Abstract
Introduction: The contribution of occupational exposure to the risk of chronic obstructive pulmonary disease COPD in population-based studies is of interest. We compared the performance of self-reported exposure to a newly developed JEM in exposure-response evaluation. Methods: We used cross-sectional data from Multi-Ethnic Study of Atherosclerosis (MESA), a population-based sample of 45–84 year olds free of clinical cardiovascular disease at baseline. MESA ascertained the most recent job and employment, and the MESA Lung Study measured spirometry, and occupational exposures for 3686 participants. Associations between health outcomes (spirometry defined airflow limitation and Medical Research Council-defined chronic bronchitis) and occupational exposure [self-reported occupational exposure to vapor-gas, dust, or fumes (VGDF), severity of exposure, and a job-exposure matrix (JEM)-derived score] were evaluated using logistic regression models adjusted for non-occupational risk factors. Results: The prevalence of airflow limitation was associated with self-reported exposure to vapor-gas (OR 2.6, 95%CI 1.1–2.3), severity of VGDF exposure (P-trend < 0.01), and JEM dust exposure (OR 2.4, 95%CI 1.1–5.0), and with organic dust exposure in females; these associations were generally of greater magnitude among never smokers. The prevalence of chronic bronchitis and wheeze was associated with exposure to VGDF. The association between airflow limitation and the combined effect of smoking and VGDF exposure showed an increasing trend. Self-reported vapor-gas, dust, fumes, years and severity of exposure were associated with increased prevalence of chronic bronchitis and wheeze (P < 0.001). Conclusions: Airflow limitation was associated with self-reported VGDF exposure, its severity, and JEM-ascertained dust exposure in smokers and never-smokers in this multiethnic study.
Background
Chronic obstructive pulmonary disease (COPD) is characterized by airflow limitation that is not fully reversible, is usually progressive and associated with inflammatory response to noxious particles and gasses (Citation1). COPD is one of the leading causes of morbidity and mortality worldwide. According to the World Health Organization, COPD affects approximately 65 million people worldwide and is responsible for approximately 3 million deaths annually. This prevalence and associated mortality is projected to increase, and by 2030 COPD is expected to be the third leading cause of death (Citation2, 3). In the U.S. in 2009, there were 137 082 deaths from chronic lower respiratory disease (primarily COPD), the third leading cause of mortality (Citation4, 5).
The American Thoracic Society (ATS) estimated that approximately 15% of COPD in the general population is attributable to occupational sources (Citation6). The estimated cost of occupational COPD in the U.S. in 1996 was approximately $5 billion (Citation7). The estimated fraction of COPD attributable to work exposure in the U.S. population is 19% overall and 31% among never smokers (Citation8). Several recent population-based studies show associations between COPD and occupational risk factors, which include vapor-gas, dust, and fume (VGDF) exposure (Citation9–15). Few of these studies, however, have included a large number of U.S. minority groups, that may be at increased risk (Citation16).
The aim of this study is to further identify and characterize occupational risk factors for COPD in an older, multiethnic U.S. sample using a newly developed job exposure matrix (JEM) and questionnaire ascertained exposure. Specifically, we aim to evaluate whether occupational exposure to VGDF as ascertained by a questionnaire and JEM are risk factors for airflow limitation, chronic bronchitis and wheeze.
Methods
Study Population
The Multi-Ethnic Study of Atherosclerosis (MESA) recruited a population-based sample of participants 45–84 years old that were free of clinical cardiovascular disease in 2000–2002 from six predominantly large urban U.S. communities located in California, Illinois, Maryland, Minnesota, New York, and North Carolina. The participation rate was 60% among those screened and deemed eligible (Citation17). The four race/ethnicity groups in this analysis were White, Black, Chinese, and Hispanic.
