Abstract
The aim of this study was to examine the value of the i-BODE index to predict hospital admission and to confirm its usefulness to predict mortality in a Danish population. The incremental shuttle walking test (ISWT) is widely used in the UK and Europe and previous work has examined the replacement of the 6MWT with the ISWT within the BODE index for predicting the prognosis of COPD (i-BODE). The 674 patients included in the analysis participated in a 7-week pulmonary rehabilitation program from 2002 to 2011. The National Health Services Central Register ascertained vital status and provided information on all hospital admissions. The mean follow-up period was 66 months (range 11–118 months). Cox proportional hazards model was used to identify factors that significantly predicted mortality and time to first hospital admission.
The i-BODE index as well as body mass index, MRC dyspnea grade, and exercise capacity (ISWT) were significantly associated with all-cause mortality. The adjusted hazard ratio for death per one point increase in the i-BODE score was 1.28 (95% confidence interval 1.20 to 1.37). The i-BODE index was also a significant predictor of hospitalization, both for all causes and COPD exacerbation. Patients in the highest i-BODE quartile had a median time to first hospitalization of 17 months compared to 51 months for patients in the lowest quartile. The i-BODE index is a significant predictor of hospital admission and thus health care utilization, and also mortality.
Introduction
Chronic obstructive pulmonary disease (COPD) is a multicomponent condition involving both local and systemic pathological processes that result in airflow limitation, loss of lean body mass, impaired muscle function, and an increased risk of cardiovascular disease (Citation1). The BODE index, a multidimensional grading scale, composed of body mass index (BMI), airflow obstruction, functional dyspnea as measured by the Modified Medical Research Council (MMRC) dyspnea scale, and exercise capacity, has been developed in an attempt to better assess severity, disability and prognosis in this complex condition (Citation2). The BODE index has been found to be better than forced expiratory volume in 1 second (FEV1) in predicting the risk of death and hospitalization among patients with COPD (Citation2, 3). In the BODE index, exercise capacity was measured by the 6-minute walking test (6MWT).
Traditionally the 6MWT has been used in the United States whereas in the UK and large parts of Europe the ISWT is more frequently used to measure exercise capacity. Recently, Williams et al. suggested that the incremental shuttle walking test (ISWT) could be substituted for the 6MWT as an alternative measure of exercise capacity within the index and introduced the i-BODE (Citation4). As field exercise tests, the ISWT and 6MWT are closely related (Citation5, 6), though ISWT is considered to be closer to a maximal exercise test (Citation7), whereas the 6MWT reflects a more functional exercise performance. ISWT correlates well with VO2max in cardiopulmonary testing (CPET) (Citation8–10) and has been widely used as a measure of exercise capacity in many clinical studies involving patients with COPD (Citation11–16) and specifically in studies on pulmonary rehabilitation (Citation17–21).
Although the i-BODE index was shown to be a predictor of the risk of death in the cohort of patients studied by Williams et al. (Citation4), it has not been validated in other cohorts. Furthermore, it is not known whether this index is a useful indicator of utilization of health-care resources caused by hospitalization. We hypothesized that the i-BODE index would better predict mortality and hospitalization for COPD than FEV1 alone and to test this hypothesis examined the predictive capacity of the i-BODE in a separate cohort of Danish patients referred for outpatient pulmonary rehabilitation.
Methods
Selection of patients
All patients included in the present analyses participated in a 7-week pulmonary rehabilitation program at Hvidovre Hospital, Copenhagen, during the period from March 2002 to March 2011. Eligibility criteria for enrolment in the rehabilitation program included stable COPD (FEV1 < 80% and FEV1/forced vital capacity < 0.70), motivation and no previous participation in pulmonary rehabilitation. Exclusion criteria were significant musculo-skeletal, cardiac, or cognitive problems.
ISWT and i-BODE
Our program has previously been described in detail (Citation22, 23). ISWT was measured at baseline and was conducted using the protocol described by Singh et al. (Citation8). Variables and point allocation used for the computation of the i-BODE index are shown in . The cut-off values for ISWT quartiles were the same as those used in the study of Williams et al. (Citation4). After the 7-week program patients were asked to continue the self-monitored daily exercise at home as the only form of maintenance training.
Table 1. Variables and point allocation used for the computation of the i-BODE index
Hospitalization and mortality
The National Health Services Central Register ascertained vital status and provided information on all hospital admissions in the follow-up period until the 24th of January 2012. A primary diagnosis of COPD (J44.x), or a primary diagnosis of respiratory failure (J96.x) with a secondary diagnosis of COPD (J44.x) at discharge was recorded as an admission due to exacerbation in COPD. The diagnoses were according to the 10th edition of the International Classification of Diseases (ICD).
