Abstract
The purpose of this pilot study was to determine the impact of time of day on the acute response to incremental exercise in chronic obstructive pulmonary disease (COPD). Fourteen subjects (nine men) aged 71 ± 7 years with moderate to severe airflow obstruction (FEV1: 58 ± 13% predicted) followed a counterbalanced randomized design, performing three symptom-limited incremental cycling tests at 8:00, 12:00, and 16:00 hours on different days, each preceded by a spirometry. COPD medications were withdrawn prior to testing. No overall time effect was found for peak exercise capacity (p = 0.22) or pulmonary function (FEV1, p = 0.56; FVC, p = 0.79). However, a large effect size (f = 0.48) was observed for peak exercise capacity and several pulmonary function parameters. For peak exercise capacity, the average within-subject coefficient of variation was 5.5 ± 3.9% and the average amplitude of change was 7 ± 5W. Seven subjects (50%) showed diurnal changes at levels equal to or beyond the minimal clinically important difference for both peak exercise capacity and pulmonary function. In this sub-group, peak exercise capacity was greatest at 16:00 hours (p = 0.03, ƒ = 1.04). No systematic time-of-day effect on peak exercise capacity was obtained in COPD patients in the present pilot study. However, based on the observed effect size and on the average amplitude of change and within-subject variations seen across testing times, the guidelines recommendation that time of day be standardized for repeat exercise testing in COPD should be maintained.
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive and partially irreversible disease associated with frequent changes in clinical status (Citation1). Within a single day, lung function and symptom perception have been shown to vary (Citation2–4). In patients with COPD, who are sensitive to small increases in airway obstruction, diurnal variations in physiological parameters are likely to have important functional repercussions (Citation5).
Cardiopulmonary exercise tests measure the global and integrative response of the major systems and provides exclusive information above what is found from resting measures alone (Citation6, 7). Current exercise testing guidelines in COPD recommend that repeated exercise tests be undertaken at the same time of day to avoid the potential confounding effect of circadian variations in exercise measures (Citation6); yet this recommendation is based on findings in healthy individuals (Citation8). In COPD, the impact of time of day on exercise performance remains to be examined.
The primary objective of the present pilot study was to evaluate the effect of time of day on peak exercise capacity in COPD. Secondary objectives were to evaluate the effect of time of day on i) resting pulmonary function, ii) resting and peak physiological responses, and iii) resting and peak symptoms perception (dyspnea and leg fatigue).
Methods
Subjects
Subjects were recruited from Hôpital du Sacré-Coeur de Montréal between August 2010 and April 2011 from a pool of patients who had completed previous studies in our laboratory and had consented to be contacted for future investigations. Eligibility criteria included: 1) clinically stable COPD; 2) aged 40 years or older; 3) smoking history of at least 10 American pack-years (20 cigarettes per pack); 4) post-bronchodilation forced expiratory volume in 1 second (FEV1) less than 80% of the predicted normal value; 5) FEV1 to forced vital capacity (FVC) ratio less than 0.7; and 6) previous experience of exercise testing. Exclusion criteria were: 1) respiratory exacerbation in the past 4 weeks (change in dyspnea or volume/colour of sputum, need for antibiotic treatment, or hospitalization); 2) contraindication to exercise testing based on guidelines from the American Thoracic Society (ATS) (Citation6); 3) active condition other than COPD that could influence exercise tolerance; 4) need for oxygen therapy; and 5) prescribed theophylline. The research protocol was approved by the institutional ethics committee and a signed informed consent was obtained from each subject.
Study design and procedure
Subjects obtained medical clearance and came to the research facility for a total of four visits. The first visit included collecting demographic and clinical information (age, sex, height, weight, and body mass index (BMI)), baseline spirometry, and a familiarization with the testing equipment and procedure.