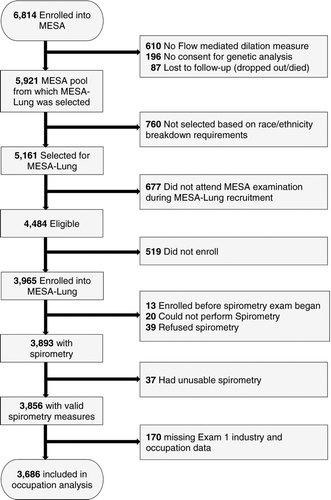
The MESA Lung Study enrolled MESA participants that were sampled from those that underwent baseline measurements of endothelial function, consented to genetic analyses, and underwent an examination during the MESA Lung Study recruitment period between 2004 and 2006 () (Citation18).
Occupational exposure assessment
We used two methods of occupational exposure assessment: (Citation1) responses to questions on exposure to VGDF; and (Citation2) JEM for the assessment of COPD risk constructed by NIOSH industrial hygienists. The JEM made use of the self-reported current occupation and industry if employed or the occupation where last employed if retired (ascertained using questions, see Appendix). The reported industry and occupation were coded by trained staff from the National Institute for Occupational Safety and Health (NIOSH) into U.S. Census 2000 occupation and industry coding.
Construction of the NIOSH JEM
To construct a generalizable JEM, we used the University of California San Francisco (UCSF) JEM Census occupational codes and added the additional MESA Census occupational codes not available in the UCSF JEM (Citation9). An industrial hygienist then constructed the NIOSH JEM by assigning an exposure score (Citation1, 2, or Citation3) representing the likelihood of the presence and severity of the VGDF exposure (low, medium, and high) for each Census occupation code based on expert opinion. In addition, the method of exposure coding applied in the Weinmann et al. (Citation14) study was adopted for assigning the exposure scores in this study (Citation19). The hygienist similarly created separate scores representing the likelihood and severity of exposure to vapor-gas, fumes, dust, and subcategories of dust (mineral; organic). Two certified industrial hygienists then reviewed the preliminary JEM scores and assigned a final consensus score. Environmental tobacco smoke was considered in the overall VGDF score for occupations with likely exposure (e.g., bartenders and waitresses), although a separate exposure score was not assigned.
Spirometry, airflow limitation, and respiratory symptoms and diseases
Spirometry tests were conducted in 2004–2006 in accordance with the ATS/European Respiratory Society (ERS) guidelines (Citation20) and all participants attempted at least three acceptable maneuvers. Tests were conducted using a dry-rolling-sealed spirometer and software that performed automated quality checks as maneuvers were performed (Occupational Marketing, Inc., Houston, TX). All spirometry exams were reviewed by one investigator and each test was graded for quality; participants with no acceptable curves were excluded (Citation21). All participants that completed an acceptable spirometry test and had industry and occupation data (n = 3686) were included in this study.
Airflow limitation was defined as the ratio of forced expiratory volume in one second to forced vital capacity (FEV1/FVC) below the lower limit of normal (LLN) and FEV1 < LLN (Citation22). NHANES-based reference equations (Citation23) were used to estimate the LLN values, however, for Asian-Americans, a correction factor of 0.88 was applied to calculate the LLN for FEV1 (Citation21). The Hankinson reference equations including the correction factors for Asian-Americans have been previously validated using MESA data (Citation21).
The other health outcomes investigated were symptoms of chronic bronchitis, wheeze, and self-reported physician-diagnosed “chronic obstructive pulmonary disease or COPD . . . or emphysema.” Chronic bronchitis (Medical Research Council) was defined as 3 or more months of chronic productive cough per year in 2 or more years (Appendix). Wheeze was defined by the question, “In the last 12 months, have you had wheezing or whistling in your chest?”
The National Heart, Lung, and Blood Institute (NHLBI) funded the MESA study and the ancillary MESA Lung Study; the JEM coding was funded by the National Institute for Occupational Safety and Health (NIOSH). The MESA study protocol was approved by the Institutional Review Board in each field center and the NHLBI; the current analysis was approved by the Institutional Review Board of NIOSH. Written informed consent was obtained from each participant.
