Abstract
Purpose: Fat free mass index (FFMI) is an independent predictor of metabolic and functional consequences in COPD. For its measurement dual energy X-ray absorptiometry (DEXA), skin-fold anthropometry (SFA), bioelectrical impedance analysis (BIA) and bioimpedance spectroscopy (BIS) are used in clinical practice. The aim of our pilot study was to analyse precisely and critically which method is most accurate and available for common use in clinical practice for measurement of FFM by assessment against relevant DEXA in patients with COPD. Methods: This was an observational cross-sectional study of consecutive COPD subjects. FFM by methods of SFA, two versions of BIA, and BIS was compared with that from clinically relevant DEXA in 41 outpatients (mean age 66.5 ± 7.7 yrs) with stable COPD, 34 men and 7 women, with mean BMI 28.2 ± 6.1 kg.m−2. Results: All methods underestimate FFM in comparison with DEXA. In the general evaluation non-significant differences with the smallest mean bias were demonstrated for SFA (1.2 kg) and BIA (3.8 kg), but there was a difference of more than 9 kg using BIS and BIA COPD methods (p < 0.0001). The best agreement between DEXA and SFA was demonstrated via Lin's concordance coefficient and Bland–Altman test. Conclusions: SFA has been demonstrated as an accurate, available and cheap method for determination of FFM and FM with application of the Durnin Womersley equation for body density and with the Siri equation for FM in patients with COPD. SFA can be easily applied in routine clinical practice.
| Abbreviations | ||
| BDT | = | bronchodilator test |
| BIA | = | Bioelectrical impedance analysis |
| BIA COPD | = | bioelectrical impedance analysis with Schols equation |
| BIS | = | Bioimpedance spectroscopy |
| BMI | = | Body mass index |
| COPD | = | Chronic obstructive pulmonary disease |
| DEXA | = | Dual energy X-ray absorptiometry |
| FEV1 | = | Forced expiratory volume in 1 sec |
| FFM | = | Fat free mass |
| FFMI | = | Fat free mass index |
| FM | = | Fat mass |
| FMI | = | Fat mass index |
| FVC | = | Forced vital capacity |
| GOLD | = | Global initiative for obstructive chronic lung disease |
| 6MWT | = | 6-minute walk test |
| OH | = | overhydration |
| PFTs | = | pulmonary function tests |
| SFA | = | Skin-fold anthropometry |
Introduction
Chronic obstructive pulmonary disease (COPD) is an important and growing cause of morbidity and mortality worldwide. Besides lung function impairment, multiple extrapulmonary and systemic effects and consequences leading to co-morbid conditions are related to COPD, such as skeletal muscle wasting, osteoporosis, cardiovascular disease, diabetes mellitus (Citation1), depression and lung cancer (Citation2). Assessment of muscle wasting in COPD is relevant as it is independently associated with metabolic and functional consequences and even survival (Citation3). Skeletal muscle loss is present not only in underweight, but also in normal or overweight patients, and could be masked by edema or fat deposits (Citation4). Muscle wasting can be assessed from fat free mass (FFM) (Citation5).
The determination of body composition in COPD patients is of high importance. The most accurate methods (such as magnetic resonance, total body potassium or in vivo neutron-activation model, double labelled water) are not suitable for common use in clinical practice due to their cost and high sophistication. Recently, dual energy X-ray absorptiometry (DEXA) has been suggested as a suitable clinical reference (“gold standard”) method for the measurement of body composition (Citation6), including in COPD (Citation7). Methods most commonly in use include skin-fold anthropometry (SFA), bioelectrical impedance analysis (BIA) and bioimpedance spectroscopy (BIS). Only a few studies have compared these methods, and their results are not clearly consistent. Miller et al. found significant difference between FFM as determined by DEXA and by other methods; both BIA and SFA overestimated FFM in comparison with DEXA (Citation7). However, other authors found no significant differences in mean FFM as determined by DEXA, BIA and SFA (Citation8), or DEXA, BIS and SFA (Citation9), although BIA and BIS tended to underestimate and SFA overestimate FFM.
DEXA is the most expensive method and the equipment is not commonly available in many hospitals and outpatient departments. The aim of this study was to analyse precisely which of the compared methods is most accurate for measurement of body composition using DEXA as a standard, and which would be available for precise measurement of FFM in patients with COPD.
