Abstract
Background: COPD is a multi-component disease that is not sufficiently reflected by FEV1 alone. We studied in patients with very severe COPD, which dimensions of the disease, including co-morbidities, dominate prognosis. Methods: In patients with FEV1 < 30% predicted, anthropometric, laboratory, spirometric and body plethysmographic data, smoking status, alcohol consumption, the level of dyspnoea and exercise performance were assessed. Co-morbidities were categorized by the Charlson-index and the COPD-specific co-morbidity test (COTE). The prognostic value of multiple dimensions was explored using uni- and multivariate survival analyses regarding death from any or respiratory cause. Results: Among 209 patients included (58/151 female/male; FEV1 25.0 (22.0–26.9)%predicted), arterial hypertension (54.1%), hyperlipidemia (38.3%) and diabetes (19.6%) were most common, 57.9% showing a COTE-index of ≥1 point. During follow-up (28 (14–45) months), 121 patients had died, mostly (56.2%) due to respiratory causes. Age, BMI, the ratio of residual volume to total lung capacity (RV/TLC), co-morbidities in terms of the COTE- and Charlson-index, but not FEV1, were significantly associated with all-cause and respiratory mortality. The association of the median values of the Charlson- (HR 1.911 [95%-CI 1.338–2.730]) and COTE-index (HR 1.852 [95%-CI 1.297–2.644], p < 0.001 each) with mortality was similar and stronger when combined with age. In multivariate analyses, only RV/TLC and co-morbidities were independent risk factors of all-cause mortality (p < 0.05 each). Conclusion: In very severe COPD, resting hyperinflation and co-morbidities provide the major prognostic information, whereas the association of the recently introduced COTE-index with mortality was similar to that of the established Charlson-index and even stronger when including age.
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of death in many parts of the world (Citation1). Historically, air-flow obstruction in terms of the forced expiratory volume in 1 second (FEV1) has been considered as most important measure to define the severity of COPD (Citation2). More recently, however, it has been recognized that FEV1 does not properly account for the complexity of the disease (Citation3). Accordingly, a broader approach has been proposed, and the most recent guidelines of the Global Initiative of Chronic Obstructive Lung Disease (GOLD) recommend the assessment of symptoms and the frequency of exacerbations, in addition to FEV1, for monitoring and therapeutic decisions (Citation4).
Similarly, it has been demonstrated that the addition of Body-Mass-Index (BMI), dyspnoea and 6-minute-walk distance (6-MWD) was superior to FEV1 alone in predicting the mortality risk (Citation5). More recently, the ADO-index (Citation6) (including age, dyspnoea, FEV1) and the DOSE-index (Citation7) (including dyspnoea, FEV1, smoking status, exacerbation frequency) have been validated for the assessment of COPD prognosis and severity. Although these indices use FEV1 to describe lung function impairment, resting hyperinflation has also been reported to be a predictor of mortality, and its power exceeded that of FEV1 (Citation8,9).
Moreover, co-morbidities of COPD are linked to health-status and survival (Citation10) and, consequently, have been included in the most recent definition of COPD (Citation4). However, integrating the risk from multiple co-morbidities is not yet established. When the Charlson-index (Citation11) was used, its predictive value regarding short- (Citation12) and long-term outcome (Citation13–16) was quite different. Recently, the COPD-specific co-morbidity test (COTE) has been proposed (Citation17), but its prognostic value has not yet been evaluated in diverse, separate populations.
The majority of investigations on predictors of long-term survival in COPD included patients with a wide range of airflow limitation (Citation18), or focused on patients receiving a specific treatment such as long-term oxygen therapy (LTOT) (Citation13) or non-invasive positive pressure ventilation (NPPV) (Citation9). To our knowledge, patients with very severe COPD, based on the criterion FEV1 < 30% predicted, have not yet been separately targeted, although co-morbidities might play a particularly important role in these patients (Citation19).
Based on these considerations, we hypothesized that in very severe COPD survival might be particularly dominated by dimensions of the disease other than airflow obstruction. To address this issue, we used the COTE-index and the Charlson-index to assess co-morbidities and took into account a broad panel of measures including body plethysmography to comprehensively characterize the patients.
Methods
Study population
Following the study protocol, all patients admitted to the Donaustauf Hospital, Center for Pneumology, within a 10-year time period (September 1999 to September 2009) who had undergone spirometry within 3 days after admission were assessed. Primary inclusion criteria were (Citation1) severe obstructive ventilatory impairment according to GOLD IV, i.e. FEV1/forced vital capacity (FVC) < 70% and FEV1 < 30%predicted, (Citation2) age 30–95 years, (Citation3) history of COPD according to medical records. Based on a comprehensive review of medical data, we excluded patients with other obstructive (e.g. asthma, cystic fibrosis, bronchiectasis) or restrictive (e.g. interstitial lung disease, thoracic deformity, lung resection, neuromuscular disease) disease, or patients after lung transplantation. Patients with current major exacerbation, based on at least one of the following criteria: C-reactive protein (CRP) > 20 mg/dl upon admission, current indication for or actual antibiotic therapy within the last 2 weeks, blood pH < 7.35, or consolidation on chest-x ray suggesting pneumonia, were also excluded.
