Abstract
Background and Objective: An association between chronic obstructive pulmonary disease (COPD) and low body mass index (BMI) has been well established in cross-sectional studies. However, there have been few cohort studies investigating this issue. We therefore aimed to address this gap. Methods: Two population-based studies, a cross-sectional study including 1818 subjects and a subsequent 4-year cohort study consisting of 759 individuals without COPD, were conducted in Guangzhou, China. Every subject was 40 years old or older at the time of recruitment and completed questionnaire interviews, anthropometric measurements and spirometry testing. As a follow-up, each subject underwent annual pre-bronchodilator spirometry testing. Subjects with a pre-bronchodilator FEV1/FVC <0.7 were required to undergo post-bronchodilator spirometry testing. Subjects with a post-bronchodilator FEV1/FVC <0.7 were diagnosed with COPD. Results: Compared to subjects with normal BMI (18.5 to 23.9 kg/m2), those with low BMI (<18.5 kg/m2) had a higher prevalence of COPD (21.1% vs. 7.5%), with an adjusted OR of 2.75 [95% confidence intervals (CI): 1.69 to 4.47]. Both low BMI and obese (≥28.0 kg/m2) subjects had lower FEV1 after adjustment. This association was further confirmed in the cohort study; non-COPD subjects with low BMI at baseline were more likely to develop COPD (RR = 2.88, 95% CI: 1.06 to 7.85), independent of smoking status and other confounders. Conclusions: Low BMI was not only a systemic consequence of COPD but also an important risk factor for the development of COPD, which raises the possibility that early intervention in subjects with low BMI may reduce the incidence of COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) primarily affects the lungs but also produces systemic consequences. One of the main systemic consequences is nutritional abnormalities, mainly represented by a low body mass index (BMI) (Citation1). BMI, the body weight (kg) to height squared (m2) ratio, as part of the BODE index (BMI, airflow obstruction, dyspnea, and exercise capacity), has been shown to be an independent indicator of poor prognosis in patients with COPD (Citation2–4). Harik-Khan has also suggested that low body weight might be a risk factor for COPD (Citation5). To date, however, there have been few cohort studies addressing the relationship between COPD and BMI. Therefore, we evaluated the association between BMI and COPD through a cross-sectional study and a subsequent cohort study in Guangzhou, Southern China.
Methods
Study design and subjects
Two population-based epidemiological studies were conducted in an urban area of Guangzhou, China, including a cross-sectional survey including 1818 subjects aged ≥40 years that was conducted in 2002 and a subsequent 4-year follow-up survey (from 2003 through 2007) of 759 subjects who did not have COPD at the baseline test. In both surveys, multistage cluster sampling strategies were used, and spirometry testing and questionnaires interviews were conducted.
The detailed study protocols have been described in previous publications (Citation6, 7). Ethical approval of the study protocols was obtained from the Medical Ethics Committee of the Guangzhou Institute of Respiratory Diseases, and informed consent was obtained from all participants. The studies adhered to the principles of the Helsinki Declaration.
Spirometry
In both surveys, spirometry testing was performed using portable spirometers (Micro Medical Ltd., Chatham, Kent, UK) by professional staff. The spirometry testing procedure recommended by American Thoracic Society (Citation8) and Enright PL et al. (Citation9) was applied to all eligible subjects. Subjects with a pre-bronchodilator FEV1/FVC <0.7 underwent post-bronchodilator testing; i.e., spirometry carried out within 15 to 20 minutes after taking a dose of 200 μg (in the cross-sectional study) or 400 μg (in the 4-year follow-up survey) of Salbutamol (Ventolin; GlaxoSmithKline, Middlesex, UK) inhaled through a 500-ml spacer.