Materials and Methods
Study design and populations
An observational cross-sectional study was performed during the period April 2011 to April 2012. All COPD subjects underwent a comprehensive examination to determine COPD severity (e.g. CAT scale, modified MRC dyspnea scale, arterial blood gases assessment, optional bronchoscopy, Beck and Zung depression scales, Epworth sleepiness scale, endocrinological and metabolic assessment), COPD phenotype, systemic and/or extrapulmonary involvement associated with COPD, and exclusion of any alternative diagnosis.
The study population consisted of 41 consecutive outpatients (mean age 66.5 ± 7.7 yrs) with stable (free of acute exacerbation of COPD and/or deterioration of internal co-morbidities more than 8 weeks prior study enrollment) COPD, 34 men and 7 women, with mean body mass index (BMI) 28.2 ± 6.1 kg.m−2. The study was reviewed and approved by the Ethical Committee of Charles University, Faculty of Medicine in Hradec Kralove (March, 2011) and all subjects gave written informed consent.
Pulmonary function assessment
Pulmonary function tests were performed and interpreted according to the ATS/ERS recommendations and methodology (ZAN 500 Body, Messgeraete GmbH, Germany). An obstructive ventilatory defect was defined as a reduced FEV1/VC ratio below the 5th percentile of the predicted value (Citation10,11). Spirometry was carried out before and after administration of ipratropium bromide 80 mcg and salbutamol 400 mcg (Citation12).
In line with the latest classification of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) patients were divided into four grades [1–4] according to post-bronchodilator airflow limitation: forced expiratory volume in 1 sec (FEV1) ≥80% of predicted value for grade 1, 50% ≤ FEV1 < 80% for grade 2, 30% < FEV1 < 50% for grade 3, FEV1 < 30% for grade 4. The pulmonary function tests (PFTs) included static and dynamic lung volumes, single-breath determination of carbon monoxide uptake. Values are expressed as a percentage of predicted values calculated from European Respiratory equations (Citation13). The six-minute walk test (6MWT) (Citation14) was used for additional objective evaluation of functional exercise capacity.
Body composition
All measurements were taken before rehabilitation commenced in the morning after a fast of 12 h. Body weight was measured in underwear to the nearest 0.1 kg (Tanita Corporation, Japan). Height was measured with a stadiometer to the nearest 0.5 cm. Both were used to calculate BMI (kg.m−2).
FM and FFM were calculated in absolute and percentage values from results yielded by the various techniques used in the study. FM and FFM were corrected for height to produce mass index (FMI, FFMI), expressed in kg.m−2.
DEXA was measured in the supine position using a total body scanner (Hologic Discovery A, Waltham, Maryland, USA). The manufacturer's software was then used to resolve body composition into bone mineral, fat and fat-free soft tissue components. FFM-DEXA was estimated as the sum of total body bone mineral and fat-free soft tissue.
For SFA, triple measurements were taken in four standard places (triceps, biceps, subscapular, suprailiac) using a caliper (Best K-501, Trystom, Czech Republic). Body density was estimated according to the Durnin and Womersley method (Citation15), and FM calculated with the Siri equation (Citation16). All measurements were performed by a sole trained examiner.
Bioelectric impedance analysis measurements were performed in three ways. BIA by dual-frequency was conducted in the standing position on two electrodes of body composition monitors (InnerScan, Body Composition Monitor, Japan). FM was calculated using the manufacturer's software. FFM (at SFA, BIA) was calculated as the difference between body weight and FM.
BIS was performed with a bioimpedance spectroscopy device with 50 frequencies from 5 kHz to 1 MHz measured in the semisupine position (Body Composition Monitor, Fresenius, Germany), with application of the Moissl equation (Citation17) for body composition and the Chamney equation (Citation18) for overhydration for determination of oedema. FM and FFM (of BIA COPD) were calculated from BIS measurement used a value of the resistance for calculation by special the Schols equation for COPD only (Citation9).
Statistical analysis
The acquired data were analyzed using programs GraphPad Prism5 (GraphPad Software, La Jolla, CA, USA) and Excel 2010 (Microsoft, Redmont, WA, USA). All parameters were evaluated by descriptive statistics. ANOVA one-way test was applied to determine differences between observed parameters for the four GOLD stages.