Measurements
In addition to anthropometric data, sex, age, smoking history and alcohol consumption (no; low (<12 g/d (f); <24 g/d), or previous alcohol consume) were assessed. Co-morbidities were recorded based on medical records, current medication or as reported by the patients. Specifically we addressed cardiovascular diseases (coronary artery disease, congestive heart failure, atrial fibrillation/flutter), cardiovascular risk factors (systemic hypertension, diabetes mellitus, hyperlipidemia), malignancies, AIDS, liver, renal, gastroenterologic, peripheral vascular, cerebrovascular, connective tissue and psychiatric diseases (depression, anxiety, dementia). Co-morbidities were categorized according to the COTE-index (Citation17) covering 12, and the Charlson-index (Citation11) covering 19 domains. Regarding the Charlson-index, we added one point for COPD to the overall score. The condition “moderate or severe renal insufficiency” was based on a GFR < 60 ml/min/1.73 m2. Moreover, we built combined age-co-morbidity scores, in which for each decade over 40 years, 1 point was added (Citation20). Current medication and treatment with LTOT or intermittent NPPV were recorded, and dyspnoea at admission was quantified via the modified Medical Research Council (mMRC) scale (Citation21).
Spirometry and body plethysmography (Masterlab; Viasys, Höchberg, Germany) were performed according to the American Thoracic Society (ATS) (Citation22) using the references of the European Community for Steel and Coal (Citation23). Blood gases from the hyperaemic earlobe during daytime without oxygen supplementation were registered. 6-MWD was assessed following ATS recommendations (Citation24) and reported in absolute and%predicted values (Citation25). Moreover, ADO- (Citation6) and BODE-index (Citation5) were computed; in the absence of 6-MWD values, the BOD-score was built (Citation26). Hemoglobin, hematocrit, leukocyte numbers, and creatinine level was done by standard methods. GFR was calculated via the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation (Citation27).
Follow-up
Patients were followed until either time of death or January 1, 2010. Information on vital status was obtained from medical records and/or via telephone interview of the patients’ relatives, physicians or hospitals. Date and specific cause of death were recorded (Citation28), whereby deaths were assigned to respiratory causes (respiratory failure, right heart failure, acute pulmonary embolism, pneumonia, pneumothorax), cardiovascular disease, cancer, others, or unknown causes. Survival times were evaluated for all-cause and respiratory mortality. Prior to the patients’ interview, written informed consent had been obtained via the patients or their relatives. This approach and the study protocol were approved by the ethics committee of the University of Regensburg, Germany (Study number 09–137).
Statistical analyses
Data were analyzed with PASW Statistics (SPSS, version 19.0, Chicago, IL, USA). According to data distributions, for continuous variables median and quartiles are shown. Differences between groups were assessed by the non-parametric Mann–Whitney U-test for continuous variables, or the chi-square test for categorical variables. The prognostic value of single parameters was evaluated by univariate survival analysis (log-rank test) and visualized in Kaplan–Meier curves, using median values as cut-offs. To identify independent risk factors of mortality, multivariate proportional hazard Cox-regression analyses were performed including factors that were significant in univariate analysis or pathophysiologically sensible. For elucidating the structure of risk factors, we additionally performed a factor analysis, using the principal component approach. Factors with eigenvalues > 1 were retained and normalized Varimax rotation was employed to derive final loadings of variables on factors. In all analyses, p-values < 0.05 were considered statistically significant.
Results
Study population
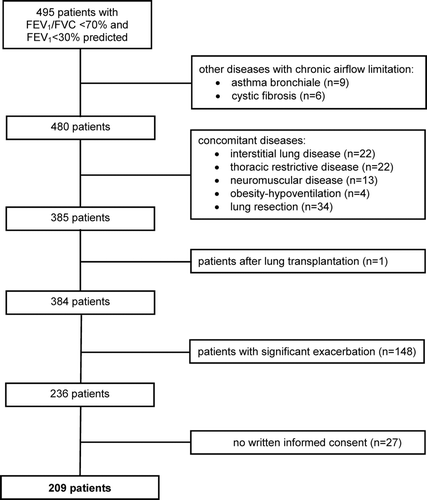
Among 495 patients with a FEV1 < 30% predicted on admission, 286 patients were excluded (). Thus, the study population comprised 209 (58 female, 151 male) patients (). Most subjects had been admitted for evaluation or follow-up investigation of LTOT or NPPV because of chronic respiratory failure (n = 121), for optimizing overall therapy in the presence of increased dyspnoea (n = 41) (e.g. evaluation for lung volume reduction, lung transplantation, or sleep apnoea) or due to mild exacerbation (n = 47) but without fulfilling the criteria mentioned here.
