Abstract
Background: The assurance of high-quality spirometry testing remains a challenge. Methods: Spirometry training consisted of standardized coaching followed by certification for 35 spirometry-naïve and 9 spirometry-experienced research assistants. Spirometry was performed before and after bronchodilator (BD) in random population samples of 5176 people aged 40 years and older from 9 sites in Canada. using the hand-held EasyOne spirometer (ndd Medical Technologies Inc., Andover, MA, USA). Pulmonary function quality assurance with over reading was conducted centrally in Vancouver: spirograms were reviewed and graded according to ATS/ERS standards with prompt feedback to the technician at each site. Descriptive statistics were calculated for manoeuvre acceptability and repeatability variables. A logistic regression model was constructed for the predictors of spirometry quality success. Results: 95% of test sessions achieved pre-determined quality standards for back extrapolated volume (BEV), time to peak flow (PEFT) and end of test volume (EOTV). The mean forced expiratory time (FET) was 11.2 seconds. Then, 90% and 95% of all manoeuvres had FEV1 and FVC that were repeatable within 150 ml and 200 ml respectively. Test quality was slightly better for post-BD test sessions compared with pre-BD for both groups of research assistants. Independent predictors of acceptable test quality included participant characteristics: female sex, younger age, greater BD responsiveness; but not study site or prior experience in completing spirometry by the technologist. Conclusions: Good quality spirometry tests are attainable in large multicenter epidemiological studies by trained research assistants, irrespective of their prior experience in spirometry.
Introduction
Accurate and reliable measures of spirometry are critical for the documentation and interpretation of chronic airflow limitation, the hallmark of COPD. The American Thoracic Society (ATS) and European Respiratory Society (ERS) (Citation1–3) goals of acceptability and repeatability for spirometry which were originally described for quality assurance of spirometry performed by trained respiratory technicians in hospital-based lung function laboratories (Citation4), have been extended for assuring quality assurance of office spirometry but with variable outcomes (Citation5). Good spirometry quality assurance was recently reported in two large field studies: the World Trade Center responders Screening program (Citation6) and the international Burden of Obstructive Lung Disease (BOLD) study (Citation7). These studies suggested that targeted training of respiratory technicians in spirometry was effective for obtaining good quality in spirometry. However, it was unclear whether prior experience by research technicians in spirometry performance is an advantage for quality assurance.
The aims of the analysis were to evaluate the ability of participants in a large multisites, population-based study to meet the quality goals for spirometry and to assess the factors contributing to good quality measurements. In particular we were interested to determine whether prior experience in completing spirometry was a prerequisite for a good quality outcome.
Methods
The Canadian Obstructive Lung Disease [COLD] study is a cross-sectional multisite, nationwide, population-based, non-interventional study on lung health, which constituted the first phase of the Canadian Cohort of Obstructive Lung Disease, CanCOLD study. Data used for the present study were collected between August 2005 to May 2009 in 9 sites across Canada. The sampling strategy and baseline study protocol of the CanCOLD study were the same as that used in the international Burden of Obstructive Lung Disease [BOLD] initiative, the full details of which have been published elsewhere (Citation8). Additional specific details of the CanCOLD study were also described in a previous publication (Citation9).
Ethics and consent
All participants gave written informed consent and the study was approved by the respective university and institutional ethical review boards: UBC/ PHC Research Ethics Board, P05-006 (Vancouver); Biomedical-C Research Ethics Board, BMC-06-002(Montreal); UHN REB, 06-0421-B (Toronto); Capital Health Research Ethics Board, CDHA-RS/2007-255 (Halifax); Conjoint Health Research Ethics Board, ID21258 (Calgary); DMED-1240-09 (Kingston); 2009519-01H (Ottawa); Bio-REB09-162(Saskatoon); CER20459 (Quebec City).
Study population and design
Briefly, random samples of non-institutionalized adults, aged 40 years and older in 9 urban sites (Vancouver, Montreal, Toronto, Halifax, Calgary, Quebec City, Kingston, Saskatoon and Ottawa) were identified by BC STATS using official census data from Statistics Canada (Survey and Analysis Section; Victoria, BC, Canada) and the recruitment by random digit dialing was conducted by NRG Research group (Vancouver, BC, Canada). Eligible individuals were then invited to attend for a clinic visit to complete interviewer-administered respiratory questionnaires and to perform pre- and post-bronchodilator spirometry conducted by trained and certified staff.