Statistics
Cox proportional hazards model was used to identify factors that significantly predicted mortality and time to first hospitalization. The variables included in the model were: age, sex, pack-years smoked, smoking status (yes/no), BMI (kg/m2), oxygen saturation at rest, MRC dyspnea score (Citation1–5), maintenance treatment oral corticosteroids (yes/no), long-term oxygen therapy (LTOT) (yes/no), FEV1% of predicted value, and ISWT distance walked. This was done to enable us to account for any additional factors not already included in the i-BODE index, which were found to also significantly predict mortality in this cohort of patients. Covariates (confounders) with a p-value < 0.10 in the univariate analysis were subsequently included in a multivariate Cox proportional hazard regression model. The Wald test was used for assessing the fit of the independent variables in the Cox model. Assumption of linearity was assessed by categorizing the variable into multiple dichotomous variables of equal units (quartiles) on the variable's scale. The estimated coefficients of each dichotomous variable were compared.
In the case of non-linearity, dichotomous variables with equal hazard ratios were combined. The results of the regression analyses are given in terms of estimated hazard ratios (HR), with corresponding 95% confidence intervals (CI). Kaplan-Meier survival curves were created for descriptive purposes using the statistical package for the social sciences (SPSS) version 13.0 (SPSS Inc., Chicago, USA). Continuous variables are presented as mean (SD) unless otherwise indicated. A p-value < 0.05 was considered statistically significant.
Results
Our study group consisted of 674 consecutive patients out of 695; 18 patients were excluded due to missing values. The majority of our patients had severe airflow obstruction (84.9% of the patients had FEV1 ≤ 50% of the predicted value). According to the GOLD classification (Citation24) 233 (34.6%) of the patients were classified as having very severe COPD (stage IV), 330 (49.0%) had severe COPD (stage III), 110 (16.3%) had moderate COPD (stage II), and) 1 (0.1%) had mild COPD (stage I).
Using the new GOLD 2011 classification (Citation25), all the patients were in group B and D as breathlessness was an inclusion criteria for participating in the pulmonary rehabilitation program. The mean value for the i-BODE index was 5.72 (SD = 1.86). Baseline characteristics for the entire cohort, those hospitalized during follow-up and those dying during follow-up, are shown in . The mean follow-up period was 66 months (range 11–118 months). During the follow-up, 325 deaths (48.2%) were observed and a total of 557 patients (82.6%) had at least one acute hospital admission for any cause. In total, 421 patients (62.5%) had at least one hospital admission due to a COPD exacerbation using these criteria.
Table 2. Baseline characteristics of patients attending the rehabilitation program according to subsequent survival and hospitalization
Survival
Multivariate Cox proportional hazards analysis revealed that, three of the four original BODE factors were individually associated with all-cause mortality: BMI (p < 0.001), MRC dyspnea grade (p < 0.001) and exercise capacity (ISWT) (p < 0.001), whereas the HR for FEV1 did not reach statistical significance (p = 0.16). The i-BODE index was found to be a significant predictor of death and more strongly associated with survival than the individual components, even after adjustment for the additional prognostic factors including age, gender, pack-years smoked, current smoking status, maintenance treatment with oral prednisolone, oxygen saturation, oxygen desaturation at exertion, and use of LTOT ().
Table 3. Predictors of all-cause mortality
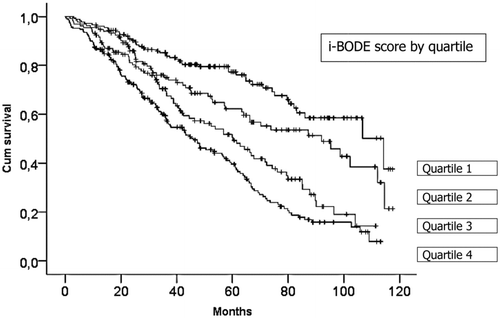
The adjusted hazard ratio for death per one point increase in the i-BODE score was 1.28 (95% CI 1.20 to 1.37, p < 0.001). Adjusted survival analysis also showed that each quartile increase in the score was significantly associated with increased mortality (p < 0.001) (). The cut-off values for the four i-BODE quartiles were: quartile one: 0–4 points, quartile two: 5 points, quartile three: 6 points, and quartile four: 7–10 points. Patients in the highest (i.e. worst) i-BODE quartile (7–10 points) had a median survival of 46 months compared to 114 months for patients in the lowest quartile (0–4 points).
