Abstract
Introduction: Severe and acute exacerbation of chronic obstructive pulmonary disease (COPD) is associated with a high mortality. Since COPD is an airway inflammatory disease, and heparin has shown anti-inflammatory effects in previous studies, we evaluated the clinical effect of low molecular weight heparin (LMWH; nadroparin) in COPD patients admitted into the hospital due to acute exacerbations. Methods: Sixty-six patients admitted to the intensive care unit (ICU) were randomly divided into control group (n = 33) and LMWH group (n = 33). The control group received conventional treatment, including oxygen therapy (non-invasive or conventional mechanical ventilation), anti-infection, atomization expectorant, spasmolysis, anti-asthmatics, and nutritional support. The LMWH group received the same treatment plus LMWH for 1 week. The levels of plasma C-reactive protein, interleukin-6, and fibrinogen were measured. The main outcomes were duration of mechanical ventilation, length of ICU stay, and hospital stay. Results: There were no significant differences between the groups with respect to demographics, severity of illness, and gas exchange variables. The levels of plasma C-reactive protein, interleukin-6, and fibrinogen were significantly decreased in the LMWH group. LMWH significantly reduced the mean duration of mechanical ventilation (6.6 days vs. 3.8 days; p < 0.01), the length of ICU stay (8.5 days vs. 5.6 days; p < 0.01) and hospital stay (14.3 days vs. 11.3 days; p < 0.01). Conclusions: The addition of LMWH to standard therapy benefits COPD patients with acute exacerbation.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by minimally reversible airflow obstruction and progressive incapacity, and is the 4th leading cause of death worldwide (Citation1). The prevalence of COPD is slowly rising in recent years; an epidemiologic study estimated a two-fold increase in deaths in the next 20 years (Citation2). Acute exacerbation is the first leading cause of decompensation, hospital admission, and death in patients with COPD (Citation3–5).
Heparin is a member of the glycosaminoglycans family, and one of the most often used anticoagulant drugs (Citation6). Heparin is currently used to prevent and treat venous thrombosis and pulmonary embolism, arterial thrombosis in patients presenting with acute myocardial infarction. It prevents re-thrombosis after thrombolysis, and prevents thrombosis in extracorporeal circuits and hemodialysis (Citation7). Although heparin is primarily used as an anticoagulant, it has shown anti-inflammatory activities in experimental and clinical studies (Citation8,9).
Heparin may serve as an endogenous anti-inflammatory molecule involved in the control and resolution of inflammatory responses (Citation10). Several clinical trials in various inflammatory diseases support the anti-inflammatory effect of heparin (Citation11–13). Heparin and related compounds have shown clinical benefit to patients with bronchial asthma, ulcerative colitis, and burns (Citation14).
Given that COPD is an airway inflammatory disease, we hypothesized that heparin may have some therapeutic benefit in patients with acute exacerbation of COPD. The aim of the present study was to evaluate the effects of low molecular weight heparin (LWMH; nadroparin (5000 U per day × 1 week, subcutaneously)) in COPD patients with acute exacerbation.
Methods
Subjects
Sixty-six patients with acute exacerbation of COPD were enrolled in this study. The eligible subjects should be older than 50 years, with at least 10 pack-years smoking history, a FEV1 < 60% predicted at the time of inclusion or an inability to perform spirometry due to dyspnea, and an admitting diagnosis of acute exacerbation of COPD (defined as an acute increase in dyspnea, sputum volume, and/or sputum purulence without other attributable cause). Patients were excluded if they had acute heart failure, abnormal coagulation function, severe immune dysfunction, liver or kidney failure.
The study was approved by the Medical Ethics Committee for Clinical Trials at Shanghai First People's Hospital of Shanghai Jiaotong University, which complied with the ethical guidelines of the 1975 Declaration of Helsinki. Informed consent was obtained from each patient before the study.
