Abstract
Background: High rates of disability associated with chronic airway obstruction may be caused by impaired pulmonary function, pulmonary symptoms, other chronic diseases, or systemic inflammation. Methods: We analyzed data from the Cardiovascular Health Study, a longitudinal cohort of 5888 older adults. Categories of lung function (normal; restricted; borderline, mild-moderate, and severe obstruction) were delineated by baseline spirometry (without bronchodilator). Disability-free years were calculated as total years alive and without self-report of difficulty performing &γτ;1 Instrumental Activities of Daily Living over 6 years of follow-up. Using linear regression, we compared disability-free years by lung disease category, adjusting for demographic factors, body mass index, smoking, cognition, and other chronic co-morbidities. Among participants with airflow obstruction, we examined the association of respiratory factors (FEV1 and dyspnea) and non-respiratory factors (ischemic heart disease, congestive heart failure, diabetes, muscle weakness, osteoporosis, depression and cognitive impairment) on disability-free years. Results: The average disability free years were 4.0 out of a possible 6 years. Severe obstruction was associated with 1 fewer disability-free year compared to normal spirometry in the adjusted model. For the 1,048 participants with airway obstruction, both respiratory factors (FEV1 and dyspnea) and non-respiratory factors (heart disease, coronary artery disease, diabetes, depression, osteoporosis, cognitive function, and weakness) were associated with decreased disability-free years. Conclusions: Severe obstruction is associated with greater disability compared to patients with normal spirometery. Both respiratory and non-respiratory factors contribute to disability in older adults with abnormal spirometry.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common chronic condition that affects an estimated 24 million U.S. adults, especially older adults (Citation1). COPD is recognized as a major cause of morbidity and functional impairment, and was the third-leading cause of death in the United States in 2010 (Citation2). Understanding functional limitations and disability in chronic illness are key priorities for research recommended by the Institute of Medicine (Citation3). Although most previous studies showing a relationship between COPD and disability were cross-sectional, a recent prospective study also found that a diagnosis of COPD was associated with development of disability (Citation4). In addition, among COPD patients, worse lung function was associated with development of disability over a 2-year period (Citation5). Yet, despite the impact of COPD on disability, few studies have characterized the factors that lead to disability in COPD (Citation5, 6).
A model of the progression from COPD to disability proposed by Jette outlines how lung disease leads to respiratory impairment (e.g. lower FEV1% predicted), functional limitation (e.g. 6-minute walk test), and ultimately to disability (Citation7). Although respiratory factors such as pulmonary function (Citation8–10) and dyspnea (Citation9–11) clearly contribute to disability, non-respiratory factors likely also play a role. In this study we examine the disease to disability pathway, based on evidence that COPD is a systemic disease (Citation12) characterized by systemic inflammation (Citation13), which may lead to muscle wasting and the development, or worsening, of co-morbid illness. In addition, COPD may lead to worsening cognitive function (Citation14). Eisner and colleagues found that non-respiratory factors such as abnormal body composition, muscle strength and cognitive function increase the 2-year risk of disability in COPD (Citation5), We therefore hypothesize that the disablement model by Jette could be broadened () to include non-respiratory impairments in other organ systems that lead to functional limitation and disability in obstructive lung disease.
Previous longitudinal studies of disability in COPD have also generally not accounted for death which likely underestimates the effect of a chronic disease on disability because survivors are likely less impaired (Citation7). Disability-free life expectancy is an outcome that summarizes the effect of both mortality and disability (Citation15). Using a similar approach to calculate disability free years (DFY) can therefore help to address mortality in longitudinal studies of older persons with chronic illness.
The aims of this study were 1) toCitation estimate the impact of airway obstruction (as a surrogate of COPD) on DFY over a 6-year period in a cohort of older adults, and 2) to identify the pulmonary and systemic determinants of DFY in patients with obstructive lung disease. Understanding the contribution of respiratory and non-respiratory factors to disability can influence clinical care and help organize health services and public health interventions to reduce disability in COPD.