Training and certification of technicians
Training for spirometry testing consisted of 3 days of standardized training followed by certification for 35 spirometry-naïve and 9 spirometry-experienced research assistants in 9 sites. All technicians received training followed by certification, irrespective of any previous training in spirometry. At least one team member of each participating site was first trained and certified centrally by a pulmonary specialist with expertise in spirometry testing and interpretation. These initial technologists were the primary group involved in subsequent training and certification. The study technologists had very different prior experiences in spirometry testing including fully trained qualified clinical technologists as well as nurses and undergraduate university students with no prior experience with spirometry. Training consisted of intense and supervised coaching such that technicians could perform up to 8 manouvers and achieve a grade A or B on the auto-display on the EasyOne spirometer (Citation7).
Data collection
Standardized questionnaires included a core questionnaire containing demographic details and questions about respiratory health and symptoms, smoking history of tobacco, health-related quality of life, respiratory related health care use, cardiovascular and other co-morbidities as well as other respiratory diseases; occupational and biomass utilization questionnaires.
Lung function data was obtained with the use of the ndd EasyOne spirometer (ndd Medical Technologies Inc., Andover, MA, USA) with performance of spirometry in a seated position, before and 15 minutes after administration of 200 μg of albuterol/salbutamol via a metered-dose inhaler with a spacer (Citation10). Pulmonary function quality assurance with over reading was conducted in which all spirograms were reviewed and graded according to ATS/ERS standards (Citation1, Citation3) with prompt feedback to the technician at each site. The maintenance of quality assurance for spirometry is detailed in previous publications (Citation8, 9). Only spirometric data that fulfilled the ATS acceptability and repeatability criteria (Citation3) were used for analyses.
Spirometry quality assurance
The quality of all test sessions was reviewed centrally in the COLD study pulmonary function reference center in Vancouver. Reports of scores were sent as feedback to each study site to assist the technologists maintain ongoing optimal average [of most recent 10 tests] scores, with additional training if necessary, similar to that in the BOLD study (Citation7).
Statistical analysis
Descriptive statistics were calculated for a) the acceptability variables of back extrapolated volume (BEV) and peak expiratory flow time (PEFT) for the maneuver with the highest FEV1, while end-of-test volume (EOTV) and forced expiratory time (FET) for the maneuver with the highest forced vital capacity (FVC); b) the repeatability of the maneuvers was computed for FVC and FEV1 by the difference between the highest and the second highest (Citation11).
The performance of the two technologist groups with and without prior experience was evaluated in two ways: a) the success rate was compared using Fisher's exact test; b) likelihood of success was compared using a logistic regression model as shown next.
A logistic regression model was used to identify the predictors of adequate overall quality of the spirometry as the dependent variable; independent variables include participant demographics, smoking status, educational level, MRC dyspnoea score, baseline spirometry, and the type of technologists classified by the presence or absence of prior training and experience in completing spirometry. All tests were two-tailed in nature and were performed using SAS software (SAS, version 9.3; SAS Institute; Cary, NC). A p value < 0.05 was considered significant.
Results
The pre- and post-bronchodilator spirometric data from all 5,176 participants from 9 sites in Canada were analyzed. The mean age was 57.7 (S.D.11.2) years; 56% were women; 19% were current smokers, 40% former smokers; the mean BMI was 28 kg/m2 with 28% being obese (>30 kg/m2); 10.9% had self-reported current asthma; 12.3% had chronic airway obstruction as defined by post-bronchodilator FEV1/FVC < 5th percentile (LLN), while 10.1% had spirometric restriction (post-BD FEV1/FVC > LLN and post-BD FVC < LLN). shows the mean, 5th and 95th percentiles for pre- and post-bronchodilator spirometric variables for all participants.
Table 1. Spirometry variables (mean, 5th, and 95th percentiles) from the 5176 participants (including those with poor spirometry results) in the Canadian Obstructive Lung Disease Study
Success rates by overall EasyOne scores
The evaluation of success rates achieved according to automatic quality assurance of the Easyone spirometer (quality grades A, B, C) (Citation7) showed that 93% for pre-bronchodilator test sessions and 95% for post-bronchodilator tests sessions had adequate quality, with variation by study sites from a low of 89% to a high of 97% for pre-bronchodilator sessions and 91% lowest to 97% highest for post-bronchodilator sessions.