Study design and protocol
Sixty-six patients were randomized into the standard therapy group (control group) or LMWH plus standard therapy group (LMWH group) with equal number. At the time of admission, all subjects received the standardized therapy, including non-invasive ventilation, albuterol and ipratropium bromide, nutritional support, and antibiotics (Citation15).
Patients were then randomized using blocks of two randomization protocol into control group receiving standardized therapy and the LMWH group receiving standardized therapy plus nadroparin (5000 U daily × 1 week subcutaneously, the prophylactic dose). Each eligible patient was sequentially assigned to either treatment group according to a randomization table generated in advance by our in-house statisticians. The study design was open-labeled, and the treatment assignment was not blind to the investigators, attending physicians, nurses, or patients.
Patients who were treated with non-invasive mechanical ventilation were considered to need tracheal intubation if they met any of the following criteria: pH < 7.20; pH = 7.20–7.25 on 2 separate measures 1 hour apart; hypercapnic coma (Glasgow Coma Scale = 8 and a PaCO2 = 60 mm Hg); a PaO500 < 45 mm Hg despite a maximum tolerated fraction of inspired oxygen; and/or cardiac arrest (Citation16).
Patients with conventional mechanical ventilation were screened each morning to assess recovery from respiratory failure and to determine whether or not they should be weaned from mechanical ventilation. In patients with non-invasive mechanical ventilation, weaning was considered successful if after at least 3 hours of breathing without ventilator assistance the following criteria were met: an arterial oxygen saturation ≥ 90% with a fraction of inspired oxygen ≤ 40%; a pH ≥ 7.35; and a respiratory rate ≤ 35 breaths/min.
C-reactive protein was measured using an automated clinical analyzer (Abbott Architect ci8200; Abbott Laboratories, Abbott Park, IL, USA) with the minimum detectable concentration of 5 mg/L. IL-6 levels were measured using the Quantikine High-sensitivity ELISA Commercial Kit (R&D Systems, Minneapolis, MN, USA) with the minimum detectable concentration of 0.039 pg/mL. Fibrinogen was assessed using a coagulation analyzer (Sysmex CA-7000; Dade-Behring) according to the Clauss method and calculated from ethylenediamine tetra-acetic acid to citrate plasma values with the minimum detectable concentration of 0.3 g/L.
Statistical analysis
Continuous variables were expressed as the mean ± standard deviation and compared between groups using the Student's t-test, and variables with a non-normal distribution were compared with the Mann–Whitney test. Categorical variables were compared with X2 test or Fisher's exact test. All statistical analyses were performed using statistical software (SPSS 14.0; SPSS, Inc., Chicago, IL, USA). Statistical significance was defined as p < 0.05.
Results
COPD is associated with cigarette smoking and old age (Citation17); therefore, all recruited patients were older than 50 years with 10 years of smoking history. The patient demographics and baseline characteristics are summarized in . There was no significant difference in the baseline values between the two treatment groups, including age, gender, weight, height, smoking history, baseline PaO2, baseline PaCO2, and SAPS II score. All of the patients in LMWH group completed the 1-week treatment, and no death occurred during this period.
Table 1 Baseline characteristics of the 66 patients according to treatment assignment
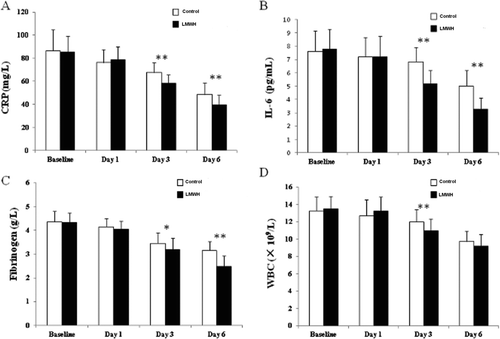
The plasma C-reactive protein levels decreased over time in the LMWH group and were significantly lower than the control group on days 3 (p < 0.01) and 6 (p < 0.01). The IL-6 levels were significantly lower in the LMWH group on days 3 (p = 0.00) and 6 (p = 0.00) compared to the control group. The fibrinogen levels were significantly lower in the LMWH group on days 3 (p = 0.02) and 6 (p < 0.01) compared to the control group. The white blood cell count was significantly lower in the LMWH group on day 3 (p < 0.01) compared to the control group ().