Methods
Study design and population
The Cardiovascular Health Study is a population-based, longitudinal study of coronary heart disease and stroke. Details of the methodology and sample are published elsewhere (Citation16). Beginning in 1989–1990, 5201 community-dwelling adults aged 65 years or older in four U.S. communities were identified based on Medicare eligibility lists. Participants needing a wheelchair or receiving hospice care, radiation treatment, or chemotherapy were excluded. During 1992–1993, 687 additional African American participants were recruited for a total of 5888 participants. Spirometry was performed during 1989–1990 (first cohort only), 1993–1994 (both cohorts), and 1996–1997 (both cohorts). Participants completed assessments of instrumental activities of daily living (IADLs) at yearly study visits. This analysis used the first year spirometry occurred to mark the participant's baseline year. Six years of follow-up data were available for analysis. When baseline variables weren't available, we used the next closest year of data. All participants provided informed consent. The VA Puget Sound Health Care System Institutional Review Board approved this analysis.
Measurement of lung function
Pulmonary function testing procedures for the CHS are detailed elsewhere (Citation17). We included only spirometry values meeting the American Thoracic Society (ATS) 1994 recommendations (grade A, B, or C), and excluded unreasonably high and low values (FEV1 > 5.5, FEV1 < 0.5, FVC > 6.0, or FVC < 0.5) (Citation17).
Reference values based on race, age, sex and height were calculated (Citation18). We used the lower limit of normal (LLN) for FEV1/FVC to define airway obstruction (Citation19). Spirometry results were classified into three mutually exclusive categories: obstruction, defined as FEV1/FVC < LLN; restriction, defined as FEV1/FVC &γτ; LLN with FVC < LLN (Citation20); and, normal, defined as FEV1/FVC &γτ; LLN with FVC &γτ; LLN.
Disability-free years
Disability was assessed by self-report of difficulty or inability to perform one or more of the following IADLs: heavy housework, light housework, shopping, preparing meals, paying bills, and using the phone. IADLs were used to indicate disability because they cover a wide range of individual activities and are detected sooner and more gradually compared to other markers of disability (Citation21). To model DFY, we summed the number of years alive without any IADL impairment, starting with the baseline year (when spirometry was performed) up to the subsequent 5 following years, with a maximum total number of years of DFY of 6 years.
Risk factors
Socio-demographic and health data were obtained from personal interviews, medication and medical record review, and clinical examination. A co-morbidity count (0, 1 or &γτ;2) was constructed from the following 6 conditions: kidney disease, liver disease, arthritis, or cancer, which were self-reported physician diagnoses; and stroke/TIA and claudication, which were each confirmed by medical record review.
Respiratory impairment and functional limitation
The degree of respiratory impairment was categorized as borderline (FEV1 &γτ; LLN); mild-to-moderate (50% predicted &λτ; FEV1, LLN); and severe (FEV1, 50% predicted) airway obstruction. Mobility-related dyspnea was measured with the ATS-DLD-78 dyspnea scale (0 = No shortness of breath; 1 = troubled by shortness of breath when hurrying or walking up a slight hill; 2 = walk slower than people your age; 3 = have to stop when walking at own pace, 4 = have to stop after walking 100 yards; 5 = too breathless to leave the house or breathless on dressing or undressing) (Citation22).
Potential non-respiratory impairments and functional limitation
Self-report of congestive heart failure and ischemic heart disease were adjudicated by review of medications and medical records (Citation23). Diabetes was based on a fasting blood glucose > 126 or if the participant was taking insulin or an oral hypoglycemic agent. Osteoporosis was measured by self-report of a doctor diagnosis. Depression status was ascertained using the 10-item Center for Epidemiologic Studies Short Depression Scale (0–30). A score > 10 was considered to be depressed (Citation24). Muscle weakness was evaluated with a hand grip test using a handheld Jamar Dynamometer. Among the initial cohort of participants, respiratory muscle strength was measured with maximal inspiratory pressure (N = 4537). A score of < 80 on the Modified Mini Mental State Exam (on a 100-point scale) was defined as a cognitive impairment (Citation25).