Table 1. Patients’ characteristics and association with mortality
The level of dyspnoea (n = 186) was mMRC 3.0 (2.0–3.0), resulting in an ADO-score of 6.0 (5.0–7.0) points. 6-MWD (n = 125) was 279.0 (210.0–346.5) m or 51.0 (38.2–62.9)% predicted (). Patients with 6-MWT were significantly older (p = 0.033), had a higher creatinine level (p = 0.024), but lower GFR (p = 0.041) compared to patients with 6-MWD. Among the 125 patients, the BODE-index amounted to 7.0 (5.0–7.0), while the BOD-score (n = 186) was 5.0 (5.0–6.0) points (). Blood gas data (pH 7.43 (7.41–7.45), partial oxygen tension 55.0 (50.0–61.0) mmHg, carbon dioxide tension 44.0 (40.0–49.0) mmHg) without oxygen supplementation were available in 109 patients (52.1%) and therefore excluded in further analyses.
Upon inclusion, 160 patients (76.6%) were on LTOT and 98 patients (46.9%) had NPPV. Among the 209 patients, 168 (80.4%) were former smokers (smoking cessation ≥ 3 months), 30 (14.4%) current smokers, and 7 (3.4%) never-smokers. Smoking status could not be reliably assessed in 4 patients (1.9%). Overall, patients had a history of 40 (Citation30–60) packyears. Regarding alcohol consumption (n = 181), 120 patients (66.3%) reported no or little consumption, 38 patients (21.0%) higher consumption, while 23 patients (12.7%) had stopped consumption. Regarding medication, 199 (95.2%) took β2-agonists, 180 (86.1%) anticholinergics, 139 (66.5%) inhaled steroids, 138 (66.0%) diuretics, 115 (55%) theophylline, 73 (34.9%) ACE or AT2-receptor-antagonists, 31 (14.8%) Ca-channel-blockers, 29 (13.9%) β-blockers, 64 (30.6%) anticoagulation drugs or platelet-inhibitors, and 26 (12.4%) digitalis.
Co-morbidities: Cardiovascular risk factors (arterial hypertension (54.1%), hyperlipidemia (38.3%), diabetes (19.6%)) and cardiac diseases (coronary artery disease (16.7%), congestive heart failure (18.7%), atrial fibrillation (16.3%)) were most frequent. Anemia was present in 23.0% of patients. Co-morbidities with a prevalence ≥ 10% are shown in . Psychiatric disorders were more frequent in female patients (p = 0.009), while male patients more often showed hyperlipidemia (p = 0.026), atrial fibrillation (p = 0.006) or any cardiac disease (p = 0.001).
Table 2. Co-morbidities and association with mortality
The Charlson-index was 2.0 (1.0–3.0); among the 19 possible conditions, “dementia,” “connective tissue disease,” “ulcer disease,” “hemiplegia,” or “AIDS” did not occur in our study population. Similarly, regarding the 12 conditions of the COTE-index, no points were allocated to “gastric/duodenal ulcers” and “pulmonary fibrosis.” The COTE-index yielded 1.0 (0.0–3.0) points, with 57.9% of patients showing an index of ≥1 (). The combined age-co-morbidity scores yielded values of 3.0 (2.0–5.0) for the COTE- and 4.0 (3.0–5.0) for the Charlson-index (). The distribution of data within the COTE and the age-combined COTE-index is depicted in .
Figure 2a. Distribution of points of the COTE-index either < (light grey column) or ≥ (dark grey columns) the median value (1.0 (0.0–3.0)).
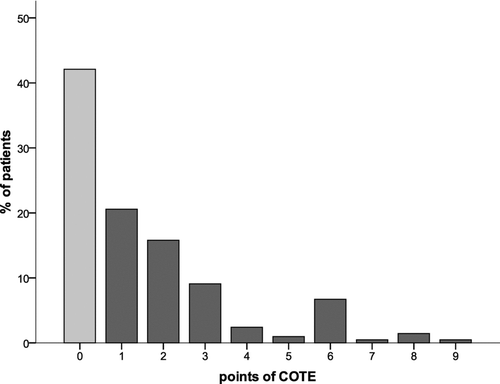
Table 3. Combined scores and the risk of mortality
Survival: During the follow-up of 28 (Citation14–45) months, 121 patients (57.9%) died; 68 (56.2%) due to respiratory causes, 31 (25.6%) from non-respiratory causes (15 cardiovascular, 12 cancer, 4 others), and 22 (18.2%) from unknown causes. Four patients died in hospital, 117 patients after discharge.