Figure 1. Mean levels of C-reactive protein (CRP) (A), Interleukin-6 (IL-6) (B), Fibrinogen (C) and white blood cell count (WBC) (D) at selected time points after treatment. The error bars indicates standard errors. (*p < 0.05, and **p < 0.01, for comparison with control group).
The clinical outcomes were summarized in . Compared to the standard therapy (control group), treatment with LMWH plus standard therapy (LMWH group) significantly reduced the mean duration of mechanical ventilation (6.6 days vs. 3.8 days; p < 0.01), the length of ICU stay (8.5 days vs. 5.6 days; p < 0.01) and hospital stays (14.3 days vs. 11.3 days; p < 0.01). Failure of non-invasive mechanical ventilation was not significantly reduced in LMWH group (13% vs. 22%; p = 0.39). In-ICU mortality was similar in the LMWH and control groups (9% vs. 12%; relative risk, 1.37; 95% CI, 0.15–3.52; p = 0.69). There was no difference in the incidence of adverse effects between these two groups. No thromboenblic or major bleeding events occurred in either group, but clinically significant non-major bleeding events were found in 1 patient (3.0%) in the LWMH group and in 2 (6.1%) in the control group (95% CI, 0.04–5.61; p = 0.55). All non-major bleeding events were hematomas at the injection site.
Table 2. Outcome measures
In LMWH group, 23 patients (22/23) were treated with non-invasive mechanical ventilation (NIMV) and 10 patients (10/23) with conventional mechanical ventilation (CMV), compared to 22 patients (22/33) with NIMV and 11 patients (11/33) with CMV in control group. The clinical outcomes in each subgroup were summarized in . Either in NIMV or CMV subgroup, the mean duration of mechanical ventilation, the length of ICU stay, and hospital stays were significantly reduced in LMWH group compared to the control group.
Discussion
This study evaluated the effects of LMWH in addition to the standard treatment in COPD patients with acute exacerbations. The results demonstrated that 1 week of LMWH treatment had a positive anti-inflammatory effect, as evidenced by decreased CRP, IL-6, and fibrinogen levels. The length of ICU and hospital stays were also reduced. Airway and systemic inflammation occurs in patients with COPD (Citation18). Stable COPD is associated with low-grade systemic inflammation manifested by an increase in blood leukocyte count, acute-phase proteins (CRP and fibrinogen), and inflammatory cytokines (Citation19–21).
IL-6 and acute-phase proteins levels further increase during acute exacerbations of COPD and decline again during the recovery (Citation20, Citation22). Clinical studies and animal experiments found an increase in the expression of neutrophil adhesion molecules and changes in neutrophil functions (Citation23–25). Heparin modulates some of the pathophysiologic effects of endotoxin and TNF-α, such as neutrophil migration, edema formation, pulmonary hypertension, and hypoxemia (Citation26). In addition, heparin has been shown to suppress specific neutrophil functions, such as superoxide generation and chemotaxis in vitro, and eosinophil migration (Citation27). One of the proposed mechanisms underneath the anti-inflammatory actions of heparin is the binding of glycosaminoglycan to adhesion molecules on the surface of activated ECs (endocytes) and/or leukocytes (Citation26).
Heparin influences immunologic responses and inhibits L- and P-selectin-mediated adhesion and TNF-α production by macrophages (Citation26, Citation28). Several recent studies have demonstrated the anti-inflammatory activity of heparin (Citation29, 30). In animal models, heparin disaccharide inhibits TNF-α production by macrophages and decreases the immune inflammation (Citation29–31). The anti-inflammatory activity of heparin has been confirmed by small clinical trials involving patients with a range of inflammatory diseases, including rheumatoid arthritis, bronchial asthma and inflammatory bowel disease (Citation32–35).