The use of short- and long-acting bronchodilators within 12 or 24 hours prior to the test, respectively, was prohibited. We determined a quality grade (A–F) based on acceptable maneuvers and repeatability of the FEV1 and FVC (Citation10). At baseline, spirometry results with Grades A, B or C (at least two acceptable maneuvers, with FEV1 values matching within 0.2 L) was considered acceptable for analysis.
In our follow-up, spirometry testing was performed annually at the same time, and a stricter standard was required for analysis: at least three acceptable and two reproducible measurements (i.e., the highest and second highest values of the forced vital capacity (FVC) and FEV1 were within 150 ml or 5%) were required for analysis. According to the current criteria of the global initiative for chronic obstructive lung disease (GOLD) (Citation11), subjects with post-bronchodilator FEV1/FVC <0.7 were diagnosed with COPD, and the stage (I-IV) of COPD was determined in each diagnosed patient. Predicted normative values of FEV1 in Chinese population were derived from ECSC93 (European Coal and Steel Community in 1993) equations and adjusted using the appropriate conversion factors (Citation12).
Questionnaire
The questionnaires used in the two studies have been published elsewhere (Citation6, 7) and include questions about demographic variables, respiratory symptoms/disease history, co-morbidities, health care utilization, activity limitation, nutritional status, smoking and other potential risk factors for COPD. Body weight was measured in light clothing to the nearest 0.1 kg with a calibrated balance beam scale; height without shoes on was measured to the nearest 0.5 cm using a vertical ruler; and BMI (kg/m2) was computed as the ratio of body weight (kg) to height squared (m2).
BMI status was classified as “low (<18.5 kg/m2),” “normal (18.5–23.9 kg/m2),” “overweight (24.0–27.9 kg/m2)” or “obese (≥ 28.0 kg/m2)” (Citation13). Smoking status was recorded as “currently smokes,” “has never smoked,” or “previously smoked” at each visit. Subjects who had smoked for at least six months or had smoked at least 100 cigarettes in their lifetime were defined as “ever smokers”(Citation14), otherwise, they were categorized as never smokers. “Former smokers” were defined as subjects who had quit smoking for at least 6 months.
Current smokers included continual smokers and those who had quit but restarted or relapsed or had quit for less than 6 months (Citation7). The following factors were measured at baseline and coded as dichotomous variables: occupational exposure (yes or no, classified with a cut-off point of occupational exposure to dusts/fumes/gases for one year), co-morbidity (any physician-confirmed COPD-related disease), and family history of respiratory disease (any parent or sibling diagnosed with chronic bronchitis, emphysema, asthma or COPD).
Statistical analysis
Statistical analyses were performed with Stata software (Version 7.0, Stata Corporation, College Station, TX, USA) and SAS version 9.1 software (SAS Institute, Cary, NC). The association between BMI and COPD was evaluated using dichotomous logistic regression, and odds ratios (ORs) and relative risk ratios (RRs) for COPD were calculated after adjustment for clusters, gender, education level, smoking status, family history of respiratory disease, history of exposure to occupational dust/fume/gases, and age. No interaction was added to the final logistic regression model. A p-value of <0.05 was considered statistically significant.
Results
Baseline characteristics of the two study populations
The mean age of the population in the cross-sectional study was 59.11 (the standard deviation (SD), 11.82) yrs; 40.2% were male; the mean FEV1 was 2.06 (SD, 0.64) L; the mean FVC was 2.58 (SD, 0.75) L; the mean FEV1/FVC was 80.00 (SD, 9.31)%; the mean BMI was 22.88 (SD, 3.50); and 35.1% of population had ever smoked cigarettes. The baseline characteristics of the cohort study and the cross-sectional study populations were similar, except for occupational exposure (p < 0.05) ().
Table 1. Baseline characteristics of the participants in the cross-sectional study and subsequent cohort study
BMI and COPD
As the cross-sectional study has shown, patients with low BMI had a higher prevalence of COPD than those with normal BMI (21.1% vs. 7.5%), with an adjusted OR of 2.75 (95% CI, 1.69 to 4.47) (). In addition, it appeared that the subjects with higher BMI had a lower likelihood of COPD (Ptrend < 0.001). This association was further identified in the follow-up cohort study, as shown in .