Evaluation of FFM, FFMI, FM and FMI from each method compared with values obtained by DEXA was assessed using Dunnet's test for multiple testing of differences, correlation analysis for demonstration of relationships, Lin´s concordance correlation test for assessment of agreement, and the Bland–Altman test for determination of limit agreements. Significance was accepted at p < 0.05. The Bonferroni test was applied for mutual multiple testing of differences between values of overhydration in the GOLD groups.
Results
The physical characteristics of the study group overall and by severity of COPD according GOLD stage is presented in . The average BMI of all 41 patients was in the overweight range 28.2 kg.m−2. In the total patient group only five patients were underweight (<20 kg.m−2), six were in the normal range (20–25 kg.m−2), fourteen were overweight (25.1–30 kg.m−2), and sixteen were obese (>30 kg.m−2). There was a small non-significant decrease in BMI with increase in GOLD stage.
Table 1. Clinical characteristics of the patient population
Dunnet's test demonstrated a statistically significant decrease of six-minute walk distance (6MWD) between patients in GOLD stages 1 and 2 and those in GOLD 3 and 4. All patients were below the physiological limit (Citation19). Both the fat mass and the fat free mass as measured by DEXA and by the comparison methods are presented in .
Table 2. Evaluation of body composition with DEXA comparison
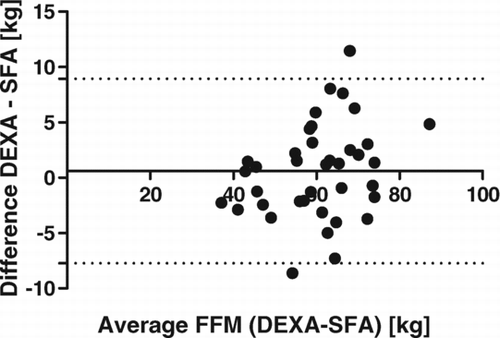
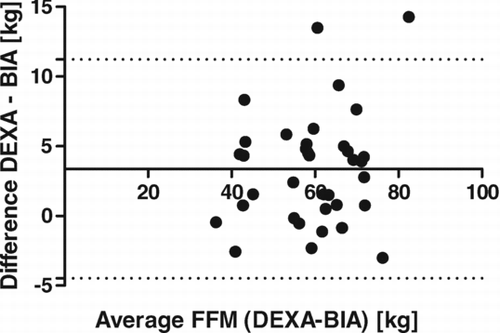
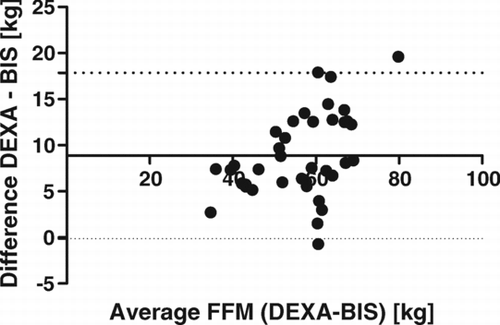
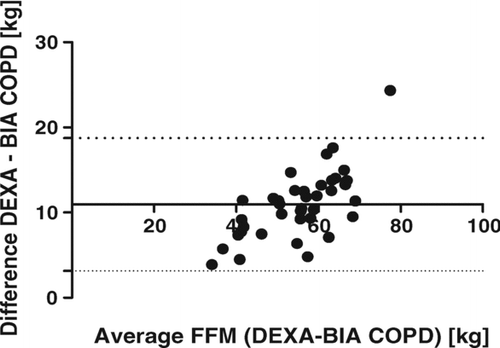
All methods underestimate FFM compared with measurements from DEXA. In the study group overall, the smallest differences were demonstrated with SFA and BIA, with non-significant mean bias, conversely higher differences were observed for measurements by BIS and BIA COPD (p < 0.0001). Similar relative results were demonstrated within the GOLD groups, although for patients at GOLD stages 3 and 4 all differences were non-significant. High correlations between DEXA and each other method were found (p < 0.0001), with r = 0.93 at SFA, r = 0.94 at BIA, r = 0.92 at BIS, and r = 0.96 at BIA COPD. Convenient application of the SFA method for determination of FFM was demonstrated by the best limit agreement relative to DEXA via Bland–Altman test compared with other methods ().