Imputation of missing variables
Ascertainment of death was complete in this cohort, and we imputed IADL scores for observation periods that were missing while the participant was alive. Data were borrowed from previous and subsequent observations for each participant, and no data were borrowed between participants (Citation26). This method of imputation had the following steps:
Standardization to self-rated health: observed data were transformed to a new scale corresponding to the probability of being healthy (ìexcellent,î ìvery goodî or ìgoodî), where 100 is perfect health and 0 is death (Citation27).
Death: the transformed variable was set to zero if the person was dead when this measure would otherwise have occurred.
Interpolation: for data missing between two observed values, we used linear interpolation to impute the missing IADL values based on the imputed self-rated health value.
Extrapolation: for missing data where the person is alive but there are no follow-up measures, we used a combination of last-observation-carried-forward, and the imputed self-rated health value to impute yearly IADL values.
To determine if this imputation method influenced the results, we conducted a sensitivity analysis with only complete cases.
Analysis
Linear regression was used to model average DFY comparing participants with restriction, borderline, mild/moderate and severe airflow obstruction to normal spirometry. We used linear regression because the outcome is expressed in years, which is clinically meaningful and understandable (Citation28). In the base model, we measured the effect of lung function on DFY after adjusting for demographic factors, smoking status, and body mass index (kg/m2). Next we added non-respiratory impairments to the model. Because we could not exclude the possibility that some patients with obstruction had poorly controlled asthma, we performed a sensitivity analysis excluding those with a self-reported physician's diagnosis of asthma.
Among participants with obstruction we examined the effect of respiratory and non-respiratory impairments and functional limitations on disability. The base model included demographic factors, smoking status, body mass index (kg/m2) and categories of airflow obstruction. Model 2 included the base model plus non-respiratory impairment. A third model added functional limitation. Finally, we re-ran the model while excluding those with a self-reported physician's diagnosis of asthma.
Because most prior studies have used logistic regression to model the risk of disability, we also performed relative risk regression to estimate the 1-year risk of disability (Citation29) (e-Tables). Analyses were conducted in Stata version 11 (StataCorp, 2009, College Station, TX: StataCorp LP).
Results
A total of 29,196 observations were made over the 6 year follow-up period on the 4,866 participants with valid baseline spirometry. Of these, 2004 (6.9%) observations were imputed, 1,624 (5.6%) by interpolation (measures existed before and after), and 371 (1.3%) by extrapolation (last observation carried forward). The mean age of the cohort was 72.6 years, 57.9% were female and 11.7% were African American. At baseline 1,048 (21.5%) participants were categorized with obstruction and 472 (9.7%) with restriction. Among those with obstruction, mean FEV1 was 1.57 L (65% predicted). Participants with obstruction were significantly more likely to be male, have lower educational attainment, and to have smoked (). Participants with abnormal spirometry were more likely to have congestive heart failure, ischemic heart disease or diabetes. Severe dyspnea was more common among participants with obstruction or restriction.
Table 1. Baseline characteristics of Cardiovascular Health Study participants by spirometry results
The average DFY for the entire cohort was 4.0 (SD = 2.1) out of a possible 6 years. Fifty four percent of participants (2,651) were alive and without disability for 5 to 6 years. Nine percent (446) were dead and/or disabled after the baseline year (0 DFY). Among participants with borderline, mild/moderate and severe airflow obstruction the average DFY was 4.1, 3.8 and 3.1, respectively. The distribution of DFY among these participants was similar to the group as a whole, although severely obstructed participants spent fewer years alive and without disability free (33.6% with 5 to 6 DFY). After adjusting for demographic risk factors (, Base model), restriction was associated with 0.78 fewer years of DFY compared to persons with normal spirometry (). Severe obstruction was associated with 1.27 fewer DFY compared with normal spirometry. The measure of effect did not change significantly after including the non-respiratory factors, or excluding participants with asthma. Using non-imputed data resulted in very similar estimates compared to the estimates presented from the imputed dataset.