A hypercoagulable state exists in COPD patients; therefore heparin's anti-coagulant effect may add more benefit to COPD patients (Citation36). Thus, heparin may have both anti-inflammatory and anticoagulant effects. In 2006, Brown et al. (Citation37) reported that subcutaneously administrated LMWH (enoxaparin) once daily for 12 weeks in combination with conventional therapy provide additional clinical benefit, such as blood gas tension, dyspnea, and supplemental salbutamol use, to COPD patients (Citation37).
Most recently, Shi et al. (Citation38) reported that addition of LMWH to the conventional therapy (oxygen inhalation, spasmolysis, asthma relief, cough relief and sputum reduction) significantly improves the pulmonary functions in COPD patients with acute exacerbation. Our study showed that 1 week administration of heparin in combination with conventional therapy reduced the inflammatory molecules levels and the hospital and ICU stay of COPD patients with acute exacerbation, further supporting the clinical benefit of heparin in COPD.
This study is the first effort to evaluate the efficacy of a LMWH (nadroparin) in COPD patients with acute exacerbations in combination with standard therapy. Our observations suggest that further investigation is warranted to evaluate the use of heparin and related drugs in the treatment of COPD patients with acute exacerbations.
Declaration of Interest Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgment
Thanks to the Shanghai Young Physician Training Fund sponsored by Shanghai Medicine and Health Development Foundation for sponsorship to Y.Q.
References
- Decramer M, Janssens W, Miravittles M. Chronic obstructive pulmonary disease. Seminar. Lancet 2012; 379:1341–1351.
- Pauwels RA, Buist AS, Calverley PM, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001; 163:1256–1276.
- Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60:925–931.
- Liu H, Zhang TT, Ye J. Analysis of risk factors for hospital mortality in patients with chronic obstructive pulmonary diseases requiring invasive mechanical ventilation. Chin Med J (Engl) 2007; 120:287–293.
- Garcia-Aymerich J, Farrero E, Felez MA, Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax 2003; 58:100–105.
- Casu B. Structure of heparin and heparin fragments. Ann NY Acad Sci 1989; 556:1–17.
- Baglin T, Barrowcliffe TW, Cohen A, Guidelines on the use and monitoring of heparin. Br J Haematol 2006; 33:19–34.
- Ludwig RJ. Therapeutic use of heparin beyond anticoagulation. Curr Drug Discov Technol 2009; 6:281–289.
- Akyol C, Ozis E, Cakmak A, Nadroparine blunts lipopolysaccharide-induced hypothermia and behavioral depression in mice. J Invest Surg 2008; 21:311–317.
- Page CP. One explanation of the asthma paradox: inhibition of natural anti-inflammatory mechanism by beta 2-agonists. Lancet 1991; 337:717–720.
- Chande N, MacDonald JK, Wang JJ, Unfractionated or low molecular weight heparin for induction of remission in ulcerative colitis: a Cochrane inflammatory bowel disease and functional bowel disorders systematic review of randomized trials. Inflamm Bowel Dis 2011 Sep; 17(9):1979–1986.
- Lever R, Page CP.Non-anticoagulant effects of heparin: an overview. Hand Exp Pharmacol 2012; 207:281–305.
- Jennewein C, Paulus P, Zacharowski K. Linking inflammation and coagulation: novel drug targets to treat organ ischemia. Curr Opin Anaesthesiol 2011 Aug; 24(4):375–380.
- Young E. The anti-inflammatory effects of heparin and related compounds. Thromb Res 2008; 122:743–752.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis,management,and prevention of chronic obstructive pulmonary disease. 2009.
- National Clinical Guideline Centre (UK). Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care. 2010.
- Mannino DM, Braman S. The epidemiology and economics of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2007; 4:502–506.