Data analysis
A logistic regression model was used to estimate the association between occupational risk factors and the dichotomous variable of airflow limitation. The occupational risk was evaluated using self-reported exposure and exposure established using the NIOSH JEM. The occupational exposure variables individually evaluated in the logistic model included self-reported exposures to vapor-gas, dust, fumes; severity of VGDF exposure, years of exposure to VGDF, and exposure scores assigned by the JEM. An additional variable (number of VGDF agents) was created to reflect the number of agents (i.e., vapor-gas, dust, and/or fumes) to which the person reported exposure (none, one, two, or three). The covariates adjusted for in the model were age, gender, race/ethnicity, education, body mass index (BMI), smoking status, pack-years, cigar-years, pipe-years, environmental tobacco smoke, and asthma. BMI was divided into four categories: < 25, 25 –<30, 30 –<35, and ≥ 35 kg/m2 to reflect the potential effect of BMI on lung function (Citation24). Since there were few participants with BMI < 18 these were combined into the <25 category. In addition, separate models for never smokers were evaluated.
To investigate the combined effect of the occupational exposure and smoking on the various health outcomes, we created interaction variables combining smoking status (ever/never) and occupational exposure status [i.e., self-reported exposure (yes/no) or JEM (no = low, yes = medium or high) for dust, vapor-gas, and fumes, and VGDF]. The data were then analyzed using a model with three dummy variables comparing those with occupational exposure only, smoking only, and both smoking and occupational exposure to those without any of the two exposures, to determine a trend in the odds ratios. In addition, to test the interaction effect on a multiplicative scale, we included in the logistic model occupational exposure, smoking, and the interaction term (Citation9, 10).
Results
Demographic data for age, race/ethnicity, smoking status, BMI, and education are presented in . There were 3667 individuals with spirometry and complete information used in this analysis out of 3686 included in the occupational sample (). The study sample was 36% non-Hispanic White, 26% African-American, 22% Hispanic and 16% Chinese-American, and 44% had never smoked cigarettes. shows the characteristics of the sample overall, stratified by race/ethnicity, and restricted to never smokers (44%). A large proportion had education beyond high school (66.7%) and 44.3% were employed in the management or professional occupational group while 19.3% were blue-collar workers. The prevalence of moderate airflow limitation (FEV1/FVC < LLN and FEV1 < LLN) was 5.7% overall, 6.7% for males, 4.7% for females, and 2.1% among never smokers.
Table 1. Characteristics of participants in the MESA Lung Study and occupational exposure as ascertained by questionnaire and NIOSH job-exposure matrix (JEM)
Frequencies of occupational exposures as ascertained by a questionnaire and by the job exposure matrix are also presented in . For all participants, 37.9% self-reported exposure to dust, 19.4% to vapor-gas, and 24.2% to fumes, and 13.7% and 5.2% reported moderate and severe VGDF exposure, respectively. According to the JEM, the medium and high exposures totaled 12.0% for dust (5.3% for mineral dust, 7.6% for organic dust), 17.6% for vapor-gas, 4.6% for fumes, and the overall medium to high VGDF exposure was 15.1%. The proportion who were retired at the time of spirometry was 41.6%.
The adjusted odds ratios for the associations of airflow limitation with occupational exposure, in the whole cohort, show significant associations with self-reported vapor-gas exposure (). The dose-responses for severity of exposure and for the number of exposures reached statistical significance at the highest categories. The number of cases was, however, too small to perform meaningful analysis using race/ethnic stratification.
Table 2. Adjusted Odds Ratios (OR) for the association of airflow limitation† with occupational exposures ascertained using questionnaire and NIOSH job-exposure matrix (JEM)
Among never-smokers, airflow limitation was associated with the highest severity of self-reported exposure to VGDF with the odds ratio almost seven times that of “All” participants (). Among never-smokers, there was also an increasing trend in the odds of airflow limitation as the number of VGDF agents (exposure to 0, 1, 2, or 3 agents) increased, P < 0.01, with exposure to three agents associated with five times higher odds of airflow limitation (OR 5.1, 95% CI 1.5–16.7). Additionally, never-smokers exposed to VGDF for 15 or more years had over triple the odds of airflow limitation (OR 3.3, 95% CI 1.3–8.7). Also among never-smokers, the odds of airflow limitation was more than doubled with self-reported exposure to vapor-gas (OR 2.7, 95% CI 1.1–6.7) and increased 4-fold with exposure to fumes (OR 4.2, 95% CI 1.7–9.9) in comparison to those not exposed.