Success rates by ATS acceptability and repeatability criteria
Results of quality checks for ATS criteria of acceptability (BEV, PEFT, FET, EOTV), and repeatability (variation of FEV1, FVC, and PEF) from pre and post–bronchodilator spirometry test sessions are shown in . The ATS criteria for acceptability are back-extrapolated volume (BEV) <150 ml, peak expiratory flow time (PEFT) <120 ms, forced expiratory time (FET) > 6 seconds, end-of-test-volume (EOTV) <40 ml depicted schematically in ; and for repeatability, variation in FEV1 (<200 ml), FVC (< 200 ml) (Citation3). In 90% of maneuvers with the highest FEV1 the criteria BEV was <150 ml, and PEFT < 100–110 ms; in 90% of maneuvers with the highest FVC: FET was > 6.8 seconds, and EOTV < 20–21 ml. In 90% of both pre- and post-bronchodilator test sessions, the FEV1 variation is <140 ml, and the FVC variation is <150 ml. In addition, when the FEV1 and FVC acceptability goals were tested in the single “best maneuver,” defined as that with the highest sum of FEV1 and FVC (Citation1), the FEV1 and FVC acceptability goals were also met by about 90% or more of the participants, for both the pre- and post-bronchodilator test sessions.
Figure 1. Acceptability Criteria [BEV, PEFT, FET, EOTV] for Spirometry tracings depicted in schematic drawings of Volume vs time and Flow vs time curves. BEV = back extrapolated volume; PEFT = time to peak expiratory flow; FET = forced expiratory time; EOTV = end of test volume as indication of presence of plateau.
![Figure 1. Acceptability Criteria [BEV, PEFT, FET, EOTV] for Spirometry tracings depicted in schematic drawings of Volume vs time and Flow vs time curves. BEV = back extrapolated volume; PEFT = time to peak expiratory flow; FET = forced expiratory time; EOTV = end of test volume as indication of presence of plateau.](/cms/asset/f2a1eb8f-e541-4f34-8900-3b1715bfcdec/icop_a_822857_f0001_b.gif)
Table 2. Results of quality checks for ATS criteria of acceptability (BEV, PEFT, FET, EOTV), and repeatability (variation of FEV1, FVC, and PEF) from pre and post–bronchodilator spirometry test sessions
Detection of ‘slow-starts’
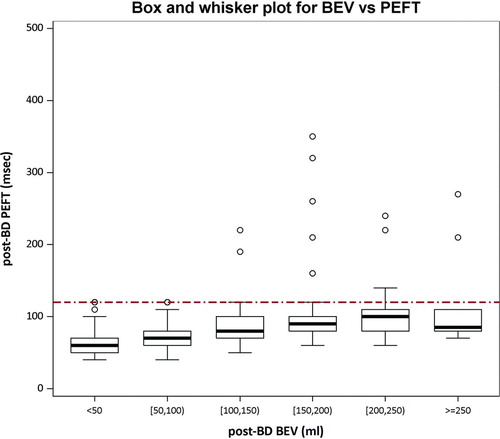
shows the relationship between back-extrapolated volume (BEV) and time to peak flow for detecting slow starts in test sessions. The cutoff goals by each criteria were BEV < 150 ml and PEFT > 120 ms. Of all those maneuvers with acceptable BEV, 97% also had acceptable PEFT. The BEV criterion was more likely than the PEFT criterion to “fail” a maneuver for slow-start: 10.4% in pre-bronchodilator sessions and 10.6% in post-bronchodilator sessions versus 3.9% and 4.4%, respectively, p < 0.001.
Figure 2. Box and whisker plots show relationship between back-extrapolated volume (BEV in mL) and time to peak flow (PEFT, msec) for detection of slow starts in post-BD test sessions. BEV was categorized in 50 mL increments. A BEV > 150 mL or PEFT > 120 ms indicates a slow start. The bottom and top of each box are the 25th and 75th percentiles, while the bottom and top whiskers are the 5th and 95th percentiles.
Evaluation of “premature termination”
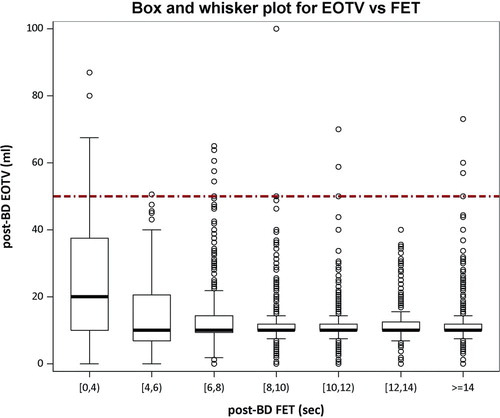
shows the relationship between end-of-test volume [EOTV] and forced expiratory time [FET] for detecting premature termination of forced vital capacity maneuvers in post-bronchodilator sessions. In maneuvers with FET > 6 seconds, 99.3% have end of test plateau. A lack of flat plateau or premature termination, as measured by an EOTV < 40 ml was seen in 20% of the maneuvers with FET < 4 seconds, 4.4% in those with FET < 6 seconds and ≥ 4 seconds, and only 0.7% of those with FET ≥ 6 seconds.