Application of Lin's concordance correlation test, applied for comparison of accuracy each measurements, demonstrated via coefficient the best agreement with DEXA for SFA (ρc = 0.9593), lower for other compared methods, for BIA (ρc = 0.9431), BIS (ρc = 0.7766) and BIA COPD (ρc = 0.6980).
Nutritional depletion was evaluated according to FFMI in the total patient group and within the GOLD groups; the threshold for depletion was taken as FFMI < 16 for men and FFMI < 15 for women (Citation20). Using this parameter an identical prevalence of 5% was observed with DEXA and SFA; the other compared methods overestimated nutritional depletion, specifically BIA 10%, BIS 20% and BIA COPD 22% (). Depletion was found in subjects with low BMI. Strong correlations were proved between FFMI (DEXA) and FEV1 (p = 0.05) and FEV1/FVC (p < 0.0001).
Table 3. Number of patients with identified nutritional depletion via compared methods
FM and FMI, as measured by SFA and BIA were well correlated with DEXA (r = 0.93), with a small non-significant mean bias. Measurement by BIS and BIA COPD did not correlate, with statistically significantly overestimation of FM and FMI in the total patient group and in all GOLD stages.
Discussion
In COPD, loss of FFM, an important non-pulmonary physiologic impairment (Citation21), is associated with poorer prognosis (Citation22). Frequently FFMI is used in clinical practice as an indicator of nutritional depletion and for evaluation of pulmonary rehabilitation. Having a reliable and accurate method of measurement of body composition is important, allowing planned interventions for the group most likely to benefit. In the current study we applied DEXA as a “gold standard” with which to compare other bedside methods for determination of body composition in a group of subjects with COPD.
The advantages of DEXA include good accuracy and reproducibility, and it can provide an assessment of regional body composition (Citation23). Importantly, it has been validated for investigation in COPD (Citation7).
In evaluation of our results in the current study we demonstrated high correlation between body composition measurements using DEXA and the other comparison methods (p < 0.0001), but only SFA and BIA had very small non-significant mean bias as proved by Dunnet's test. Also, BIS and BIA COPD methods produced significantly different results from that of DEXA, and from each other (Table2). The best agreement between SFA and DEXA were also confirmed by other applied statistical methods (Lin´s concordance coefficient, Bland-Altman test). Similar results at each GOLD grade were demonstrated.
Our data support results from other studies (Citation7, 8, Citation24). SFA provides a very attractive alternative to other methods when evaluating body composition because of its relative simplicity, low cost and minimal equipment requirement. The accuracy of this method at COPD was demonstrated with measurement of tissue overhydration in this study by BIS. Both ANOVA and the Bonferroni test showed no differentiation in overhydration, on average 0.3l (p = 0.71) and did not exceed in average max. 1l, in each GOLD group in our research. This proved that measured skinfolds contained no excessive accumulation of extracellular water, which could contribute to overestimation of thickness, and which is typical for other types of disease (e.g. sepsis, polytrauma). Vitally important for maximum accuracy were triple exact measurements of each skinfold, with exact localization of the skinfolds on the body.
The application of BIS in this study consistently underestimated FFM. BIS measures impedance at 50frequencies, and is described as an accurate method for measurement of extracellular and intracellular fluids via the Moissl equation (Citation17). Calculation of fat mass with lean tissue mass is based on the assumption of normally hydrated tissue. The previous study of Miller etal. described a variation in hydration of FFM according to GOLD severity. This knowledge can explain underestimation of FFM and FFMI with BIS observed also in other studies (Citation7, 8, Citation25).
Measurements using BIS COPD with application of the special Schols equation for calculation of FFM from resistance in COPD patients, based on specific hydration of FFM, showed a significant and the highest mean bias, confirmed also by the Bland–Altman test.
A surprise was the concordance in the measurement of FM with FFM by the most common and accessible method BIA with leg-leg pressure contact, that has a limited ability to distinguish the distribution of TBW into intracellular and extracellular compartments (Citation26). This complementary and relatively inexpensive method showed behind SFA only small non-statistical differences in the present results relative to DEXA.