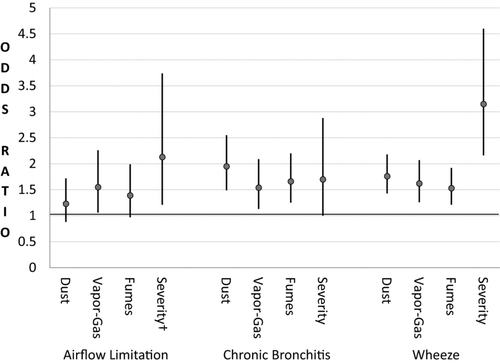
For associations with exposure to dusts as ascertained by the JEM, the odds ratios for airflow limitation doubled for highest level of exposure, especially among males, and nearly tripled for organic dust exposure among females (). We did not find significant association between airflow limitation and the JEM VGDF score (results not shown). There was a low Spearman rank correlation (0.21, P < 0.001) between self-report of VGDF and the JEM VGDF. shows the odds ratios for self-reported exposures (vapor-gas, dust, fumes, and the severity of exposure to VGDF) and airflow limitation.
Figure 2. Adjusted Odds Ratios for airflow limitation, chronic bronchitis, wheeze and self-reported exposures. †Severity is the most severe exposure to VGDF.
Odds ratios for the associations between airflow limitation and the combined effect of smoking and each of the occupational exposures are reported in . For all the exposures (vapor-gas, dust, fumes, and VGDF) there was an increasing trend in the odds ratios, including for the category with both exposures present. However, none of the odds ratios for the interaction effect were statistically significant at p < 0.05 level, rejecting the hypothesis that there is multiplicative interaction. The combined associations were similar for males and females.
Table 3. Adjusted Odds Ratios (OR) for association of airflow limitation† with the interaction of smoking and occupational exposures from questionnaire and NIOSH job-exposure matrix (JEM).
Self-reported doctor-diagnosed COPD associations reached statistical significance with self-reported dust exposure among “All” participants (OR 1.7, 95% CI 1.0–2.6), vapor-gas among “All” participants (OR 2.0, 95% CI 1.2–3.3), especially among females, and self-reported years of exposure to VGDF for 1–15 and over 15 years (). COPD also was associated with self-reported exposure to all three VGDF agents among “All” participants (OR 2.6, 95% CI 1.4–5.0), among females (OR 6.0, 95% CI 1.9–19.2), and among never-smokers (OR 14.2, 95% CI 2.1–94.9). COPD was associated with severity of exposure, especially in the highest severity category (OR 2.8, 95% CI 1.3–6.1).
Table 4. Adjusted Odds Ratios for Self-reported Physician Diagnosis of COPD,† with occupational exposure from questionnaire
Chronic bronchitis was associated with self-reported dust, vapor-gas, fumes, severity of exposure, years of exposure and the number of VGDF agents (, ), but not with the JEM exposure scores. Likewise, wheeze was associated with self-reported dust, vapor-gas, fumes, severity of exposure, years of exposure, and the number of VGDF agents (, ), but not with the JEM exposure scores.
Table 5. Adjusted Odds Ratios for chronic bronchitis† and wheeze‡ with occupational exposure from questionnaire
For chronic bronchitis, the combined effect of smoking and self-reported VGDF exposure or JEM assigned VGDF exposure was evaluated in a manner similar to airflow limitation. Similarly, for all the exposures (vapor-gas, dust, fumes, and VGDF) there was an increasing trend in the odds ratios, including for the category with both exposures present (results not shown). However, none of the odds ratios for the interaction effect were statistically significant at p < 0.05 level, rejecting the hypothesis that there is multiplicative interaction.