Hospital admission
The i-BODE index was also found to be a significant predictor of hospitalization, both for all cause admission and for an exacerbation of COPD. As for mortality, i-BODE was more strongly associated with hospitalization than the individual components of the index (). Each quartile increase in the score was significantly associated with increased risk of being admitted with an exacerbation in COPD at least once (p < 0.001) (). Patients in the highest i-BODE quartile (7–10 points) had a median time to first hospitalization of 17 months compared to 51 months for patients in the lowest quartile (0–4 points).
Figure 2. (a) Time to first admission for all causes (Kaplan-Meier). Log Rank = 32.5, p < 0.001. Quartile 1 is a score of 0 to 4, quartile 2 is a score of 5, quartile 3 is score of 6 and quartile 4 is a score of 7 to 10. (b) Time to first admission for COPD exacerbation (Kaplan-Meier). Log-Rank = 42.2; p < 0.001. Quartile 1 is a score of 0 to 4, quartile 2 is a score of 5, quartile 3 is score of 6 and quartile 4 is a score of 7 to 10.
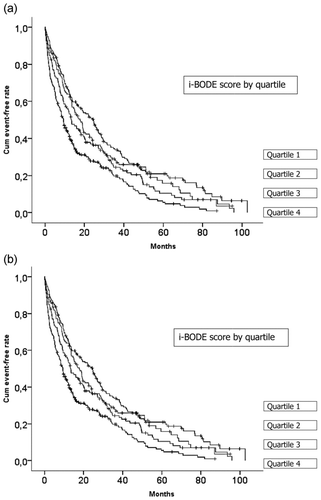
Table 4. Adjusted risk of hospitalization due to exacerbation in COPD and all causes
Discussion
We found an association between i-BODE and hospitalization, which we believe has not been reported before. We also found that i-BODE was a better predictor of mortality than the individual variables (BMI, FEV1, MRC, and ISWT). In the present cohort of patients with COPD participating in pulmonary rehabilitation, the adjusted hazard ratio for death per one point increase in the i-BODE score was 1.28. We also found that i-BODE was a better predictor of hospital admission than the individual variables (BMI, FEV1, MRC, and ISWT) and that the adjusted hazard ratio for all-cause hospitalization per one point increase in the i-BODE score was 1.15 and for hospitalization due to exacerbation in COPD 1.21. Two components of the i-BODE (MRC and ISWT) provided the majority of the predictive power of both mortality and hospitalization. These components express physical functioning, which is known to be a strong prognostic predictor in COPD (Citation26).
Our findings regarding mortality are in accordance with the Leicester study that found a hazard ratio of 1.27 (Citation4) and also with the original BODE paper, where Celli et al. reported a hazard ratio of 1.34 (Citation2). This is interesting because our study population differed from the patients included in the Leicester study (Citation4), as we have included more females and patients with a lower mean ISWT distance and FEV1% predicted. This meant that 73.6% (496) of the patients in our study had an i-BODE score of 5–10 (more severe disease) compared to 50% in the Leicester study. This difference is explained because patients with milder COPD are referred to pulmonary rehabilitation in the community in Denmark. Despite the variability in patient population composition between the two centres, we found similar results, and we interpret this as a demonstration of the BODE index's consistent ability to predict the vital prognosis.
The relationship between the original BODE and hospital admission has only been examined in a few previous studies. These studies have shown that, as expected, hospitalization prior to calculation of the BODE index increases the score i.e. a ‘worse’ score (Citation27, 28). Three studies have assessed the value of the BODE index as a predictor of hospitalization. In the first study, 275 patients with COPD were followed every 6 months up to 8 years (Citation29). Exacerbations were graded according to either: treatment in primary care, emergency room visit, or hospitalization. The BODE index was a good predictor of both the number and the severity of exacerbations in COPD, especially in those exacerbations that required hospital admission. In the second study of 127 COPD patients (91% males), all hospital admissions due to COPD were recorded. The definition was broad; e.g. admissions with the primary diagnosis of cardiac failure or tuberculosis and a secondary diagnosis of either COPD or respiratory failure were included. The incidence rate ratio increased by 1.20 (1.15 to 1.25) per one unit of the BODE index score. However, there was a lower risk at scores 5–6 than 0–2 and 3–4. The authors suggest that this could be caused by differences in patient characteristics, other confounders, or the fact that this group with BODE scores 5–6 had the highest HR for mortality of the four quartiles (Citation3). Thus, the results were not conclusive. In the third study, the aim was to find predictors of hospital admission in COPD with severe emphysema and FEV1 of 45% predicted or lower (Citation30). All patients completed 6–10 weeks pulmonary rehabilitation and were then randomized to either continued medical treatment or lung volume reduction surgery. This study followed the 610 patients in the medical arm and found that the BODE index was not associated with hospital admission. However, the follow up period was only 12 months and exacerbations in COPD were not assessed separately.