Subjects then entered the counterbalanced design (visits 2–4) where they each completed a spirometry followed by a symptom-limited incremental cycling exercise test, each conducted on a different day, at a different time (08:00, 12:00, and 16:00 hours ± 15 min). The selected testing times were chosen to cover the range of hours when exercise tests are typically conducted in clinical practice. Study visits were separated by at least 36 hours, but no more than 1 week. To limit a potential training effect, the order of testing times was determined through block randomization; however, participant availability and accessibility to medical supervision were considered. To limit the potential confounding effect of the timing of COPD medications on exercise capacity at different times of day, subjects were asked to withhold their respiratory medications 6 to 24 hours before visits 2–4 (). All other pharmacological treatment remained unchanged and subjects returned to their original regimen upon completion of their last study visit.
Table 1. Medication restrictions before visits 2, 3, and 4
Assessments
Pulmonary function testing
Spirometry was performed at baseline and before each evaluation according to recommended techniques (Citation9). Values were compared to predicted normal values from the European Community for Coal and Steel/European Respiratory Society (ERS) (Citation10).
Exercise testing
The symptom-limited incremental cycling exercise test was selected as it is currently the most frequently used in respirology (Citation11). Subjects were seated on an electromagnetically braked cycle ergometer (Ergoselect 200P, Ergoline, Germany) and connected through a mouthpiece to a cardio-respiratory circuit, which consisted of a digital volume sensor (TripleV), oxygen and carbon dioxide analyzers, and 12-lead electrocardiogram (Jaeger Oxycon Pro, CareFusion, Germany). After 5 minutes of rest followed by 3 minutes of unloaded pedalling, the workload was increased in a stepwise manner every minute, up to the individual's maximum capacity. The workload was increased by 5 or 10 watt (W) increments (5 W for subjects with a predicted peak work rate < 50 W; 10 W for those with a predicted peak work rate > 50 W, as determined from each subjects previous experience with exercise testing). Gas exchange parameters (minute ventilation (e), oxygen consumption (O2), and carbon dioxide production (CO2)), pulse oximetry (SpO2), and heart rate (HR) were measured at rest and during exercise on a breath-by-breath basis. Blood pressure (BP) (Tango, SunTech Medical, USA), inspiratory capacity (IC) (in accordance to the ATS/ERS guidelines (Citation9, Citation12)), and dyspnea and leg fatigue (modified 10-point Borg scale (Citation13)) were measured at rest and every other minute during exercise. Peak exercise capacity was defined as the highest work rate maintained at a pedalling speed of at least 50 revolutions per minute for a minimum of 30 seconds. All tests were completed under medical supervision.
Special attention was taken to optimize reproducibility of measurements as outlined in the ATS and American College of Sports Medicine (ACSM) guidelines (Citation6, Citation14). Prior to each evaluation, the cardiometabolic equipment and gas analyzers were calibrated. Laboratory temperature (22.2 ± 0.6°C) and humidity (37.6 ± 14.6%) were maintained within recommended ranges for all tests. Instructions given to participants followed the ACSM guidelines (Citation14), encouragement during the test were standardized, and all tests were conducted by the same two exercise physiologists.
Statistical analyses
To verify the distribution of normality for the primary and secondary outcomes, measures of skewness and kurtosis were used. For our primary objective, the overall effect of time of day (08:00, 12:00 and 16:00 hours) on peak exercise capacity was assessed with one-way repeated-measures analyses of variance (ANOVAs) using the General Linear Model. If a significant effect was obtained, pairwise comparisons with Bonferroni's corrections were conducted to identify time points showing significant differences. For our secondary objectives, we did the same analyses for parametric data, and Friedman tests were performed for non-parametric data. To test the presence of a potential order effect, results from the first and third sessions were compared using paired t-tests. Within-subject variability over the three testing sessions was estimated with the coefficient of variation (standard deviation [SD]/mean × 100), the average amplitude of change (Δmax-min), and the percent variation ([∆max-min/min] × 100).
Subjects showing changes at or beyond the suggested minimal clinically important difference (MCID) were identified for peak exercise capacity (MCID of 10 W (15)), FEV1 (MCID between 100–150 ml (Citation9, Citation16)), and FVC (MCID between 150–325 ml (Citation9, Citation17)). Exploratory a posteriori analyses were conducted to characterize subjects with clinically important variations on all three outcomes. More specifically, these subjects –which were considered as having high diurnal variability –were compared to the remaining subjects (low variability) on age, sex, BMI, exercise capacity, and pulmonary function using t-tests for independent samples. In the subgroup with high diurnal variability, a repeated-measures ANOVA was conducted to test the presence of a time-of-day effect.