Discussion
We found that airflow limitation, physician-diagnosed COPD, chronic bronchitis, and wheeze were associated with self-reported occupational exposure to vapors, gas, dust and fumes in a large, multi-ethnic study of participants 45 to 84 years of age when initially recruited during 2000–2002. Associations were particularly strong among never smokers and for the severity of exposure. This study adds to our understanding about the association of VGDF exposure on non-malignant respiratory conditions and about the two methods of ascertainment of occupational exposure: self-reported using a questionnaire and a JEM.
We found that airflow limitation was associated with JEM-assigned scores for VGDF, dust and organic dust. To compare results for self-reported dust exposure with those for JEM-assigned dust exposure (low, medium, or high), the self-reported exposure had elevated odds while with the JEM the highest assigned dust category was significant (). The increased risk associated with highest JEM dust exposure category was primarily in males. This result may suggest that the JEM's three levels of exposure categorization may better capture the highest exposure level while the dichotomous self-reported dust exposure cannot differentiate the exposure levels.
Using the JEM, we also investigated the association of airflow limitation with exposure to organic dust for which we did not have self-reported data, and found that for “All” participants there was an increasing trend for airflow limitation and the level of exposure (i.e., low, medium, and high) (X2 = 3.8, P = 0.05). The association was statistically significant in females. This indicates that the JEM method may be useful also in the evaluation of occupational exposures for which self-report data are not available.
Among never-smokers (), associations with airflow limitation were observed for self-reported severity of VGDF exposure, the number of self-reported agents, and number of years exposed to VGDF agents. These findings are consistent with a study reported by Blanc et al. (Citation9) where self-reported VGDF and JEM assigned VGDF exposure were found to be associated with COPD. However, in our study we did not find a significant association between airflow limitation and the JEM VGDF. The low Spearman rank correlation between self-reported VGDF and the JEM VGDF may indicate that the participants’ own knowledge of their exposure was better than the JEM when the information about the longest held job was not available.
Self-reported doctor-diagnosed COPD was associated with self-reported dust, vapor-gas, number of years of exposure to VGDF, increasing severity of exposure to VGDF, and increasing number of agents (CitationTable 4). Some of the odds of COPD were increased 2-fold or greater among never-smokers. This result is consistent with a study by Blanc et al. (Citation9).
Chronic bronchitis is a major component of COPD; therefore Medical Research Council (MRC)-defined chronic bronchitis as determined by several questions was evaluated for association with occupational exposure (Appendix). Chronic bronchitis was associated with self-reported occupational exposure to dust, vapor-gas, fumes, severity of exposure to VGDF, years of exposure to VGDF, and with the increasing number of agents (none, one, two, or three) of vapor-gas, dust, and/or fumes (). This result reinforces the well-known association between occupational exposure and “industrial bronchitis” (Citation15, Citation25–27). We also investigated the association of chronic bronchitis with the combined effect of smoking and each exposure. The results (not tabulated) were similar to those for airflow limitation.
Wheeze is a symptom often associated with airflow limitation (Citation28, 29). In our study, wheeze within the last year was associated with occupational exposures to vapor-gas, dust, fumes, a progressive gradient of severity of exposure to VGDF, and with the increased number of the VGDF agents reported by participants. These associations were also consistent by gender and among never smokers (). The associations of chronic bronchitis and wheeze with occupational exposure were stronger than with airflow limitation.
This study has some limitations, which are: 1) Temporality cannot be determined; 2) Questionnaire data pertaining to past occupational exposure may be prone to recall bias in that respondents with disease or symptoms may have considered the presence and more severe exposure than respondents without symptoms. The use of both questionnaire self-reported exposure and JEMs should help reduce potential for misclassification bias (Citation9, Citation30, Citation31). Since participants may not be aware that they have airflow limitation, differential misclassification would be less likely for this outcome than for reported symptoms of chronic bronchitis and wheezing. 3) For some outcomes, the highest JEM exposure category had insufficient sample size to determine a significant trend. 4) Another important limitation is that the JEM assessment was based on the most recent job. Almost 42% of the MESA-Lung participants were retired at the time of spirometry and only the most recent job prior to retirement was reported. This lack of information on the longest held job may have decreased concordance between the JEM and self-reported exposure and reduced the ability of the JEM to detect the effects of occupational exposure on disease.