Our study included well-characterized subjects with only few missing data. All patients were followed and managed in one setting with similar access to health care. However, a limitation of our study was that it only included patients that had participated in pulmonary rehabilitation; consequently, most of these patients were diagnosed with severe COPD. Very few had an FEV1 > 50% predicted, MRC 1–2, or good physical tolerance. It should also be acknowledged that admission data over a protracted period of data collection can be challenging. In Denmark, however, no major structural changes have been implemented and therefore there have not been any considerable changes in processes and thresholds for admission.
In many settings, the ISWT is the standard method of measuring exercise capacity. Introducing i-BODE modifies the well-established BODE index so it can also be used in centres preferring to use the ISWT rather than the 6 MWT. We have demonstrated that the i-BODE is a predictor of admission not only due to COPD exacerbations but also for all causes. This finding suggests that patients with a high BODE score are vulnerable not only in relation to their pulmonary disease. This is important as patients with severe COPD often have several co-morbidities (Citation31–34). An approach to this issue has been proposed by Divo et al., who developed the COPD specific CO-morbidity Test (COTE) index—a risk stratification co-morbidity tool; the COTE index added complementary information to the BODE index and also seemed to increase the predictive value of the BODE index (Citation35).
It would be of interest to examine the impact of for example, cardiovascular disease, both in terms of events and mortality in relation to the predictive value of the BODE index. The strongest i-BODE components regarding prognosis are the MRC and the ISWT. Therefore the i-BODE is likely to work as well in COPD with cardiovascular disease since symptoms and exercise capacity are also associated with prognosis in cardiovascular disease.
Declaration of Interest Statement
Mia Moberg was funded by TrygFonden. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Conclusion
Our study of 674 Danish COPD patients undergoing pulmonary rehabilitation has shown a predictive value of the modified i-BODE index regarding hospital admission and has confirmed that the index is a valid predictor of mortality in patients with severe COPD.
References
- Agusti AG. COPD, a multicomponent disease: implications for management. Respir Med 2005 Jun; 99(6):670–682.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004 Mar 4; 350(10):1005–1012.
- Ong KC, Earnest A, Lu SJ. A multidimensional grading system (BODE index) as predictor of hospitalization for COPD. Chest 2005 Dec;128(6):3810-6.
- Williams JE, Green RH, Warrington V, Steiner MC, Morgan MD, Singh SJ. Development of the i-BODE: Validation of the incremental shuttle walking test within the BODE index. Respir Med 2012 Mar; 106(3):390–396.
- Turner SE, Eastwood PR, Cecins NM, Hillman DR, Jenkins SC. Physiologic responses to incremental and self-paced exercise in COPD: a comparison of three tests. Chest 2004 Sep; 126(3):766–773.
- Luxton N, Alison JA, Wu J, Mackey MG. Relationship between field walking tests and incremental cycle ergometry in COPD. Respirology 2008 Nov; 13(6):856–862.
- Onorati P, Antonucci R, Valli G, Berton E, De Marco F, Serra P, Non-invasive evaluation of gas exchange during a shuttle walking test vs. a 6-min walking test to assess exercise tolerance in COPD patients. Eur J Appl Physiol 2003 May;89(3–4): 331–336.
- Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 1992 Dec; 47(12):1019–1024.
- Singh SJ, Morgan MD, Hardman AE, Rowe C, Bardsley PA. Comparison of oxygen uptake during a conventional treadmill test and the shuttle walking test in chronic airflow limitation. Eur Respir J 1994 Nov; 7(11):2016–2020.
- Arnardottir RH, Emtner M, Hedenstrom H, Larsson K, Boman G. Peak exercise capacity estimated from incremental shuttle walking test in patients with COPD: a methodological study. Respir Res 2006; 7:127.
- Gagnon P, Saey D, Provencher S, Milot J, Bourbeau J, Tan WC, Walking exercise response to bronchodilation in mild COPD: a randomized trial. Respir Med 2012 Dec; 106(12):1695–1705.
- Bedard ME, Brouillard C, Pepin V, Provencher S, Milot J, Lacasse Y, Tiotropium improves walking endurance in COPD. Eur Respir J 2012 Feb; 39(2):265–271.
- Rea H, McAuley S, Stewart A, Lamont C, Roseman P, Didsbury P. A chronic disease management programme can reduce days in hospital for patients with chronic obstructive pulmonary disease. Intern Med J 2004 Nov; 34(11):608–614.
