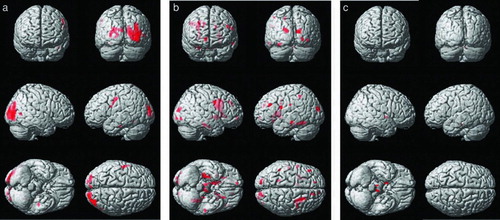
Table 3. Results of Voxel-Wise Comparisons of Trace Maps among Three Subject Groups of Cognitive Normal, Moderate, and Severe COPD (Note: areas with z-score more than 3.63 were listed in this table)
Discussion
The main finding of this study was a significant change in the axonal integrity in multiple brain regions in COPD cases. FA decreased in prefrontal lobes in moderate COPD compared with normal control subjects. Severe COPD showed extensive regions with significantly lower FA and higher trace in gray and white matter than control groups. VBM and DTI allow for the assessment of microstructural changes in the brain, which cannot be revealed on conventional structural MRI. However, these had not been applied in the research for cognition impairment in COPD. Although there had been efforts to investigate perfusion status or metabolic change in brain with COPD using SPECT or MR spectroscopy, these techniques had several limitations to accurately demonstrate the regional neuronal damage (Citation19–21). The significance of this research is that it is the first study that assesses brain damage in patients with COPD by using advanced MRI techniques.
In this study, we could find positive results from voxel based analysis of two indices, FA and trace, derived from DTI data. The significant lower FA in the brain region most likely results from a change in tissue architecture due to subtle neuronal alteration, demyelination of axonal structure, and possible gliosis (Citation22–25). Although the precise pathologic correlates of elevated trace are not known with respect to neuronal degeneration, elevated trace results from increased extracellular water content due to loss of neurons, axons and dendrites. The abnormal trace elevation in the white matter may be associated with increased interspace between the microscopic barriers such as neuronal fibers and myelin. In many white matter diseases, a negative correlation was found between trace and FA in the brain regions. Chronic hypoxia is a noteworthy cause of neuronal damage in COPD, as the proinflammatory cytokines including such as interleukin-6 and tumor necrosis factor-α may mediate the neuronal damage (Citation26, 27). The changes of the DTI parameter in gray matter suggested that neuronal dysfunction can lead to the disconnection of the axons in gray matter.
A previous SPECT study of cerebral perfusion suggested that the decrease of the cerebral perfusion in bilateral anterior frontal, left middle frontal, and left parietal lobe of COPD might result in cognitive impairment (Citation19). In our results, the alteration of FA in bilateral prefrontal lobes was presented in the earlier stage of COPD. This finding suggests a correlation with a significant decrease in frontal function in severe COPD subjects. Decreased perfusion on SPECT represents decreased neuronal activity in functional circuits as well as the region with greater sensitivity to chronic hypoxia or hypercapnea. Therefore, lower brain perfusion in the left frontal lobe in SPECT might be exaggerated compared with actual neuronal damage.
In the present study, the volume of regions with lower FA and higher trace value were gradually increased, according to the severity of COPD. Interestingly, although there is no significant difference between the controls and moderate COPD groups in neuropsychological tests, the comparison of two DTI indices showed regional difference between two groups. This finding suggested that the assessment of DTI may be helpful to diagnose the microstructural change of brain in COPD patients at subclinical stage.
Another noteworthy result in the present study was that the DTI change in severe COPD patients was globally involved in the brain. These results can match with the pattern of cognitive decline in COPD patients. Neuropsychologic decline in COPD patients is most commonly seen as global deterioration, rather than deterioration in a specific pattern. This is consistent with age-related psychologic decline. Chronic hypoxia, or hypercapnea or prevalence of a hypoxic episode in COPD can lead to the generation of free radicals, an inflammatory mediated neurotoxic effect, and oxygen dependent enzyme, which can result in the global involvement of neuronal injury. All this could explain the observed widespread change in DTI.
The aging process in white matter may be another cause of global decrease of FA. It is well known that the FA value is inversely correlated with age. In this study, despite no statistical difference in age between groups and adjustment of age with covariation, severe COPD patients showed a significant decrease of FA globally, and this pattern was analogous to the result of research about age-related changes in the FA value (Citation28). Previous investigators postulated that the combination of age- and disease-related declines can lead to the impairment in cognitive function (Citation20). Our results may support this presumption.