Figure 3. Box and whisker plots show the relationship between the end-of-test volume (EOTV in mL) and the forced expiratory time (FET) for detecting premature terminations of FVC maneuvers. Acceptability thresholds are < 45 mL for EOTV and > 6.0 s for FET. The bottom and top of each box are the 25th and 75th percentiles, while the bottom and top whiskers are the 5th and 95th percentiles. After 6 seconds, only 0.7% of maneuvers had an unacceptable EOTV (shown as a flat plateau on the volume-time curve).
Comparison of success at repeatability by ATS 1994 versus ATS/ERS2005 criteria
The success at achieving repeatability goals was also examined, according to two repeatability cutoffs: the ATS 1994 criteria (< 200 ml) and ATS/ERS 2005 (< 150 ml). The participants met ATS 1994 repeatability goal in 96.2% and 94.0% for dFEV1 and dFVC in pre-bronchodilator tests and 97.3% and 95%, respectively, for post-bronchodilator tests. The corresponding values for dFEV1 and dFVC according to ATS/ERS 2005 repeatability goals (< 150 ml) were lower (p < 0.0001): 92.8% and 87.5% in pre-BD tests and 94.5% and 90.8% in post-BD tests.
Predictors of success in meeting quality criteria
shows the univariate and multivariate results of the logistic regression model in determining the independent predictors of success in meeting overall quality goals in post-bronchodilator test sessions according to the EasyOne A, B, C grades (based on ATS 1994 criteria). The independent predictors for quality success in pre and post-BD sessions were female sex, younger age, while higher bronchodilator response responsiveness was a predictor in post-BD session and lower baseline FEV1 a predictor in pre-BD sessions. Participant characteristics including smoking status, the severity of exertional dyspnoea according to MRC score, education grade and the prior experience of the spirometry technologist were not significant independent predictors.
Table 3. Summary of logistic regression model# for predicting adequate post-bronchodilator spirometry
Comparison of performance between technologists with and without prior experience
showed that the proportion of adequate spirometry was higher for the ATS 1994 repeatability criteria (cutoff < 200 ml) (Citation3) versus the ATS/ERS 2005 criteria (cutoff < 150 ml) (Citation1) for both groups of technologists. There was no significant difference in the success rate between the two groups of technologists in meeting repeatability criteria for spirometry (all p values > 0.159, Fisher's exact test) although both groups showed improved performance in post-BD sessions compared with pre-BD sessions (all p values < 0.0005).
Figure 4. Comparison of success rates in meeting ATS/ERS acceptability and repeatability criteria in spirometry test sessions for the two groups of technologists (naïve versus non-naïve). Naïve is defined as having prior experience as a respiratory technologist in a formal pulmonary function laboratory. Both groups showed improved rates in post-bronchodilator sessions compared to pre-bronchodilator sessions, but there was no difference in rates between the two groups. NS = not significant (all p values > 0.159, Fisher's exact test).
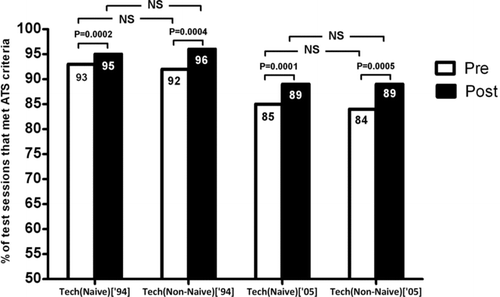
further confirmed that prior experience of completing spirometry did not have an impact on the overall likelihood of achieving successful spirometry maneuvers in the study (p = 0.3354), even after adjusting for potential differences in study participants tested by the two groups of technologists with [non-naive] or without [naïve] prior experience in completing lung function.
Discussion
Despite the widespread use of spirometry testing, the ability to achieve and maintain high quality spirometry tests remains a challenge. The results of this study showed that good quality spirometry testing that meets ATS acceptability and repeatability criteria are attainable in large multicenter epidemiological studies with quality success rates of >90% that are comparable to those achieved in the best hospital-based formal lung function laboratories (Citation4, Citation12). This study further indicated that this success was mainly attributable to a standardized protocol of rigorous coaching in spirometry, accreditation and continuous quality feedback and that the possession of previously acquired skills in completing spirometry before study-based training was not a prerequisite for predicting successful ability of performing spirometry in the study. These feasbility data are encouraging in the present climate of recommendations by global and national guidelines (Citation13–17) for more widespread use of spirometry testing for the better detection and confirmation of suspected obstructive airway diseases such as asthma and chronic obstructive lung diseases in primary care and in the community.