- Menadue C, Alison JA, Piper AJ, Flunt D, Ellis ER. Non-invasive ventilation during arm exercise and ground walking in patients with chronic hypercapnic respiratory failure. Respirology 2009 Mar; 14(2):251–259.
- Omachi TA, Eisner MD, Rames A, Markovtsova L, Blanc PD. Matrix metalloproteinase-9 predicts pulmonary status declines in alpha1-antitrypsin deficiency. Respir Res 2011; 12:35.
- Sukisaki T, Senjyu H, Oishi K, Rikitomi N, Ariyoshi K. Single dose of inhaled procaterol has a prolonged effect on exercise performance of patients with COPD. Physiother Theory Pract 2008 Jul-Aug; 24(4):255–263.
- Egan C, Deering BM, Blake C, Fullen BM, McCormack NM, Spruit MA, Short term and long term effects of pulmonary rehabilitation on physical activity in COPD. Respir Med 2012 Dec; 106(12):1671–1679.
- Albores J, Marolda C, Haggerty M, Gerstenhaber B, Zuwallack R. The use of a home exercise program based on a computer system in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev 2013 Jan; 33(1):47–52.
- Garrod R, Ford K, Daly C, Hoareau C, Howard M, Simmonds C. Pulmonary rehabilitation: analysis of a clinical service. Physiother Res Int 2004; 9(3):111–120.
- Spencer LM, Alison JA, McKeough ZJ. Do supervised weekly exercise programs maintain functional exercise capacity and quality of life, twelve months after pulmonary rehabilitation in COPD? BMC Pulm Med 2007; 7:7.
- Liu WT, Wang CH, Lin HC, Lin SM, Lee KY, Lo YL, Efficacy of a cell phone-based exercise programme for COPD. Eur Respir J 2008 Sep; 32(3):651–659.
- Singh SJ, Smith DL, Hyland ME, Morgan MD. A short outpatient pulmonary rehabilitation programme: immediate and longer-term effects on exercise performance and quality of life. Respir Med 1998 Sep; 92(9):1146–1154.
- Ringbaek T, Brondum E, Martinez G, Lange P. Rehabilitation in COPD: the long-term effect of a supervised 7-week program succeeded by a self-monitored walking program. Chron Respir Dis 2008; 5(2):75–80.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007 Sep 15; 176(6):532–555.
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: GOLD Executive Summary. Am J Respir Crit Care Med 2013 Feb 15; 187(4):347–365.
- Celli BR. Predictors of mortality in COPD. Respir Med 2010 Jun;104(6):773–779.
- Alcazar B, Garcia-Polo C, Herrejon A, Ruiz LA, de Miguel J, Ros JA, Factors associated with hospital admission for exacerbation of chronic obstructive pulmonary disease. Arch Bronconeumol 2012 Mar; 48(3):70–76.
- McKellar A, Cottrell WN, Whelan A. BODE score is a useful predictor of hospital admission in rural patients with chronic obstructive pulmonary disease. Respirology 2008 May; 13(3):438–443.
- Marin JM, Carrizo SJ, Casanova C, Martinez-Camblor P, Soriano JB, Agusti AGN, Prediction of risk of COPD exacerbations by the BODE index. Respir Med 2009; 103(3):373–378.
- Benzo RP, Chang CC, Farrell MH, Kaplan R, Ries A, Martinez FJ, Physical activity, health status and risk of hospitalization in patients with severe chronic obstructive pulmonary disease. Respiration 2010; 80(1):10–18.
- Corsonello A, Antonelli Incalzi R, Pistelli R, Pedone C, Bustacchini S, Lattanzio F. Comorbidities of chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2011 Dec;17 Suppl 1:S21–28.
- Antonelli Incalzi R, Fuso L, De Rosa M, Forastiere F, Rapiti E, Nardecchia B, Co-morbidity contributes to predict mortality of patients with chronic obstructive pulmonary disease. Eur Respir J 1997 Dec; 10(12):2794–2800.
- Luppi F, Franco F, Beghe B, Fabbri LM. Treatment of chronic obstructive pulmonary disease and its comorbidities. Proc Am Thorac Soc 2008 Dec 1; 5(8):848–856.
- Crisafulli E, Gorgone P, Vagaggini B, Pagani M, Rossi G, Costa F, Efficacy of standard rehabilitation in COPD outpatients with comorbidities. Eur Respir J 2010 Nov; 36(5):1042–1048.
- Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto-Plata V, Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012 Jul 15; 186(2):155–161.