Table 2. Respiratory impairment and disability-free years
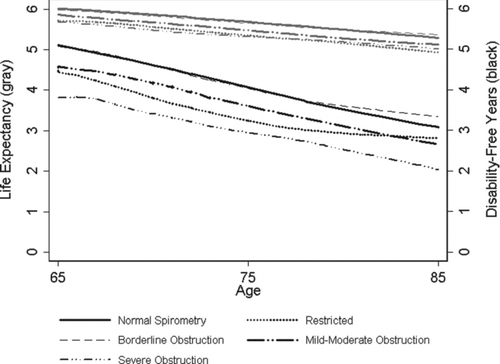
shows the predicted life expectancy and DFY based on the model including demographic characteristics, spirometry and non-respiratory factors by age category. During the 6 years of follow-up, the impact of baseline lung function on DFY increased with higher baseline age. Severe obstruction was associated with fewer DFY. There was little difference between normal lung function and borderline obstruction.
Figure 2. Predicted life expectancy and disability-free life expectancy (of a possible 6 years) by baseline age and lung function. Note: Predicted values are lowess smoothed. In this figure, we excluded participants with baseline age > 85, because of small numbers in the upper age categories (<3% of total participants).
For the 1048 participants with obstruction, all respiratory and non-respiratory variables studied were associated with decreased DFY, except heart failure (). Maximal inspiratory pressure was not associated with disability in a model including grip strength (p = 0.82, data not presented). In the final model that excluded participants with asthma, severe airflow obstruction was associated with 0.66 fewer DFY compared to borderline obstruction. Non-respiratory factors including diabetes, depression, osteoporosis, and cognitive dysfunction were each associated with roughly half a year less DFY. The most severe category of dyspnea was associated with 2.7 fewer DFY compared to participants without dyspnea. We repeated the analysis to predict 1-year relative risk of disability, and found similar results (e-Tables).
Table 3. Respiratory and non-respiratory impairments, functional limitations and disability-free years in individuals with baseline airflow obstruction
Discussion
We found that obstruction was associated with disability, measured by loss of disability-free years of life. The effect was greatest among participants with severe obstruction, who showed about one less disability-free year of life over 6 years, controlling for sociodemographic and health-related factors. Among those with obstruction, respiratory factors such as lung function and dyspnea were the largest contributors to DFY. Non-respiratory factors including heart disease, diabetes, muscle weakness, depression and cognitive ability also contributed significantly to DFY.
Most prior large studies that reported a strong association between airway obstruction and disability were cross-sectional, and few prospective studies have used objective measures of airway obstruction (Citation5,Citation20). In addition, accounting for missing values and death is important in studies with self-reported outcomes, since the patients who have complete data tend to be healthier and have better outcomes (Citation7). Our longitudinal analysis is the first to focus on the relationship between lung function and disability-free life expectancy, an outcome that incorporates death and provides an estimate of the total time alive and without disability (Citation15).
Based on ATS guidelines (Citation19), we categorized participants with airway obstruction based on the fifth percentile lower limit of normal for the FEV1/FVC ratio to minimize the risk of misclassification (Citation20). Patients with restriction (a low FVC) have increased functional impairment in cross-sectional studies, which may be related to several different etiologies including obesity, muscular weakness, and (rarely) interstitial lung diseases (Citation30). Patients with restriction had fewer DFY than those with normal lung function, even after adjusting for obesity and weakness (low handgrip strength).
Similar to others, we found that dyspnea was an important respiratory contributor to disability in those with obstruction. Pulmonary function impairment, and especially the resulting dyspnea, is therefore a key independent component contributing to disability in older adults with airway obstruction.
In addition, we also found that non-respiratory factors contribute significantly to disability. People with COPD are more likely to develop non-respiratory chronic illness such as acute myocardial infarction, stroke (Citation31), and diabetes (Citation32) when compared to people without COPD. The prevalence and incidence of depression are high in COPD (Citation33). Cognitive impairment (Citation14) and skeletal muscle strength (Citation12) may also be affected by COPD. These findings build on recent work showing that non-pulmonary impairments including body composition and muscle weakness contribute to disability (Citation5).