The ability of participants in this Canadian Obstructive Lung Disease study to meet goals for spirometry test sessions are similar to that found in the multisite Burden of Obstructive Lung Disease (BOLD) study (Citation7). This study showed that both the earlier (< 200 ml repeatability) and later and more stringent (< 150 ml repeatability) versions of ATS/ERS goals could be met in at least 90% of post-bronchodilator test sessions by trained researchers without prior training in carrying out spirometry, to a standard required for experienced technologists in an accredited hospital-based pulmonary function laboratory (Citation1, Citation4).
The individual technologists have been shown in previous studies to be the most common source of variability in quality (Citation4, Citation12, Citation18–20). We believe that the good quality spirometry for all sites in this study was due to the reduction of this variability by the rigorous personalized coaching of the technologists to optimally interact with the participant in perfecting the three phases [maximal inhalation, maximal expiration and prolonged exhalation] of a forced expiratory maneuver with careful explanation of the consequences of submaximal inhalation, submaximal expiratory effort and a premature termination on the accuracy and repeatability of the various components of spirometry, in particular the FEV1 and FVC (Citation21, 22).
This standardized teaching strategy was used in the BOLD initiative in which Vancouver participated as the Canadian site. The subsequent multisite Canadian COLD study replicated the same approach with similar success rates for all sites, thus emphasizing that the success rested in the quality of training and continuous supervision rather than the characteristic or background experience of individual technologist.
This study provided a systematic detailed assessment of spirometry success by the separate analysis of the components of acceptability and repeatability. A lack of maximal inhalation in the first phase of the spirometric maneuver, could not be objectively analyzed but was inferred from poor repeatability across several maneuvers. For the second and third phase of the forced expiratory maneuver, direct objective assessment of the quality checks showed that in this study the standard was at least as good as or better than the quality in the BOLD study (Citation7).
As in the BOLD study, we found that a slow start or hesitation, a characteristic of suboptimal expiratory effort, could be detected almost exclusively by increased BEV (> 120 ml), and that an increased PEFT (> 150 ms) did not improve the detection. In the third phase, premature termination was also entirely detected by a short FET (< 6 seconds) without the need to utilize a high EOTV as an additional determinant.
This study has added new data to the literature when compared with other studies on spirometry quality (Citation19, Citation23–25). The success rate for all tests in this study were comparable to that in earlier studies reporting spirometry quality in middle aged men in Bergen, Norway (Citation19), in people around 47 years old and 71 years old in the same region (Citation24); in office workers of both sexes in Canada (Citation25) and in population based samples from six cities in the United States (Citation23) and in the international multisite BOLD study (Citation7). However, the analyses in this study as in the BOLD study included detailed objective analyses of success according to the different acceptability criteria.
The protocol in the Canadian COLD study was the same as that in the BOLD study (Citation8) but the results showed several similarities and some differences (Citation7). In this study the success rates for attaining spirometry repeatability goals for pre- and post-bronchodialtor tests sessions were same as that in the BOLD study, but the lack of end of test plateau was negligible and several times lower than that in BOLD study. Further, the independent predictors of adequate post-bronchodilator spirometry included female sex, younger age in both studies, but higher education and lower pre-bronchodilator FEV1 were not found to be consistent independent predictors in our study.
These differences could be due to the fact that the population in this study was perhaps more homogenous as the sites were from one country Canada rather than international sites in the BOLD study. Another novel finding shown in the COLD study but not examined in the BOLD study was that prior spirometry skill in a technologist was not a prerequisite for good spirometry quality, which could be achieved with standardized acute personalized coaching followed by focused feedback. This observation is reassuring in the light of the current guideline recommendation for more widespread use of spirometry testing in the community for case finding of COPD (Citation13) and for surveillance of individuals in high-risk occupations (Citation26).
There are some limitations to the study. Our study population included people 40 years and older though we do not expect inferior performance for younger people, based on our risk factor analyses. Our results may vary for people with severe airway obstruction, which accounted for less than 1% of the population studied. Finally, we could not address the issue of what was the key item needed to assure long-term technician competence. Because we adopted a holistic multistage strategy for achieving technician competence, it is difficult to tease out the relative importance of the different components of our quality assurance program.
It is likely that the high rates of good quality spirometry seen in our study were the additive impact of the different components. Indeed, other authors have found that a single session of spirometry training was inadequate to maintain a good long term outcome and that a continuous quality improvement program of monitoring and feedback on the quality of the tests are essential to sustain this (Citation27). There is a need for further research to evaluate the relative effectiveness of different strategies (Citation28), such as the incorporation of automatic quality checks and quality grades (A to F) in office spirometers; the development of inexpensive, performance-based spirometry “certificates” for staff of primary care practitioners with the potential for regular recertification to maintain competence; and the provision of a continuous quality-improvement program by certified trainers to primary care staff who perform spirometry.