Table 2. Association between BMI and COPD in a population-based cross-sectional study
Table 3. Association between BMI and COPD in population-based cohort study
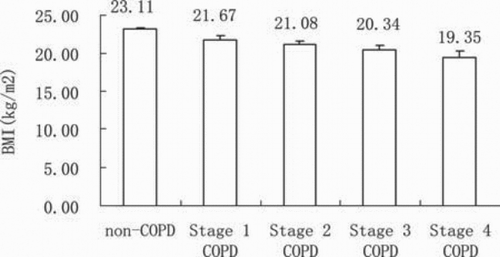
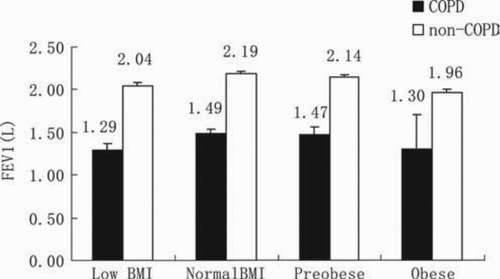
Non-COPD subjects with low BMI at baseline were more prone to developing COPD than were those without low BMI, with an RR of 2.88 (95% CI, 1.06 to 7.85). In addition, as shown in , males, elderly subjects, smokers and subjects with a family history of respiratory disease were more likely to develop COPD. Non-COPD subjects with low FEV1/FVC at baseline and those with a higher smoking index were also more likely to develop COPD (). Finally, we found that patients with COPD had lower BMIs than those without COPD, and patients with stage IV COPD had the lowest BMIs (). The baseline survey showed that both low BMI and obese subjects with or without COPD had lower FEV1 after adjustment for covariates ().
Discussion
The major finding of this study was that low BMI was associated with an increased incidence of COPD, and that this association was not merely observed at a later stage of COPD. A major strength of the present study compared to previous studies is that it provides comprehensive data about COPD and BMI in the general population using both cross-sectional and prospective approaches. With regard to the association between COPD and low BMI, researchers have previously suggested that low BMI was secondary to COPD (Citation15) and could be attributed to the systemic inflammation, imbalance of oxidative status and tissue hypoxia present in COPD patients (Citation16–18).
In contrast to this hypothesis, we observed that subjects with low BMI were at a substantially elevated risk of COPD even after adjusting for other potential risk factors. Our results were consistent with the reports of Harik-Khan and Higgins. Harik-Khan (Citation5) has indicated that men with a low BMI are at increased risk of developing COPD, and Higgins (Citation19) has demonstrated that the incidence of obstructive airway disease, defined as a predicted FEV1 < 65%, is highest in lean men and lowest in overweight men.
The association between low BMI and an excessive incidence of COPD may be explained by several factors. First, poor nutritional status at birth or during early infancy is associated with impaired lung function or the development of COPD in adulthood (Citation20, 21). Although we assessed low adulthood BMI rather than low birth weight, both of these observations suggest that malnutrition reduces respiratory muscle and is likely to increase the likelihood of chronic lung infections (Citation22, 23). Second, increases in BMI over time provoked greater decreases in FVC than in FEV1; therefore, the ratios of FEV1/FVC increased as BMI increased because both FVC and FEV1 decreased (Citation24).
Third, the lower caloric intake by cigarette smokers may contribute to the finding that low BMI subjects were more susceptible to COPD. However, smoking-induced low caloric intake cannot completely account for low BMI because the association of leanness with a higher risk of respiratory mortality was also observed in non-smokers (never smokers) (Citation25, 26). Hence, weight loss in COPD is unlikely to be due to simple malnutrition. In addition, deficits in cell-mediated immunity and circulating T-lymphocyte numbers caused by protein-energy malnutrition can lead to an increased susceptibility to infection (Citation27), which exacerbate declines in pulmonary function and are considered important risk factors for COPD (Citation23).