We used the model of the disablement process developed by Verbrugge and Jette (Citation34), and adapted for pulmonary disease (Citation6). Our findings further support broadening the model () to include impairments in other organ systems that can lead to functional limitation and disability. The model proposed by Jette also includes factors such as social support, home environment, respiratory medications and intra-individual factors (e.g. psychological factors or sensitivity to dyspnea) that were not available as part of this study, but may have contributed to disability among those with obstruction.
Strengths of this study include a large population representative cohort, with objective classification of lung function. We accounted for a broad range of important confounders and had 6 years of longitudinal follow-up for subjects with yearly assessment of IADL disability and accounted for death by focusing on DFY.
Several limitations should be considered. COPD is defined by post-bronchodilator airway obstruction, and since our participants did not receive post-bronchodilator spirometry, we may have included those with poorly controlled asthma. Some participants with normal spirometry or restriction had self-reported asthma which may have contributed to disability. However, models excluding asthma did not noticeably change the coefficients for DFY for participants with airway obstruction. The CHS only included participants &γτ; 65 years, thus limiting the generalizability of the results to older persons. As mentioned above, other factors that were not measured as part of this analysis such as the home environment or sensitivity to dyspnea may also contribute to disability. Further research is therefore necessary to better elucidate the disablement pathway for patients with airflow obstruction.
Conclusion
In summary, participants with airway obstruction have spent more years with disability compared to those with normal lung function, and worsening lung function and dyspnea were strongly associated with decreased DFY. In addition to respiratory impairment, non-respiratory factors related to chronic lung disease including co-morbidity, cognitive impairment and muscle weakness also contributed significantly to time spent with disability among those with airway obstruction.
Declaration of Interest Statement
There is no conflict of interest associated with this manuscript. This material is the result of work supported by resources from the VA Puget Sound Health Care System, Seattle, Washington. The research reported in this article was supported by contracts HHSN268201200036C, N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
Authorship contributions: Authors of this manuscript made the following contributions: concept and design: VSF, ST, EL; data analysis and interpretation: EL, VSF, ST, PD, SRH; drafting the manuscript: VSF, EL; preparing the data: AGW, RAK, PD; and, review of the manuscript: all authors.
References
- 2009 Chart Book on Cardiovascular, Lung, and Blood Diseases: National Institutes of Health, National Heart Lung and Blood Institute, Bethesda, MD, 2009.
- Murphy SL, Xu JQ, Kochanek KD. Deaths: Preliminary Data for 2010. Hyattsville, MD: National Center for Health Statistics, 2012.
- Iglehart JK. Prioritizing comparative-effectiveness research—IOM recommendations. N Engl J Med 2009; 361(4):325–328.
- Chaudhry SI, McAvay G, Ning Y, Allore HG, Newman AB, Gill TM. Geriatric impairments and disability: the cardiovascular health study. J Am Geriatr Soc 2010; 58(9):1686–1692.
- Eisner MD, Iribarren C, Blanc PD, Development of disability in chronic obstructive pulmonary disease: beyond lung function. Thorax 2011; 66(2):108–114.
- Jette DU, Manago D, Medved E, Nickerson A, Warzycha T, Bourgeois MC. The disablement process in patients with pulmonary disease. Phys Ther 1997; 77(4):385–394.
- Diehr P, Johnson LL, Patrick DL, Psaty B. Methods for incorporating death into health-related variables in longitudinal studies. J Clin Epidemiol 2005; 58(11):1115–1124.
- Coultas DB, Mapel D, Gagnon R, Lydick E. The health impact of undiagnosed airflow obstruction in a national sample of United States adults. Am J Respir Crit Care Med 2001; 164(3):372–377.