Conclusions
Good quality spirometry tests are attainable in large multicenter epidemiological studies by trained research assistants, irrespective of their prior experience in spirometry. Further research is needed to define the relative effectiveness of different components of a quality assurance program to meet the needs of more widespread use of spirometry testing for the better detection and confirmation of suspected obstructive airway diseases such as asthma and chronic obstructive lung diseases in primary care and in the community.
Acknowledgements
We thank the men and women who participated in the study and individuals in the *CanCOLD Collaborative Research Group: Executive Committee - Jean Bourbeau, (McGill University, Montreal, QC, Canada); Wan C. Tan, J. Mark FitzGerald; D.D. Sin. (UBC, Vancouver, BC, Canada); D. D. Marciniuk (University of Saskatoon, Saskatoon, SASK, Canada); D. E. O'Donnell (Queen's University, Kingston, ON, Canada); Paul Hernandez (University of Halifax, Halifax, NS, Canada); Kenneth R Chapman (University of Toronto, Toronto, ON, Canada); Robert Cowie (University of Calgary, Calgary, AB, Canada); Shawn Aaron (University of Ottawa, Ottawa, ON, Canada); F. Maltais (University of Laval, Quebec City, QC, Canada); International Advisory Board- Jonathon Samet (the Keck School of Medicine of USC, CA, USA); Milo Puhan (John Hopkins School of Public Health, Baltimore, USA); Qutayba Hamid (McGill University, Montreal, Quebec, Canada); James C. Hogg (UBC James Hogg Research Center, Vancouver, BC, Canada). Operations Center - Jean Bourbeau (PI), Carole Jabet, Maria Sedona, Palmina Mancino, Yvan Fortier (University of McGill, Montreal, Quebec, Canada); Wan C Tan(PI), Don Sin, Joe Comeau, Adrian Ng, Harvey Coxson, Tara Candido, Jonathon Leipsic (University of British Columbia James Hogg Research Center, Vancouver, BC, Canada). Economic Core - Andrea Benedetti (McGill University, Montreal, QC, Canada); Carlo Mara, Mohsen Savafi (University of British Columbia, Vancouver, BC). Data Management and Quality Control - Wan C. Tan, Harvey Coxson, (UBC, Vancouver, BC, Canada); Jean Bourbeau, ZL Pei, Yvan Fortier, Carole Jabet, Andrea Benedetti (McGill University, Montreal, QC, Canada), Denis O'donnell (Queen's University, Kingston, ON, Canada. Field Centers - Wan C. Tan (PI), Christine Lo, Wen Wang, Dianna Louie, Aimee Jon, Shen Gao, Eddy Wang, Jerry Chiu, Rita So, Jeong Min, Carly Moy, Sean Ling, Marina Wada, Yuexin Li, Sheena Tam, Anna La Lau, Ashleigh Sran, Ebony Swanson, Ying Yuan, Daniel Chen, Lu Zheng, Tina Yang, Junior Chuang, Best Guo [UBC James Hogg Research Center, Vancouver, BC]; Jean Bourbeau (PI), Palmina Mancino, Maria Sedona, Carmen Darauay, David Latrieille, Myriam Costa, Ashley Rycroft, Sabrina Pacheco, Marie-Paule Tran, Marie-Pierre Tran, Fatimah Momoh, Filipe Ruiz, Zhi-Li Pei [McGill University, Montreal, QC, Canada]; Kenneth Chapman (PI), Patricia McClean, Heather Sporn, Sviatlana Davychanka, Nadeen Audiosho, Ana Bradi [University of Toronto, Toronto, ON, Canada]; Robert Cowie (PI), Ann Cowie, Curtis Dumonceaux, Jessica Moore, Rustem Celami [University of Calgary, Calgary, AB, Canada]; Paul Hernandez (PI), Scott Fulton, Jasna Topek, Maria Yorke, Natalie Fiorotos, Ashley Rowe, Denise Wiggerus [University of Halifax, Halifax, NS, Canada]; Shawn Aaron (PI), Kathy Vandemheen, Gay Pratt, Jeevitha Srighanthan (University of Ottawa, Ottawa, ON, Canada); Denis O'Donnell (PI), Kathy Webb, Naparat Amornputtisathaporn, Kate Cheung, Kate Whelan, Jenny Cheng (Queen's University, Kingston, ON, Canada); Francois Maltais (PI), Joanie Couture, Luciana Garcia Pereira, Marie-Josée Breton, Cynthia Brouillard (University of Laval, Quebec City, QC, Canada); Darcy Marciniuk (PI), Ron Clemens, Janet Baran (University of Saskatoon, Saskatoon, SK, Canada).