A somewhat surprising result of this study is our finding that subjects with high baseline BMI had a lower risk of developing COPD, despite their lower FEV1. We had initially suspected that the predominantly restrictive effects of central obesity would eventually lead to a reduction in both FVC and FEV1.
However, this study has several limitations that must be acknowledged. First, there were insufficient subjects with low BMI and newly developed COPD for an independent analysis of the female subgroup in the cohort study. Second, like many other prospective studies, this study has an inevitable survivor bias. However, the influence of this effect should be minor because COPD is typically not a fatal condition.
In addition, the assessment of nutritional status based on BMI has several inherent limitations, in that the loss of skeletal muscle mass is the main cause of weight loss in patients with COPD, and this commonly occurs at an earlier stage of COPD than does the decrease in BMI. Recent data have suggested that fat-free mass index (FFMI) and mid-arm muscle area (MAMA) can provide information beyond that provided by BMI (Citation28–30).
In summary, we have found that low BMI is an important risk factor for development of COPD, independent of patient age, smoking status, and other potential risk factors. The source of this relationship is unclear, but it raises the possibility that early intervention in patients with low BMI may reduce the occurrence of COPD.
Declaration of Interest Statement
Supported by Chinese Central Government key research projects of the 10th national 5-year development plan grants 2001BA703B03(A) (P.R.) and The National Key Technology R&D Program of the 12th National Five-year Development Plan 2012BAI05B01 (P.R.).
The researchers were also independent from funders. The study funders were independent from the study in design, collection, analysis, interpretation of data, writing of the report, and in the decision to submit the article for publication. The study protocol was approved by the Medical Ethics Committee of Guangzhou Institute of Respiratory Diseases on 20 May 2002.
Yumin Zhou collected the data and monitored data collection, planned the statistical analysis, analysed the data, and drafted the manuscript. Dali Wang, Shengming Liu, and Jiachun Lu implemented the trial. Jingping Zheng and Nanshan Zhong conducted and monitored data collection. Pixin Ran initiated and designed the project, monitored data, and drafted the paper. Yumin Zhou and Pixin Ran are guarantors.
Acknowledgements
We are grateful to Prof. Shiliang Liu (Health Surveillance and Epidemiology Division, Health Promotion and Chronic Disease Prevention Branch, Public Health Agency of Canada, Ministry of Health, Ottawa) for their assistance in English.
References
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176: 532–555.
- Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 160: 1856–1861.
- Yang L, Zhou M, Smith M, Yang G, Peto R, Wang J, Body mass index and chronic obstructive pulmonary disease-related mortality: a nationally representative prospective study of 220,000 men in China. Int J Epidemiol. 2010; 39:1027–1036.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350:1005–1012.
- Harik-Khan RI, Fleg JL, Wise RA. Body mass index and the risk of COPD. Chest 2002; 121:370–376.
- Zhong N, Wang C, Yao W, Chen P, Kang J, Huang S, Prevalence of Chronic Obstructive Pulmonary Disease in China –A Large Population-based spirometry based Cross-sectional Survey. Am J Respir Crit Care Med 2007; 176:753–760.
- Zhou Y, Hu G, Wang D, Wang S, Wang Y, Liu Z, Community based integrated intervention for prevention and management of chronic obstructive pulmonary disease (COPD) in Guangdong, China: cluster randomised controlled trial. BMJ 2010; 341:c6387.
- American Thoracic Society Statements: Standardization of Spirometry 1994 Update. Am J Respir Crit Care Med 1995; 152:1107–1136.
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Interpretative strategies for lung function tests. Eur Respir J 2005; 26:948–968.