The MESA study was designed prospectively to study subclinical factors for cardiovascular disease (CVD) and thus individuals with existing clinical CVD were excluded at the onset of the study (Citation17). CVD is one of the factors that occur with the highest COPD comorbidity (Citation32–34). This exclusion may have introduced a survivor bias among elderly participants; yet, it is unlikely to have affected participants under 65 years. When investigating the prevalence of airflow limitation and self-report of severity, the sensitivity analysis results for the most severe category were similar for those under 65 years (n = 2133) and those over 65 years (n = 1375) (OR 1.8, 95% CI 0.9–3.6 vs. OR 2.2, 95% CI 0.9–5.8). Therefore, with respect to airflow limitation, survivor bias appears to be less likely in this population. However, Sin et al. reported that reduced FEV1 is a risk for cardiovascular mortality; therefore excluding those with cardiovascular disease may also exclude individuals with reduced FEV1 and COPD in this study resulting in reduced odds ratios for airflow limitation (Citation32).
Conclusion
We found significant associations between the prevalence of airflow limitation and occupational exposure ascertained by both self-report and JEM. We did not find an association between COPD and the combined effect of occupational exposure and smoking on the multiplicative scale, but there was an indication of an increased risk of COPD when both exposures were present. We developed an expanded COPD JEM based on the methodology of a previously developed JEM for use in future studies. Chronic bronchitis and wheeze each were associated with self-reported exposures (vapor-gas, dust, fumes, and VGDF exposure severity, years of exposure, and number of agents). Smoking cessation programs combined with the reduction of occupational exposures should be pursued to reduce the prevalence of COPD in workers.
Declaration of Interest
No conflicts of interest exist. The authors are responsible for the content and the writing of this paper.
Appendix: Pertinent Questions from Questionnaires
A trained interviewer administered a Respiratory Questionnaire to the MESA-Lung participants. Information from the MESA-Lung study in this analysis was supplemented through other MESA questionnaires where participants were asked to identify race/ethnicity using the 2000 Census questions. The respondents were then categorized into one of four racial/ethnic groups.
The script provided to the interviewers for the question was extracted from page 13 of the “MESA-Lung Field Center Manual of Operations and Procedures Version 1.9 April 8, 2005. The interviewers were instructed to read every question EXACTLY as written, including the responses. For clarification, they were instructed to re-read the question and answers for the participant. If the participant had questions regarding the definitions for the three questions, they were only allowed to provide the definitions in the “Note” section. The number of times (interviews) that the note for the questions was used was not captured.
The occupational exposure was determined by the following questions:
Q.13. Have you ever been exposed at work to:
(This is “ever” exposure, and it does not have to be daily exposure).
Vapors or gas Answer “yes” or “no” or “don't know”.
Note: vapors are really synonymous with gas, that is the form into which liquids are naturally converted by the action of a sufficient degree of heat.
Dust Answer “yes” or “no” or “don't know”.
Note: dust is fine particulate matter which is light enough to be to be easily raised and carried up into the air.
Fumes Answer “yes” or “no” or “don't know”.
Note: fumes are volatile matter produced by and usually accompanying combustion (where there's smoke, there's fumes –although the converse is not completely true).
If yes to any of the above, answer the following:
For how many years were you exposed at work to vapors, gas, dust or fumes?
Provide the number of years. ___ years
How long ago was your last exposure?
Select “current” or provide the number in months OR in years.
____ current OR ___ months OR ____years
Was the exposure “mild” or moderate” or severe’? ___ mild ___ moderate ___severe
Select one. Note that the exposure is in order of increasing level.
Wheezing was determined by the following question:
In the last 12 months, have you had wheezing or whistling in your chest?
Yes
No
Asthma was determined by the following question:
Have you ever had asthma?
Yes
No
COPD was determined by emphysema questions and the following question:
Has a doctor ever told you that you had any of the following:
Chronic obstructive pulmonary disease or COPD?
Yes
No
Don't know
MRC Bronchitis is based on cough and phlegm questions from the spirometry questionnaire:
“Do you usually bring up phlegm from your chest on most days for 3 or more months during the year?”