Declaration of Interest Statement
Wan C. Tan has received funding for the operations of the Canadian Obstructive Lung Disease epidemiological study from unrestricted educational grants from GlaxoSmithKline, Pfizer, Boehringer Ingelheim, AstraZeneca.
Dr. Kenneth Chapman holds the GSK-CIHR Research Chair in Respiratory Health Care Delivery at the University Health Network, Toronto, Canada.
In the past 5 years, Dr. Darcy Marciniuk received funding support to attend a symposium from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Nycomed, and Pfizer; for speaking from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Nycomed, and Pfizer; for consulting from AstraZeneca, Canadian Agency for Drugs and Technology in Health, GlaxoSmithKline, Health Canada, Health Quality Council, Novartis, Nycomed, Pfizer, Public Health Agency of Canada, Saskatchewan Medical Association and the Saskatoon Health Region; for research (directed to the University of Saskatchewan) from AstraZeneca, Boehringer Ingelheim, Canadian Agency for Drugs and Technology in Health, Canadian Institute of Health Research, Dalhousie University, Forest Research, GlaxoSmithKline, Lung Association of Saskatchewan, McGill University, Nycomed, Saskatchewan Health Research Foundation, Saskatchewan Ministry of Health, Schering, Pfizer, and the University of Alberta; and for organizing education from the American College of Chest Physicians, Boehringer Ingelheim, Canadian Thoracic Society, Merck, Nycomed, and the University of Saskatchewan. Dr. Marciniuk is an employee of the University of Saskatchewan.
A. S. Buist has received unrestricted educational grants from GlaxoSmithKline (GSK), AstraZeneca (AZ), Novartis, Chiesi, Merck, Boehringer Ingelheim to the BOLD operation Center at Kaiser Center for health Research, Portland, Oregon, and served on advisory boards for GSK, Merck, Novartis and AZ.
Denis O'Donnell has served on advisory boards for Boehringer Ingelheim, Pfizer, GSK, Novartis, and Nycomed; has received lecture fees from Boehringer Ingelheim, Astra Zeneca, Pfizer, and GSK; and has received industry-sponsored grants from Boehringer Ingelheim, GSK, and Merck Frosst Canada and from Novartis and Pfizer.
Paul Hernandez has participated during the past five years on medical advisory boards for Actelion, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck, Novartis, Nycomed, and Pfizer; has conducted clinical research funded by Actelion, AstraZeneca, Boehringer Ingelheim, CSL Behring, Eli Lilly, GlaxoSmithKline, Novartis, Nycomed, and Pfizer; and, received support to travel to ERS Congress from AstraZeneca.
JB, FM, DS, RC, JMF, SA have no conflicts of interest to declare.
All authors are responsible for the content and writing of the paper.
References
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, ATS/ERS task force. Standardisation of spirometry. Eur Respir J 2005 Aug; 26(2):319–338.
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Interpretative strategies for lung function tests. Eur Respir J 2005 Nov; 26(5):948–968.
- Anon. Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 1995 Sep; 152(3):1107–1136.
- Enright PL, Beck KC, Sherrill DL. Repeatability of spirometry in 18,000 adult patients. Am J Respir Crit Care Med 2004 Jan 15; 169(2):235–238.
- Leuppi JD, Miedinger D, Chhajed PN, Buess C, Schafroth S, Bucher HC, Quality of spirometry in primary care for case finding of airway obstruction in smokers. Respiration 2010; 79(6):469–474.
- Enright PL, Skloot GS, Cox-Ganser JM, Udasin IG, Herbert R. Quality of spirometry performed by 13,599 participants in the World Trade Center Worker and Volunteer Medical Screening Program. Respir Care 2010 Mar; 55(3):303–309.
- Enright P, Vollmer WM, Lamprecht B, Jensen R, Jithoo A, Tan W, Quality of spirometry tests performed by 9893 adults in 14 countries: the BOLD Study. Respir Med 2011 Oct; 105(10):1507–1515.
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007 Sep 1; 370(9589):741–750.
- Tan WC, Bourbeau J, FitzGerald JM, Cowie R, Chapman K, Hernandez P, Can age and sex explain the variation in COPD rates across large urban cities? A population study in Canada. Int J Tuberc Lung Dis 2011 Dec; 15(12):1691–1698.