Evaluation of FFMI as a sign of muscle wasting via SFA demonstrated 5%, the same outcome as with DEXA. Other compared methods in the current study overestimated this parameter. We are aware of the low incidence of muscle wasting in this study, to which patient numbers and diverse BMIs in each GOLD category could contribute.
Some study limitations should be addressed. First, in this pilot-study, there were sufficient subjects (41), because the ratios of variability in observed parameters were similar to those using DEXA. Second, a smaller number of women were recruited, corresponding to a lower incidence of COPD among females. Opinion about inter-gender differences between DEXA FM with FFM measurements and other techniques has been mixed. In the studies of Kyle et al. (Citation27) and Steiner (Citation9), no sex differences were detected, but such differences were seen in the study of Engelen et al. (Citation28). In the present study, body composition of both men and women were evaluated together because no significant inter-gender differences between DEXA and other methods were demonstrated. Analysis of men and women together allowed evaluation according to GOLD airflow severity classification (GOLD 1–4), and did not diminish the validity of the results.
Third, participating subjects had diverse BMIs, which contributed to the testing and evaluation of measurement at different levels of FFM with FM in patients with COPD, because each method has some limitations. DEXA estimates of FM are influenced by “trunk thickness,” with the error increasing as the individual's trunk thickness increases (Citation23). The veracity of SFA is related to overhydration and the application of predictive equations for body density and for FM. With application of other equations can be expected other accuracy. The validity of BIA is influenced by the degree of fatness, TBW and relative ECW being greater in obese individuals compared with normal-weight subjects (Citation29). Similarly, compared with DEXA, BIS underestimated FFM in normal-weight individuals and overestimated it in obese individuals (Citation30).
In conclusion, skinfold anthropometry has been demonstrated as an accurate, available and cheap method for determination of FFM and FM in patients with COPD, by application of the Durnin-Womersley equation for body density and the Siri equation for FM. SFA offers a safe non-radiation alternative for repeated investigation of body composition as an indicator of nutritional depletion among cachectic COPD phenotype subjects and for evaluation of pulmonary rehabilitation benefits. BIA bipedal measurement is convenient for examination dependent on the instrument. On the contrary BIS and BIA COPD were attested with low agreement for observed determinations in comparison with DEXA.
Declaration of Interest Statement
There are no financial conflicts of interest for any author. Supported by the project (Ministry of Health, Czech Republic) for conceptual development of research organization 00179906 and research grant of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) –Czech Republic and from Charles University in Prague [17/2012/UNCE], and Faculty of Pharmacy [SVV 267 003] are gratefully acknowledged.
Acknowledgements
We thank all participating patients with COPD, contributors B. Malinova, M. Jensik, V. Krcmarova and K. Krcmarova. The authors are grateful to Ian McColl MD PhD for assistance with the manuscript.
References
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004; 364(9434): 613–620.
- de Torres JP, Marín JM, Casanova C, Cote C, Carrizo S, Cordoba-Lanus E, Lung cancer in patients with chronic obstructive pulmonary disease—incidence and predicting factors. Am J Respir Crit Care Med 2011; 184(8):913–919.
- Rutten EP, Spruit MA, Wouters EF. Critical view on diagnosing muscle wasting by single-frequency bio-electrical impedance in COPD. Respir Med 2010; 104(1):91–98.
- De Benedetto F, Del Ponte A, Marinari S, Spacone A. In COPD patients, body weight excess can mask lean tissue depletion: a simple method of estimation [abstract]. Monaldi Arch Chest Dis 2000; 55(4):273–278.
- Sanchez FF, Faganello MM, Tanni SE, Lucheta PA, Pelegrino NG, Hasegawa SH, Anthropometric midarm measurements can detect systemic fat-free mass depletion in patients with chronic obstructive pulmonary disease. Braz J Med Biol Res 2011; 44(5):453–459.
- Van Loan MD. Is dual-energy X-ray absorptiometry ready for prime time in the clinical evaluation of body composition? Am J Clin Nutr 1998; 68(6):1155–1156.
- Miller A, Strauss BJ, Mol S, Kyoong A, Holmes PH, Finlay P, etal. Dual-energy X-ray absorptiometry is the method of choice to assess body composition in COPD. Respirology 2009; 14(3):411–418.
- Lerario MC, Sachs A, Lazaretti-Castro M, Saraiva LG, Jardim JR. Body composition in patients with chronic obstructive pulmonary disease: which method to use in clinical practice? Br J Nutr 2006; 96(1):86–92.