Huynh-Feldt correction was applied for analyses on more than two levels, but original degrees of freedom are reported. The effect size was calculated for each statistical test according to established methods (Citation18) and categorized as small (≤ 0.1), medium ( = 0.25), or large (≥ 0.4). Statistical tests were two-tailed and conducted at the 5% level of significance. They were performed with SPSS version 18.0 (Chicago, IL).
Results
Subjects
Fourteen subjects (nine men) accepted to participate and completed all measurements. Baseline characteristics of the study group are presented in and are representative of moderate to severe disease in subjects with COPD.
Table 2. Demographics and baseline characteristics of the 14 subjects (9 men, 5 women)*
Overall diurnal variations
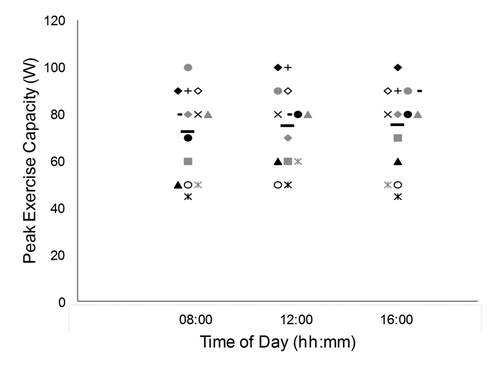
Individual results and group means for peak exercise capacity are shown in . There was no statistically significant effect of time of day for peak exercise capacity measured at 08:00, 12:00 and 16:00 hours (mean ± SD wattage of 72.5 ± 18.3, 75.0 ± 17 and 75.4 ± 17.6, respectively) (F2,26 = 1.61, p = 0.22), but the effect size was large (f = 0.48). There was no effect of testing order, based on the comparison between the first and last exercise tests (t13 = −0.19, p = 0.85, d = 0.02).
Figure 1. Peak exercise capacity at the three testing times. Each subject is identified by a specific symbol. Group means are represented by black horizontal bars.
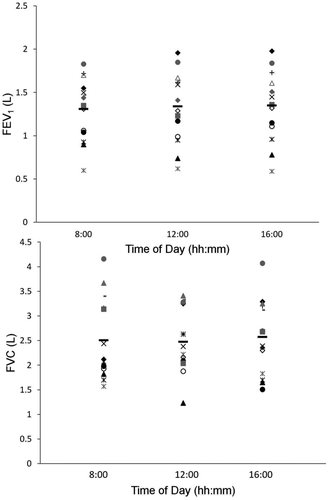
Individual data and group means for FEV1 and FVC values obtained at rest prior to each exercise test are shown in . No significant effect of time of day was detected for FEV1 (F2,26 = 0.59, p = 0.56, ƒ = 0.27), FVC (F2,26 = 0.24, p = 0.79, ƒ = 0.19), or the ratio of FEV1/FVC (F2,26 = 0.08, p = 0.92, ƒ = 0.11).
Figure 2. Individual data for forced expiratory volume in one second (FEV1) (upper panel) and forced vital capacity (FVC) (lower panel) at the three testing times. Each subject is identified by the same symbol. Group means are represented by black horizontal bars.
Group means for resting and peak e, respiratory rate (RR), tidal volume (VT), IC, O2, CO2, respiratory exchange ratio (RER), SpO2, HR, and BP are shown in . A significant effect of time of day was observed for RER under resting conditions (F2,26 = 8. 64, p = 0.005, ƒ = 1.16); resting RER was higher in the morning than at the other two time points. A trend for a time-of-day effect was detected for resting VT (F2,26 = 2.75, p = 0.08, ƒ = 0.62), which tended to decrease over the course of the day. Finally, a trend was also found for the effect of testing time on peak HR (F2,26 = 3.26, p = 0.08, ƒ = 0.68), which tended to increase throughout the day.
Table 3. Physiological response (mean ± SD) at rest and at peak exercise capacity at the three testing times
Subjects’ ratings of dyspnea and leg fatigue increased from rest to peak (p < 0.001 for both), but no time effect was found for either measure ().