- Buist AS, Vollmer WM, Sullivan SD, Weiss KB, Lee TA, Menezes AM, The Burden of Obstructive Lung Disease Initiative (BOLD): rationale and design. COPD 2005 Jun; 2(2):277–283.
- Enright PL, Connett JE, Kanner RE, Johnson LR, Lee WW. Spirometry in the Lung Health Study: II. Determinants of short-term intraindividual variability. Am J Respir Crit Care Med 1995 Feb; 151(2 Pt 1):406–411.
- Herpel LB, Kanner RE, Lee SM, Fessler HE, Sciurba FC, Connett JE, Variability of spirometry in chronic obstructive pulmonary disease: results from two clinical trials. Am J Respir Crit Care Med 2006 May 15; 173(10):1106–1113.
- Anon. Global Strategy for Diagnosis, Management, and Prevention of COPD. From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011. Available from: http://www.goldcopd.org./ April 11, 2011.
- Levy ML, Quanjer PH, Booker R, Cooper BG, Holmes S, Small I. Diagnostic spirometry in primary care: Proposed standards for general practice compliant with American Thoracic Society and European Respiratory Society recommendations: a General Practice Airways Group (GPIAG)1 document, in association with the Association for Respiratory Technology & Physiology (ARTP)2 and Education for Health3 1 www.gpiag.org 2 www.artp.org 3 www.educationforhealth.org.uk. Prim Care Respir J 2009 Sep; 18(3):130–147.
- O'Donnell DE, Hernandez P, Kaplan A, Aaron S, Bourbeau J, Marciniuk D, Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease –2008 update –highlights for primary care. Can Respir J 2008 Jan–Feb; 15 Suppl A:1A–8A.
- Price D, Crockett A, Arne M, Garbe B, Jones RC, Kaplan A, Spirometry in primary care case-identification, diagnosis and management of COPD. Prim Care Respir J 2009 Sep; 18(3):216–223.
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, Diagnosis and Management of Stable Chronic Obstructive Pulmonary Disease: A Clinical Practice Guideline Update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011; 155(3):179–191.
- Enright P. FEV1 and FVC repeatability goals when performing spirometry. Prim Care Respir J 2010 Jun; 19(2):194.
- Humerfelt S, Eide GE, Kvale G, Gulsvik A. Predictors of spirometric test failure: a comparison of the 1983 and 1993 acceptability criteria from the European Community for Coal and Steel. Occup Environ Med 1995 Aug; 52(8):547–553.
- Kunzli N, Ackermann-Liebrich U, Keller R, Perruchoud AP, Schindler C. Variability of FVC and FEV1 due to technician, team, device and subject in an eight centre study: three quality control studies in SAPALDIA. Swiss Study on Air Pollution and Lung Disease in Adults. Eur Respir J 1995 Mar; 8(3):371–376.
- Hegewald MJ, Lefor MJ, Jensen RL, Crapo RO, Kritchevsky SB, Haggerty CL, Peak expiratory flow is not a quality indicator for spirometry: peak expiratory flow variability and FEV1 are poorly correlated in an elderly population. Chest 2007 May; 131(5):1494–1499.
- Krowka MJ, Enright PL, Rodarte JR, Hyatt RE. Effect of effort on measurement of forced expiratory volume in one second. Am Rev Respir Dis 1987 Oct; 136(4):829–833.
- Eisen EA, Dockery DW, Speizer FE, Fay ME, Ferris BG, Jr. The association between health status and the performance of excessively variable spirometry tests in a population-based study in six U.S. cities. Am Rev Respir Dis 1987 Dec; 136(6):1371–1376.
- Lehmann S, Vollset SE, Nygaard HA, Gulsvik A. Factors determining performance of bronchodilator reversibility tests in middle-aged and elderly. Respir Med 2004 Nov; 98(11):1071–1079.
- Ng'Ang'a LW, Ernst P, Jaakkola MS, Gerardi G, Hanley JH, Becklake MR. Spirometric lung function. Distribution and determinants of test failure in a young adult population. Am Rev Respir Dis 1992 Jan; 145(1):48–52.
- Townsend MC. ACOEM position statement. Spirometry in the occupational setting. American College of Occupational and Environmental Medicine. J Occup Environ Med 2000 Mar; 42(3):228–245.
- Borg BM, Hartley MF, Fisher MT, Thompson BR. Spirometry training does not guarantee valid results. Respir Care 2010 Jun; 55(6):689–694.
- Enright PL. Respiratory therapists should offer spirometry expertise to local primary care providers. Respir Care 2010 Jun; 55(6):780–781.