- Steiner MC, Barton RL, Singh SJ, Morgan MD. Bedside methods versus dual energy X-ray absorptiometry for body composition measurement in COPD. Eur Respir J 2002; 19(4):626–631.
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R,et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26(5):948–968.
- Kreider ME, Grippi MA. Impact of the new ATS/ERS pulmonary function test interpretation guidelines. Respir Med 2007; 101(11):2336–2342.
- Hanania NA, Sharafkhaneh A, Celli B, Decramer M, Lystig T, Kesten S, Acute bronchodilator responsiveness and health outcomes in COPD patients in the UPLIFT trial. Respir Res 2011; 12:6.
- Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, General considerations for lung function testing. Eur Respir J 2005; 26(1):153–161.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166(1):111–117.
- Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 1974; 32(1):77–97.
- Siri WE. Body composition from fluid space and density. In Brozek J, Hanschel A, eds. Techniques for measuring body composition. Washington, DC: National Academy of Science; 1961:223–244.
- Moissl UM, Wabel P, Chamney PW, Bosaeus I, Levin NW, Bosy-Westphal A, Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas 2006; 27(9):921–933.
- Chamney PW, Wabel P, Moissl UM, Müller MJ, Bosy-Westphal A, Korth O, A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am J Clin Nutr 2007; 85(1):80–89.
- Miyamoto S, Nagaya N, Satoh T, Kyotani S, Sakamaki F, Fujita M, Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med 2000; 161(2 Pt 1):487–492.
- Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr 2005; 82(1):53–59.
- Thibault R, Le Gallic E, Picard-Kossovsky M, Darmaun D, Chambellan A. Assessment of nutritional status and body composition in patients with COPD: comparison of several methods. Rev Mal Respir 2010; 27(7):693–702.
- Andreassen H, Vestbo J. Chronic obstructive pulmonary disease as a systemic disease: an epidemiological perspective. Eur Respir J Suppl 2003; 46:2s–4s.
- Williams JE, Wells JC, Wilson CM, Haroun D, Lucas A, Fewtrell MS. Evaluation of Lunar Prodigy dual-energy X-ray absorptiometry for assessing body composition in healthy persons and patients by comparison with the criterion 4-component model. Am J Clin Nutr 2006; 83(5):1047–1054.
- Corcoran C, Anderson EJ, Burrows B, Stanley T, Walsh M, Poulos AM, Comparison of total body potassium with other techniques for measuring lean body mass in men and women with AIDS wasting. Am J Clin Nutr 2000; 72(4):1053–1058.
- Freitas Júnior IF, Rupp de Paiva SA, de Godoy I, Smaili Santos SM, Campana AO. Comparative analysis of body composition assessment methods in healthy men and in chronic obstructive pulmonary disease patients: anthropometry, bioelectrical impedance and dual-energy X-ray absorptiometry. Arch Latinoam Nutr 2005; 55(2):124–131.
- Nuñez C, Gallagher D, Visser M, Pi-Sunyer FX, Wang Z, Heymsfield SB. Bioimpedance analysis: evaluation of leg-to-leg system based on pressure contact footpad electrodes. Med Sci Sports Exerc 1997; 29(4):524–531.
- Kyle UG, Pichard C, Rochat T, Slosman DO, Fitting JW, Thiebaud D. New bioelectrical impedance formula for patients with respiratory insufficiency: comparison to dual-energy X-ray absorptiometry. Eur Respir J 1998; 12(4):960–966.
- Engelen MP, Schols AM, Heidendal GA, Wouters EF. Dual-energy X-ray absorptiometry in the clinical evaluation of body composition and bone mineral density in patients with chronic obstructive pulmonary disease. Am J Clin Nutr 1998; 68(6):1298–303.
- Pateyjohns IR, Brinkworth GD, Buckley JD, Noakes M, Clifton PM. Comparison of three bioelectrical impedance methods with DXA in overweight and obese men. Obesity (Silver Spring) 2006; 14(11):2064–2070.
- Ward LC, Dyer JM, Byrne NM, Sharpe KK, Hills AP. Validation of a three-frequency bioimpedance spectroscopic method for body composition analysis. Nutrition 2007; 23(9):65.