Table 4. Symptoms (mean ± SD) of perceived dyspnea and leg fatigue as measured by the modified 10-point Borg scale at the three testing times
Within-subject variability
For peak exercise capacity, the average within-subject coefficient of variation was 5.5 ± 3.9% (range, 0 to 11%), the average amplitude of change was 7 ± 5 W (range, 0 to 10 W), and the average percent change was 10 ± 7% (range, 0 to 20%). Nine out of the 14 subjects showed a change beyond the suggested MCID of 10 watts for peak exercise capacity between the different testing times.
The average within-subject coefficient of variation in FEV1 was 4.8 ± 3.5% (range, 0 to 13%), the average amplitude of change was 0.12 ± 0.10 L (range, 0.02 to 0.43 L), while the average percent change was 9 ± 6% (range, 1 to 22%). The average within-subject coefficient of variation for FVC was 14 ± 7.8% (range, 4 to 23%), the average amplitude of change was 0.6 ± 0.3 L (range, 0.1 to 1.2 L), with a corresponding percent change of 22 ± 11% (range, 2 to 36%). Nine of the 14 patients (64%) showed a variability ≥ 100 ml in FEV1 between visits, while three subjects showed changes ≥ 150 ml. Twelve of the 14 subjects (86%) showed a variability ≥ 150 ml in FVC between visits, and 10 subjects (71%) showed changes ≥ 325 ml.
Exploratory analyses on subgroups having high or low diurnal variability
In seven of the 14 subjects (50%), diurnal variability exceeded the lowest suggested MCID value in all three measures; peak exercise capacity, FEV1 and FVC. These subjects were identified as having high diurnal variability and exploratory analyses were conducted to characterize this sub-group compared to the seven with lower diurnal variability, using t-tests for independent samples. There was no significant difference for age, sex, exercise capacity, or pulmonary function (FEV1, FVC, FEV1/FVC, IC). The only significant difference was for BMI (t12 = −2.30, p = 0.04, d = 1.23), which was greater in subjects with high diurnal variability. In the sub-group showing high diurnal variability, repeated-measures ANOVA were conducted to test the presence of a time-of-day effect. For these subjects, peak exercise capacity increased over the day (F2,12 = 3.80, p = 0.05, ƒ = 1.04) from 08:00 (74.3 ± 15.1 W) to 12:00 (78.6 ± 16.8 W) to 16:00 hours (81.4 ± 13.5 W), with a significant difference between 08:00 and 16:00 hours (p = 0.03). No significant time-of-day effect was detected for FEV1, FVC and FEV1/FVC in this sub-group.
Discussion
This pilot study was designed to investigate the effect of time of day on incremental exercise testing measures in COPD patients. Standardization of exercise testing times is recommended in the ATS guidelines (Citation6), yet this study is the first, to our knowledge, to investigate diurnal variability in exercise performance in a COPD patient population. Though not statistically significant, the size of the effect of time of day on peak exercise capacity was large (Citation18). Resting RER was greatest in the morning than at the other two time points. No significant time-of-day effect was detected for pulmonary function or symptom perception.
Within-subjects variability
The within-subjects variability across the three times of day was relatively high, as shown by a mean coefficient of variation of 5.5%. According to the ATS/ACCP guidelines for cardiopulmonary exercise testing (Citation6), this value falls within the range reported in reproducibility studies of peak exercise capacity in COPD patients (3.7 to 13.8%). However, the studies cited in the guidelines were conducted more than 20 years ago (Citation19–23) when both methodologies and equipment were likely less reliable than those available today. A recent well-designed study using modern equipment found a coefficient of variation of 2.3% for repeated measures maximal incremental cycle tests conducted at the same time of day within a period of 8 to 10 days in 10 COPD patients (Citation24). Therefore, the variation of 5.5% measured in the present study suggests that variability of the results increases when the tests are conducted at different times of day.