“For how many years have you brought up phlegm from your chest like this?”
“Do you usually have cough on most days for 3 or more months during the year?”
If respondent has a cough on most days for 3 or more months during the year, usually brings up phlegm from chest on most days for 3 or more months during the year and did this for 2 or more years, it was considered MRC Bronchitis.
Emphysema was based on the Medical History interview administered questionnaires from Exam 1 –4. During Exam 1, the interviewer asked, “Has a doctor ever told you that you have any of the following;
Q.1 Emphysema?”
Yes
No
Don't know
During Exams 2–4, the interviewer asked,
Q.2 Has a doctor told you that you have developed any of the following since your last MESA visit on____: Emphysema?
Yes
No
Don't know
The occupation was determined by the following four open-ended questions from the survey:
Q.9 For whom do/did you work? (name of company, business, organization or other employer) If you are not working now, please respond regarding your main occupation before you stopped working.
Q.10 What type of business or industry is/was this? (e.g., hospital, newspaper publishing, mail order house, auto repair shop, bank, etc.)
Q.11 What kind of work do/did you do or what was your job title? (e.g. registered nurse, personnel manager, auto mechanic, accountant, grinder operator, etc.)
Q.12 What are/were your most important activities or duties? (e.g. patient care, directing hiring policies, repairing automobiles, reviewing financial records, operating grinding mill, etc.)
| Abbreviations | ||
| CI confidence interval | = | |
| MESA Multi-Ethnic Study of Atherosclerosis | = | |
| JEM job exposure matrix | = | |
| UCSF University of California San Francisco | = | |
| FEV1 forced expiratory volume in one second | = | |
| FEV1/ FVC ratio of forced expiratory volume in one second to forced vital capacity | = | |
| LLN lower limit of normal –the LLN approximates the one-sided 95% confidence limit for the expected value, where 5% of healthy persons who have never smoked would be identified as abnormal using the LLN | = | |
| MRC Medical Research Council | = | |
Acknowledgments
Financial support: The Multi-Ethnic Study of Atherosclerosis (MESA) and the ancillary MESA Lung study were funded by the National Heart, Lung, and Blood Institute (NHLBI) (NIH HL077612, HL075476, N01-HC-95159 through N01-HC-95165, and N01-HC-95169). The coding of occupational information was conducted by the National Institute for Occupational Safety and Health (NORA FY08 CRN SLB8).
Disclaimer The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- Celli BR, MacNee W, Agusti A, ATS/ERS Task Force Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Resp J 2004; 23:932–946.
- World Health Organization (WHO). Burden of COPD [Online]. 2007, [cited 2012]. Available from: http://www.who.int/respiratory/copd/burden/.
- World Health Organization (WHO). Chronic obstructive pulmonary disease (COPD) Fact sheet [Online]. Nov 2011, [cited 2012]. Available from: http://www.who.int/mediacentre/factsheets/fs315/en/index.html.
- Brown DW, Croft JB, Greenlund KJ, Deaths from chronic obstructive pulmonary disease—United States, 2000–2005. MMWR Surveill Summ 2008; 57(45):1–16.
- Kochanek KD, Xu JQ, Murphy SL, Minino AM, Kung HC. Deaths: preliminary data for 2009. Natl Vital Stat Rep 2011; 59(4). Hyattsville, MD: US Department of Health and Human Services, CDC, National Center for Health Statistics; 2011. Available at http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_04.pdf. Accessed May 29, 2012.
- Balmes J, Becklake M, Blanc P, et. al. American Thoracic Society Statement: Occupational contribution to the burden of airway disease. Am J Respir Crit Care Med 2003; 167:787–797.
- Leigh JP, Romano PS, Schenker, MB, Kreiss K. Costs of occupational COPD and asthma. CHEST 2002; 21:264–272.
- Hnizdo E, Sullivan PA, Bang KM, Wagner G. Association between chronic obstructive pulmonary disease and employment by industry and occupation in the US population: A study of data from the Third National Health and Nutrition Examination Survey. Am J Epidem 2002; 156:738–746.