- Graydon JE, Ross E, Webster PM, Goldstein RS, Avendano M. Predictors of functioning of patients with chronic obstructive pulmonary disease. Heart Lung 1995; 24(5):369–375.
- Lee RN, Graydon JE, Ross E. Effects of psychological well-being, physical status, and social support on oxygen-dependent COPD patients’ level of functioning. Res Nurs Health 1991; 14(5):323–328.
- Tinetti ME, McAvay G, Chang SS, Effect of chronic disease-related symptoms and impairments on universal health outcomes in older adults. J Am Geriatr Soc 2011; 59(9):1618–1627.
- Barnes PJ, Celli BR. Systemic manifestations and co-morbidities of COPD. Eur Respir J 2009; 33(5):1165–1185.
- Agusti A. Systemic effects of chronic obstructive pulmonary disease: what we know and what we don't know (but should). Proc Am Thorac Soc 2007; 4(7):522–525.
- Thakur N, Blanc PD, Julian LJ, COPD and cognitive impairment: the role of hypoxemia and oxygen therapy. Int J Chron Obstruct Pulmon Dis 2010; 5:263–269.
- Jagger C, Matthews R, Matthews F, Robinson T, Robine JM, Brayne C. The burden of diseases on disability-free life expectancy in later life. J Gerontol A Biol Sci Med Sci 2007; 62(4):408–414.
- Fried LP, Borhani NO, Enright P, The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991; 1(3):263–276.
- Jiang R, Burke GL, Enright PL, Inflammatory markers and longitudinal lung function decline in the elderly. Am J Epidemiol 2008; 168(6):602–610.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159(1):179–187.
- Pellegrino R, Viegi G, Brusasco V, Interpretative strategies for lung function tests. Eur Respir J 2005; 26(5):948–968.
- Miller MR, Quanjer PH, Swanney MP, Ruppel G, Enright PL. Interpreting lung function data using 80% predicted and fixed thresholds misclassifies more than 20% of patients. Chest 2011; 139(1):52–59.
- Nikolova R, Demers L, Beland F. Trajectories of cognitive decline and functional status in the frail older adults. Arch Gerontol Geriatr 2009; 48(1):28–34.
- Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis. 1978; 118(6 Pt 2):1–120.
- Ives DG, Fitzpatrick AL, Bild DE, Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol 1995; 5(4):278–285.
- Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med 1994; 10(2):77–84.
- Kurella M, Chertow GM, Fried LF, Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol 2005; 16(7):2127–2133.
- Engels JM, Diehr P. Imputation of missing longitudinal data: a comparison of methods. J Clin Epidemiol 2003; 56(10):968–976.
- Diehr P, Lafferty WE, Patrick DL, Downey L, Devlin SM, Standish LJ. Quality of life at the end of life. Health Qual Life Outcomes 2007; 5:51.
- Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Ann Rev Publ Health 2002; 23:151–169.
- Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159(7):702–706.
- Mannino DM, Ford ES, Redd SC. Obstructive and restrictive lung disease and functional limitation: data from the Third National Health and Nutrition Examination. J Intern Med 2003; 254(6):540–547.
- Feary JR, Rodrigues LC, Smith CJ, Hubbard RB, Gibson JE. Prevalence of major co-morbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax 2010; 65(11):956–962.
- Rana JS, Mittleman MA, Sheikh J, Chronic obstructive pulmonary disease, asthma, and risk of type 2 diabetes in women. Diabetes Care 2004; 27(10):2478–2484.
- Fan VS, Giardino N. Anxiety and Depression. In: Nici L, Zuwallack R, eds. Chronic Obstructive Pulmonary Disease: Co-Morbidities and Systemic Consequences. New York, NY: Humana Press; 2012:95–118.
- Verbrugge LM, Jette AM. The disablement process. Soc Sci Med 1994; 38(1):1–14.
![Figure 1. Disablement process in obstructive lung disease [adapted from Jette et al. 1997(6)].](/cms/asset/72fb7681-1e56-42de-b479-b431166b43a2/icop_a_781148_f0001_b.gif)