In addition to the coefficient of variability, the average amplitude of change can be used to compare time-of-day effects against those of widespread treatments. For example, pulmonary rehabilitation has been shown to elicit an average improvement (common effect) of 5.5 W (95% CI: +0.5 to 10.2 W) in peak exercise capacity in moderate to very severe COPD (Citation25), while bronchodilation has been associated with changes ranging up to 7 W (Citation26). In the present study, peak exercise capacity varied, on average, by 7 ± 5 W, with a range of 0 to 10 W across the 14 subjects. Therefore, our results show that, in some patients with COPD, the amplitude of diurnal variations in peak exercise capacity associated with different testing times can exceed the amplitude of changes expected with bronchodilation and even pulmonary rehabilitation. Consequently, even if there was no systematic time-of-day effect in our group of subjects, the guidelines recommendation to repeat exercise testing at the same time of day should be maintained, as diurnal variations may have clinically significant effects in certain patients with COPD.
In the present study, subjects were instructed to refrain from ingesting food within 3 hours of testing, in accordance to guidelines from the American College of Sports Medicine (Citation14). However, based on patient-reported information on time since last dietary intake, the interval between the last intake and the exercise test was shorter for the morning test as compared to the other time points. This may explain why resting RER was greatest in the morning. Indeed, prior food intake has been shown to increase RER values, but not peak exercise capacity, in a healthy population (Citation27). Together, these findings highlight the importance of standardizing instructions given to participants prior to an exercise test.
Between-subject variability in light of MCID values
An important observation made in this pilot study was the heterogeneity in the amplitude of variations in subjects’ response to tests conducted at different times of day. To explore potential differences between subjects showing high or low diurnal variability, they were classified according to previously suggested MCID values for peak exercise capacity (Citation15) and resting pulmonary function (FEV1 and FVC) (Citation9, Citation16, Citation17). The MCID is the smallest clinical difference in a measure that can either be perceived by the patient or that is believed to be clinically pertinent by expert opinion (Citation28). Interestingly, most subjects showing diurnal variations equal or larger than the MCID for peak exercise capacity also showed clinically significant changes for resting pulmonary function. These subjects showing high diurnal variations on all three measures constituted exactly half of our sample (seven of 14 subjects).
When the sub-groups showing high or low diurnal variability were compared, no difference emerged for disease severity or for average exercise capacity. Age, gender and smoking status were also evenly distributed in the two sub-groups. The only significant difference was for BMI, which was higher in the sub-group showing more diurnal variability. The clinical significance of this observation will have to be determined. A higher BMI –which may reflect better preserved muscle mass –has been shown to have a protective effect on lung function decline in individuals at risk of developing COPD and to predict better survival in those suffering from COPD (Citation29–31).
The diurnal maximum observed at 16:00 hours for peak exercise capacity in patients with high diurnal variability is similar to the peak observed in circadian rhythms in healthy subjects for measures of exercise performance such as peak oxygen consumption (Citation32) and exercise heart rate (Citation32–34). This observation suggests that this diurnal maximum is not a random variation but may rather reflect a circadian rhythm of larger amplitude in patients with high variability. A larger amplitude may result from a better internal synchrony between underlying physiological functions (Citation35) and, as in other pathologies (Citation36–38), high variability may therefore predict a better clinical outcome in COPD patients. This hypothesis cannot be confirmed with only three time points and in the absence of independent circadian measures. Future studies will be needed to determine whether the combination of high diurnal variability and high BMI could be used to define a specific phenotype of patients and predict clinically meaningful outcomes such as symptom exacerbations, progression of disease and response to therapy (Citation29). These studies should include a precise measurement of body composition (e.g. duel energy X-ray absorptiometry) to assess the relative contribution of muscle and fat mass in this potential phenotype.
Strengths, limitations, and perspectives
To our knowledge, this study is the first to address diurnal variations in peak exercise capacity in patients with COPD. It was conducted with the highest methodological standards, including carefully calibrated equipment and standardized procedures. To limit a potential training effect, all selected subjects had previous experience with testing procedures, and the testing order for the three times of day was counterbalanced across the subjects. In addition, great care was taken to avoid direct effects of the timing of COPD medication in relation to exercise testing, as all COPD medications were withdrawn at least 6 hours before each testing session.