- Blanc PD, Iribarren C, Trupin L, Occupational exposures and the risk of COPD: dusty trades revisited. Thorax 2009; 64:6–12.
- Blanc PD, Eisner MD, Earnest G, Further exploration of the links between occupational exposures and chronic obstructive pulmonary disease. J Occup Environ Med 2009; 51(7):1–7.
- Blanc PD, Menezes AMB, Plana E, Occupational exposures and COPD: an ecological analysis of international data. Eur Respir J 2009; 33:298–304.
- Rodriquez E, Ferrer J, Marti S, Zock JP, Plana E, Morell F. Impact of occupational exposure on severity of COPD. CHEST 2008; 136(6):1237–43.
- Trupin L, Earnest G, San Pedro M, The occupational burden of chronic obstructive pulmonary disease. Eur Respir J 2003; 22:462–469.
- Weinmann S, Vollmer WM, Breen V, COPD and occupational exposures: a case-control study. J Occup Envirn Med 2008; 50(5):561–569.
- Blanc PD, Toren K. Occupation in chronic obstructive pulmonary disease and chronic bronchitis: an update. Int J Tuberc Lung Dis 2007; 11(3):251–257.
- Eisner MD, Blanc PD, Omachi TA, Socioeconomic status, race and COPD health outcomes. J Epidemiol Community Health 2011; 65:26–34.
- Bild DE, Bluemke DA, Burke GL, Multi-ethnic study of atherosclerosis: objectives and design. Am J Epi 2002; 156(9):871–881.
- Barr RG, Bluemke DA, Ahmed FS, Percent emphysema, airflow obstruction, and impaired left ventricular filling. New Engl J Med 2010; 362(3):217–227.
- Graziani M, Doney B, Hnizdo E, Assessment of lifetime occupational exposures in an epidemiologic study of COPD. The Open Epi J 2012; 5:27–35.
- Miller MR, Hankinson J, Brusasco V, Standardisation of spirometry. Eur Respir J 2005; 26:319–38.
- Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovasky KH, Barr RG. Performance of American Thoracic Society-Recommended spirometry reference values in a multiethnic sample of adults. CHEST 2010; 137(1):138–145.
- Pellegrino R, Viegi G, Brusasco V, Interpretative strategies for lung function tests. Eur. Respir J 2005; 26:948–968.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 1999; 159:179–87.
- Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol 2010; 108:206–211.
- Beckett WS. Occupational respiratory diseases. N Engl J Med 2000; 342(6):406–13.
- Morgan WKC. Industrial bronchitis. British J of Ind Med 1978; 35:285–291.
- Boggia B, Farinaro E, Grieco L, Lucariello A, Carbone U. Burden of smoking and occupational exposure on etiology of chronic obstructive pulmonary disease in workers of Southern Italy. J Occup Envirn Med 2008; 50(3):366–370.
- American Thoracic Society (ATS). What Are the Symptoms of COPD? [Online]. Feb 2012, [cited 2012]. Available from: http://www.thoracic.org/clinical/copd-guidelines/for-patients/what-are-the-signs-and-symptoms-of-copd.php.
- Mannino DM. COPD. CHEST 2002; 121:121S–126S.
- Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic Research Principles and Quantitative Methods. New York, NY: John Wiley & Sons, Inc. 1982; pp. 190–1.
- Quinlan PJ, Earnest G, Eisner MD, Performance of self-reported occupational exposure compared to a job-exposure matrix approach in asthma and chronic rhinitis. Occup Environ Med 2009; 66:154–160.
- Sin DD, LieLing W, Man SFP. The relationship between reduced lung function and cardiovascular mortality. CHEST 2005; 127:1952–1959.
- Hansell AL, Walk JA, Soriano JB. What do chronic obstructive pulmonary disease patients die from? A multiple cause coding analysis. Eur Respir J 2003; 22:809–814.
- Martinez CH, Han MK. Contribution of the environment and comorbidities to chronic obstructive pulmonary disease phenotypes. Med Clin N Am 2012; 96:713–727.