The pattern of neuropsychologic impairment of COPD may be different from that seen in Alzheimer disease (AD) or vascular dementia. However, cognitive impairment in COPD may show some analogies with the neuropsychologic decline of AD or vascular dementia (Citation29, 30). Because individual heterogeneity in the neurologic dysfunction of some patients with COPD is not distinguishable from that of AD patients (Citation31), it requires an effort to differentiate between COPD and AD. When our results compared with previous investigation of AD, the regions having lower FA in COPD patients differed from the regions that had been known as prevalent in AD. In contrast to the global change of FA and trace in COPD, the specific regions related with AD are known to include the hippocampus, amygdala, cingulate gyrus, and white matter of the parietal lobes. Therefore, a different pathophysiologic abnormality associated with various diseases may induce their own characteristic change in the brain. The pattern of change that is revealed on DTI in COPD may be useful to distinguish from that seen in AD or other neurocognitive disorder.
A major limitation of this study is the small number of participants. As a result, differences in VBM among cases of COPD and the control group were not statistically significant. Another limitation is that we did not consider the correlation between neuropsychologic tests and regional DTI change. The present study did not allow us to confirm the brain region matched with deterioration of speech, executive, and frontal functions. Further study about the regional analysis in cognitive decline will help to assess the pathophysiology and to characterize the specific pattern of regional change in cases of COPD. Another limitation is that we couldn't completely correct the bias according to the clinical characteristics including the difference of smoking history.
In this study, the smoking history was different between the controls and moderate COPD patients. We found several articles those presented the change of white matter integrity in smoker (Citation32–34). Possible explanations for the change of white matter integrity in smokers are the stimulating effect of nicotine on nicotine receptors and the negative effect to cerebral small-vessel disease (Citation33–35).
Conclusions
In summary, the present study demonstrated that COPD could affect the diffusion anisotropic and trace value changes in the brain, and the severity of COPD might be correlated with the extent of the microstructural change in the brain. The significance of this study is that a voxel-based analysis of DTI demonstrated the gradational change of white matter integrity from normal controls to severe COPD patients and a regional change between normal and moderate COPD without the difference of the cognition decline. This result suggests several possible approaches to assess and diagnose the cognitive decline in COPD patients. Advanced brain MRI may allow preclinical detection of COPD-induced brain damage, and it may be helpful to decide the time and strategy of management of cognition decline in COPD patients. For differential diagnosis, imaging-based workup may result in a more accurate and objective evaluation compared to neuropsychologic testing, because cognitive decline in COPD can commonly overlap with other causes, such as AD or age. The information about the detailed regions that is presented by advanced MRI will be useful to investigate the mechanism of neuronal damage in COPD.
Declaration of Interest Statement
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A092125). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Grant I, Prigatano GP, Heaton RK, McSweeny AJ, Wright EC, Adams KM. Progressive neuropsychologic impairment and hypoxemia. Relationship in chronic obstructive pulmonary disease. Arch Gen Psych 1987; 44(11):999–1006.
- Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J 2010; 35(4):913–922.
- Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008(2): CD005381.
- Heaton RK, Grant I, McSweeny AJ, Adams KM, Petty TL. Psychologic effects of continuous and nocturnal oxygen therapy in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med. 1983; 143(10):1941–1947.
- Watanabe M, Kohzuki M, Meguro K, Goto Y, Sato T. Marked improvement of neuropsychological impairment in a patient with chronic obstructive pulmonary disease after lung volume reduction surgery. Tohoku J Exp Med. 2001; 193(1):67–72.
- Busatto GF, Diniz BS, Zanetti MV. Voxel-based morphometry in Alzheimer's disease. Expert Rev Neurother 2008; 8(11):1691–1702.
- Whitwell JL, Josephs KA. Voxel-based morphometry and its application to movement disorders. Parkinsonism Relat Disord. 2007;13 Suppl 3:S406-16.
- Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage 2000; 11(6 Pt 1):805–821.
- Mascalchi M, Filippi M, Floris R, Fonda C, Gasparotti R, Villari N. Diffusion-weighted MR of the brain: methodology and clinical application. Radiol Med. 2005; 109(3):155–197.
- Maclullich AM, Ferguson KJ, Reid LM, Deary IJ, Starr JM, Seckl JR, Higher systolic blood pressure is associated with increased water diffusivity in normal-appearing white matter. Stroke 2009; 40(12):3869–3871.
- Nath K, Saraswat VA, Krishna YR, Thomas MA, Rathore RK, Pandey CM, Quantification of cerebral edema on diffusion tensor imaging in acute-on-chronic liver failure. NMR Biomed 2008; 21(7):713–722.
- Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis—A technical review. NMR Biomed. 2002;15(7-8):456–67.
- Torelli F, Moscufo N, Garreffa G, Placidi F, Romigi A, Zannino S, Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage 2010.
- Zhang XQ, Lu BX, Li TP. [Correlation between white matter lesion and memory impairment in patients with obstructive sleep apnea syndrome]. Nan Fang Yi Ke Da Xue Xue Bao. 2009; 29(4):825–829.
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995; 152(5 Pt 2):S77–121.
- Spitzer RL. Values and assumptions in the development of DSM-III and DSM-III-R: an insider's perspective and a belated response to Sadler, Hulgus, and Agich's “On values in recent American psychiatric classification”. J Nerv Ment Dis 2001; 189(6):351–359.
- Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjogren M, A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001;32(6):1318–1322.
- Ahn HJ, Chin J, Park A, Lee BH, Suh MK, Seo SW, Seoul Neuropsychological Screening Battery-dementia version (SNSB-D): A useful tool for assessing and monitoring cognitive impairments in dementia patients. J Korean Med Sci 2010; 25(7):1071–1076.
- Antonelli Incalzi R, Marra C, Giordano A, Calcagni ML, Cappa A, Basso S, Cognitive impairment in chronic obstructive pulmonary disease—A neuropsychological and spect study. J Neurol 2003; 250(3):325–332.
- Ortapamuk H, Naldoken S. Brain perfusion abnormalities in chronic obstructive pulmonary disease: comparison with cognitive impairment. Ann Nucl Med 2006; 20(2):99–106.
- Shim TS, Lee JH, Kim SY, Lim TH, Kim SJ, Kim DS, Cerebral metabolic abnormalities in COPD patients detected by localized proton magnetic resonance spectroscopy. Chest 2001; 120(5):1506–1513.
- Bronge L, Bogdanovic N, Wahlund LO. Postmortem MRI and histopathology of white matter changes in Alzheimer brains. A quantitative, comparative study. Dement Geriatr Cogn Disord 2002; 13(4):205–212.
- Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol 1986; 19(3):253–262.
- Englund E. Neuropathology of white matter changes in Alzheimer's disease and vascular dementia. Dement Geriatr Cogn Disord. 1998;9 Suppl 1:6–12.
- Rose SE, Janke AL, Chalk JB. Gray and white matter changes in Alzheimer's disease: a diffusion tensor imaging study. J Magn Reson Imaging. 2008;27(1):20-6.
- Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003; 107(11):1514–1519.
- Li Y, Chongsuvivatwong V, Geater A, Liu A. Are biomarker levels a good follow-up tool for evaluating obstructive sleep apnea syndrome treatments? Respiration 2008; 76(3):317–323.
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. AJNR Am J Neuroradiol 2007; 28(2):226–235.
- Grant I, Heaton RK, McSweeny AJ, Adams KM, Timms RM. Neuropsychologic findings in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med 1982; 142(8):1470–1476.
- Incalzi RA, Gemma A, Marra C, Muzzolon R, Capparella O, Carbonin P. Chronic obstructive pulmonary disease. An original model of cognitive decline. Am Rev Respir Dis 1993; 148(2):418–424. Epub 1993/08/01.
- Incalzi RA, Gemma A, Marra C, Capparella O, Fuso L, Carbonin P. Verbal memory impairment in COPD: its mechanisms and clinical relevance. Chest 1997; 112(6):1506–1513.
- Liao Y, Tang J, Deng Q, Deng Y, Luo T, Wang X, Bilateral fronto-parietal integrity in young chronic cigarette smokers: A diffusion tensor imaging study. PloS One 2011; 6(11):e26460.
- Gons RA, van Norden AG, de Laat KF, van Oudheusden LJ, van Uden IW, Zwiers MP, Cigarette smoking is associated with reduced microstructural integrity of cerebral white matter. Brain 2011; 134(Pt 7):2116–2124.
- Paul RH, Grieve SM, Niaura R, David SP, Laidlaw DH, Cohen R, Chronic cigarette smoking and the microstructural integrity of white matter in healthy adults: a diffusion tensor imaging study. Nicotine Tobacco Res: Off J Soc Res Nicotine Tobacco 2008; 10(1):137–147.
- Gazdzinski S, Durazzo TC, Studholme C, Song E, Banys P, Meyerhoff DJ. Quantitative brain MRI in alcohol dependence: preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumes. Alcoholism Clin Exper Res 2005; 29(8):1484–1495.