This study was a pilot study and its main limitation is the modest sample size. The large effect size found for time of day on peak exercise capacity suggests that significant results may emerge in a larger group of subjects. A larger group would also allow for the characterization of potential sub-groups based on time of day variability. The diurnal maximum observed at 16:00 hours for peak exercise capacity suggests a larger circadian amplitude in patients with high variability. This hypothesis cannot be confirmed with only three time points and in the absence of a valid circadian marker. Further studies will be needed to confirm if greater variability can indeed predict better outcome in COPD. Finally, MCID estimates were used in exploratory analyses to identify subjects in whom clinically significant changes were observed across the three times of day. The limitation of using MCID estimates as cut-off thresholds to dichotomize individuals as “responders” or “non-responders” after an intervention has been demonstrated (Citation39). However, in the absence of a reproducibility criterion for peak exercise capacity, the MCID was deemed the alternative of choice. Caution is nonetheless warranted in the interpretation and generalization of these findings.
Conclusion
No systematic time-of-day effect on peak exercise capacity was obtained in COPD patients in the present pilot study. However, our findings on the average amplitude of change and within-subject variations seen across testing times support the guidelines recommendation to standardize time of day for repeat exercise testing in patients with COPD. The presence of high or low diurnal variability in peak exercise capacity needs to be further investigated to examine its potential clinical significance.
Declaration of Interest
The authors report no financial, consulting or personal relationships that have influenced their work.
The authors alone are responsible for the content and writing of the paper.
Acknowledgments
The authors want to acknowledge the contribution of the respirology unit at the Hôpital du Sacré-Coeur de Montréal for the medical supervision. We would also like to acknowledge the contribution of the participants without whom clinical studies would not be possible.
References
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2013 Update. Available from http://www.goldcopd.com
- Lewinsohn HC, Capel LH, Smart J. Changes in forced expiratory volumes throughout the day. Br Med J 1960 Feb 13; 1(5171):462–464.
- Hruby J, Butler J. Variability of routine pulmonary function tests. Thorax 1975 Oct; 30(5):548–553.
- Kessler R, Partridge MR, Miravitlles M, Cazzola M, Vogelmeier C, Leynaud D, Ostinelli J. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J 2011 Feb; 37(2):264–272.
- Medarov BI, Pavlov VA, Rossoff L. Diurnal variations in human pulmonary function. Int J Clin Exp Med 2008; 1(3):267–273.
- ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003 Jan 15; 167(2):211–277.
- Pepin V, Maltais F, Lacasse Y. Goals of Treatments and Measurement of Their Efficacy: From Clinical Trials to Real World Practice In: Rennard SI, Huchon G, Rodriguez-Roisin R, Roche N, editors. Clinical Management of Chronic Obstructive Pulmonary Disease. 2nd ed. New York, NY: Taylor & Francis Group; 2007: 239–261.
- Garrard CS, Emmons C. The reproducibility of the respiratory responses to maximum exercise. Respiration 1986; 49(2):94–100.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J 2005 Aug; 26(2):319–338.
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993 Mar; 16:5–40.
- Jones NL, Makrides L, Hitchcock C, Chypchar T, McCartney N. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis 1985 May; 131(5):700–708.
- O'Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998 Nov; 158(5 Pt 1):1557–1565.
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14(5):377–381.
- Thompson WR, Gordon NF, Pescatello LS, editors. ACSM's Guidelines for Exercise Testing and Prescription, 8th ed. New York: Lippincott Williams & Wilkins; 2010.
- Sutherland ER, Make BJ. Maximum exercise as an outcome in COPD: minimal clinically important difference. COPD 2005 Mar; 2(1):137–141.
- Donohue JF. Minimal clinically important differences in COPD lung function. COPD 2005 Mar; 2(1):111–124.
- Herpel LB, Kanner RE, Lee SM, Fessler HE, Sciurba FC, Connett JE, Wise RA. Variability of spirometry in chronic obstructive pulmonary disease: results from two clinical trials. Am J Respir Crit Care Med 2006 May 15;173(10):1106–1113.
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed.: Routledge New York; 1988.