- Wouters EF, Reynaert NL, Dentener MA, Systemic and local inflammation in asthma and chronic obstructive pulmonary disease: is there a connection? Proc Am Thorac Soc 2009; 6:638–637.
- Dev D, Wallace E, Sankaran R, Value of C-reactive protein measurements in exacerbations of chronic obstructive pulmonary disease. Respir Med 1998; 92:664–667.
- Wedzicha JA, Seemungal TA, MacCallum PK, Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemost 2000; 84:210–215.
- Undas A, Kaczmarek P, Sladek K, Fibrin clot properties are altered in patients with chronic obstructive pulmonary disease. Beneficial effects of simvastatin treatment. Thromb Haemost 2009; 102:1176–1182.
- Dentener MA, Creutzberg EC, Schols AM, Systemic anti-inflammatory mediators in COPD: increase in soluble interleukin 1 receptor II during treatment of exacerbations. Thorax 2001; 56:721–726.
- Cai C, Zhang HY, Le JJ, Inflammatory airway features and hypothalamic-pituitary-adrenal axis function in asthmatic rats combined with chronic obstructive pulmonary disease. Chin Med J(Engl) 2010; 123:1720–1726.
- Wouters EF. Chronic obstructive pulmonary disease. 5: systemic effects of COPD. Thorax 2002; 57:1067–1070.
- Miao JB, Hou SC, Li H, Clinical study of inflammatory factors in sputum induced early after lung volume reduction surgery. Chin Med J (Engl) 2008; 121:1796–1799.
- Mousa SA. Heparin and low-molecular weight heparins in thrombosis and beyond. Methods Mol Biol 2010; 663:109–132.
- Hiebert LM, Liu JM. Heparin protects cultured arterial endothelial cells from damage by toxic oxygen metabolites. Atherosclerosis 1990; 83:47–51.
- Derhaschnig U, Pernerstorfer T, Knechtelsdorfer M, Evaluation of antiinflammatory and antiadhesive effects of heparins in human endotoxemia. Crit Care Med 2003; 31:1108–1112.
- Lantz M, Thysell H, Nilsson E, On the binding of tumor necrosis factor (TNF) to heparin and the release in vivo of the TNF-binding protein I by heparin. J Clin Invest 1991; 88:2026–2031.
- Nelson RM, Cecconi O, Roberts WG,et al. Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood 1993; 82:3253–3258.
- Tyrell DJ, Kilfeather S, Page CP. Therapeutic uses of heparin beyond its traditional role as an anticoagulant. Trends Pharmacol Sci 1995; 16:198–204.
- Shin K, Nigrovic PA, Crish J, Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J Immunol 2009; 182:647–656.
- Kanabar V, Page CP, Simcock DE, Heparin and structurally related polymers attenuate eotaxin-1 (CCL11) release from human airway smooth muscle. Br J Pharmacol 2008; 154:833–842.
- Dotan I, Hallak A, Arber N, Low-dose low-molecular weight heparin (enoxaparin) is effective as adjuvant treatment in active ulcerative colitis: an open trial. Dig Dis Sci 2001; 46:2239–2244.
- Torkvist L, Thorlacius H, Sjoqvist U, Low molecular weight heparin as adjuvant therapy in active ulcerative colitis. Aliment Pharmacol Ther 1999; 13:1323–1328.
- Alessandri C, Basili S, Violi F, Hypercoagulability state in patients with chronic obstructive pulmonary disease. Chronic Obstructive Bronchitis and Haemostasis Group. Thromb Haemost 1994; 72:343–346.
- Brown RA, Allegra L, Matera MG, Additional clinical benefit of enoxaparin in COPD patients receiving salmeterol and fluticasone propionate in combination. Pulm Pharmacol Ther 2006; 19:419–424.
- Shi X, Li H. Anticoagulation therapy in patients with chronic obstructive pulmonary disease in the acute exacerbation stage. Exp Ther Med 2013; 5:1367–1370.