- Enright PL, Studnicka M, Zielinski J. Spirometry to detect and manage chronic obstructive pulmonary disease and asthma in the primary care setting. In: Wouters EF, Gosselink R, Stam H, editors. Lung function testing. Eur Respir Mon 2005; 31:1–14.
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease, GOLD Executive Summary. Am J Respir Crit Care Med 2012; Aug 9. [Epub ahead of print].
- Zheng J, Zhong N. Normative values for pulmonary function testing in Chinese adults. Chin Med J (Engl) 2002; 115:50–54.
- Zhou B; Coorperative Meta-Analysis Group Of Working Group On Obesity In China. Prospective study for cut-off points of body mass index in Chinese adults. Zhonghua Liu Xing Bing Xue Za Zhi (Chinese) 2002; 23:431–434.
- Yang G, Fan L, Tan J, Qi G, Zhang Y, Samet JM, Smoking in China, finding of the 1996 national prevalence of survey. JAMA 1999; 282:1247–1253.
- Guerra S, Sherrill DL, Bobadilla A, Martinez FD, Barbee RA. The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest 2002; 122:1256–1263.
- Vibhuti A, Arif E, Deepak D, Singh B, Qadar Pasha MA. Correlation of oxidative status with BMI and lung function in COPD. Clin Biochem 2007; 40:958–963.
- Creutzberg EC, Schols AM, Bothmer-Quaedvleig FC, Prevalence of an elevated resting energy expenditure in patients with chronic obstructive pulmonary disease in relation to body composition and lung function. Eur J Clin Nutr 1998; 52:396–401.
- Donahoe M, Rogers RM, Wilson DO, Pennock BE. Oxygen consumption of the respiratory muscles in normal and in malnourished patients with chronic obstructive pulmonary disease. Amer Rev Respir Dis 1989; 140:385–391.
- Higgins MW, Keller JB, Becker M, Howatt W, Landis JR, Rotman H, An index of risk for obstructive airways disease. Am Rev Respir Dis 1982; 125:144–151.
- Lechner AJ. Perinatal age determines the severity of retarded lung Development induced by starvation. Am Rev Respir Dis 1985; 131:638–643.
- Matsui R, Thurlbeck WM, Fujita Y, Yu SY, Kida K. Connective tissue, mechanical, and morphometric changes in the lungs of weanling rats fed a low protein diet. Pediatr Pulmonol 1989; 7:159–166.
- Arora NS, Rochester DF. Respiratory muscle strength and maximal voluntary ventilation in undernourished patients. Am Rev Respir Dis 1982; 126:5–8.
- Waaler HT. Weight and mortality: the Norwegian experience. Acta Med Scand 1984; 215(suppl):1–56.
- Paek YJ, Jung KS, Hwang YI, Lee KS, Lee DR, Lee JU. Association between low pulmonary function and metabolic risk factors in Korean adults: the Korean National Health and Nutrition Survey. Metabolism 2010; 59:1300–1306.
- Singh PN, Lindsted K. Body mass and 26-year risk of mortality from specific diseases among women who never smoked. Epidemiology 1998; 9:246–254.
- Lindsted KD, Singh PN. Body mass and 26 y risk of mortality among men who never smoked: A re-analysis among men from the Adventist Mortality Study. Int J Obes Relat Metab Disord 1998; 22:544–548.
- Chandra RK. Cell-mediated immunity in nutritional imbalance. Fed Proc 1980; 39:3088–3092.
- Marquis K, Debigaré R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 166:809–813.
- Soler-Cataluña JJ, Sánchez-Sánchez L, Martínez-García MA, Sánchez PR, Salcedo E, Navarro M. Mid-arm muscle area is a better predictor of mortality than body mass index in COPD. Chest 2005; 128:2108–2115.
- Ischaki E, Papatheodorou G, Gaki E, Papa I, Koulouris N, Loukides S. Body mass and fat-free mass indices in COPD: relation with variables expressing disease severity. Chest 2007; 132:164–169.