- Noseda A, Carpiaux JP, Prigogine T, Schmerber J. Lung function, maximum and submaximum exercise testing in COPD patients: reproducibility over a long interval. Lung 1989; 167(4):247–257.
- Brown SE, Fischer CE, Stansbury DW, Light RW. Reproducibility of VO2max in patients with chronic air-flow obstruction. Am Rev Respir Dis 1985 Mar; 131(3):435–438.
- Swinburn CR, Wakefield JM, Jones PW. Performance, ventilation, and oxygen consumption in three different types of exercise test in patients with chronic obstructive lung disease. Thorax 1985 Aug; 40(8):581–586.
- Cox NJ, Hendriks JC, Binkhorst RA, Folgering HT, van Herwaarden CL. Reproducibility of incremental maximal cycle ergometer tests in patients with mild to moderate obstructive lung diseases. Lung 1989; 167(2):129–133.
- Owens MW, Kinasewitz GT, Strain DS. Evaluating the effects of chronic therapy in patients with irreversible air-flow obstruction. Am Rev Respir Dis 1986 Nov; 134(5):935–937.
- Poulain M, Durand F, Palomba B, Ceugniet F, Desplan J, Varray A, Prefaut C. 6-minute walk testing is more sensitive than maximal incremental cycle testing for detecting oxygen desaturation in patients with COPD. Chest 2003 May; 123(5):1401–1407.
- Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006(4):CD003793.
- Liesker JJ, Wijkstra PJ, Ten Hacken NH, Koeter GH, Postma DS, Kerstjens HA. A systematic review of the effects of bronchodilators on exercise capacity in patients with COPD. Chest 2002 Feb; 121(2):597–608.
- Bougard C, Bessot N, Moussay S, Sesboue B, Gauthier A. Effects of waking time and breakfast intake prior to evaluation of physical performance in the early morning. Chronobiol Int 2009 Feb; 26(2):307–323.
- Make B, Casaburi R, Leidy NK. Interpreting results from clinical trials: understanding minimal clinically important differences in COPD outcomes. COPD 2005 Mar; 2(1):1–5.
- Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, Fabbri LM, Goldin JG, Jones PW, Macnee W, Make BJ, Rabe KF, Rennard SI, Sciurba FC, Silverman EK, Vestbo J, Washko GR, Wouters EF, Martinez FJ. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 2010 Sep 1; 182(5):598–604.
- Harik-Khan RI, Fleg JL, Wise RA. Body mass index and the risk of COPD. Chest 2002 Feb; 121(2):370–376.
- Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr 2005 Jul; 82(1):53–59.
- Hill DW. Effect of time of day on aerobic power in exhaustive high-intensity exercise. J Sports Med Phys Fitness 1996 Sep; 36(3):155–160.
- Reilly T, Brooks GA. Investigation of circadian rhythms in metabolic responses to exercise. Ergonomics 1982 Nov; 25(11):1093–1107.
- Reilly T, Robinson G, Minors DS. Some circulatory responses to exercise at different times of day. Med Sci Sports Exerc 1984 Oct; 16(5):477–482.
- Ruger M, Scheer FA. Effects of circadian disruption on the cardiometabolic system. Rev Endocr Metab Disord 2009 Dec; 10(4):245–260.
- Pati AK, Parganiha A, Kar A, Soni R, Roy S, Choudhary V. Alterations of the characteristics of the circadian rest-activity rhythm of cancer in-patients. Chronobiol Int 2007; 24(6):1179-97.
- Tranah GJ, Blackwell T, Stone KL, Ancoli-Israel S, Paudel ML, Ensrud KE, Cauley JA, Redline S, Hillier TA, Cummings SR, Yaffe K. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol 2011 Nov; 70(5):722–732.
- Perez-Lloret S, Risk M, Golombek DA, Cardinali DP, Sanchez R, Ramirez A. Blunting of circadian rhythms and increased acrophase variability in sleep-time hypertensive subjects. Chronobiol Int 2008 Feb; 25(1):99–113.
- Dolmage TE, Hill K, Evans RA, Goldstein RS. Has my patient responded? Interpreting clinical measurements such as the 6-minute-walk test. Am J Respir Crit Care Med 2011 Sep 15; 184(6):642–646.