Smoking status or the numbers of packyears were not significantly related to mortality. The same was true for the information whether patients consumed either < 12/24 g alcohol, or more, or were currently abstinent. Moreover, the presence of a mild exacerbation at the time of assessment was not related to survival. Similarly, it was not associated with survival whether or not patients had been admitted for initiation or follow-up of LTOT or NPPV. Survival probability regarding all-cause (HR 0.929 (95%-CI 0.655–1.329)) and respiratory mortality (0.744 (0.462; 1.199)) of patients without versus with 6-MWD was not statistically different from each other.
Risk factors of death from any cause: Next to age and BMI, hemoglobin, hematocrit and leukocyte number were associated with death in univariate analyses of survival (p < 0.05 each). Among lung function measures, the association of RV/TLC (absolute values) with death was strongest (, ), but failed statistical significance for FEV1 (absolute values or as%predicted) (, ).
Figure 2b. Distribution of points of the age-COTE-index either < (light grey column) or ≥ (dark grey columns) the median value (3.0 (2.0–53.0)).
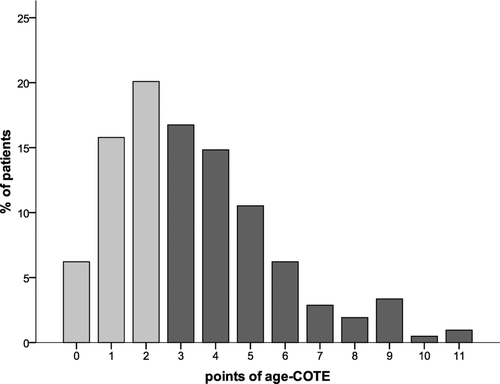
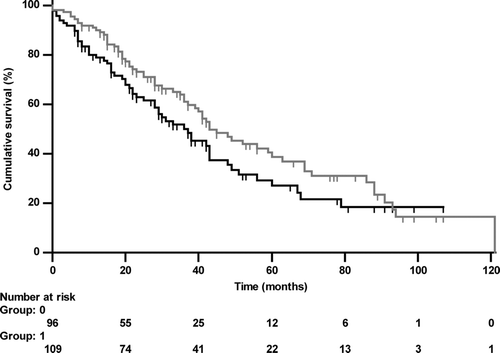
Figure 3a. Kaplan-Meier curve of RV/TLC either < (grey line, group 0) or ≥ (black line, group 1) the median value (76.84%; HR 2.095 (1.445–3.038), p < 0.001) regarding all-cause mortality.
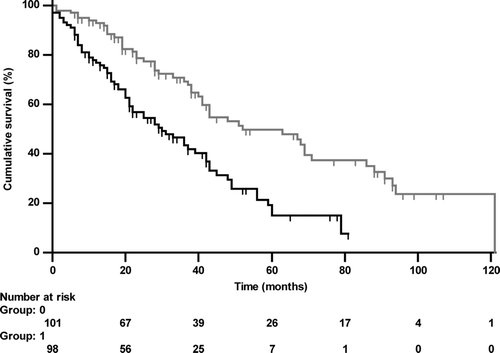
Figure 3b. Kaplan-Meier curve of FEV1%predicted either < (black line, group 0) or ≥ (grey line, group 1) the median value (25.0%; HR 0.749 (0.521–1.077), p = 0.106) regarding all-cause mortality.
When co-morbidities were evaluated separately, only malignancies, obesity and underweight were related to death from any cause (p < 0.05 each), but atrial fibrillation and left-heart failure failed statistical significance. As overall measures, COTE- and Charlson-index were associated (p < 0.05 each) with all-cause mortality ( and ). These associations were stronger when using the age-combined scores ().
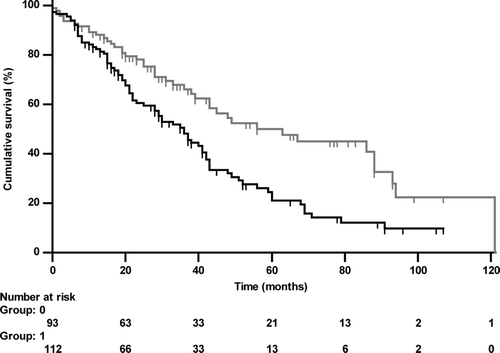
Figure 3c. Kaplan-Meier curve of the COTE-index either < (grey line, group 0) or ≥ (black line, group 1) the median value (1.0; HR 1.911 (1.338–2.730), p < 0.001) regarding all-cause mortality.
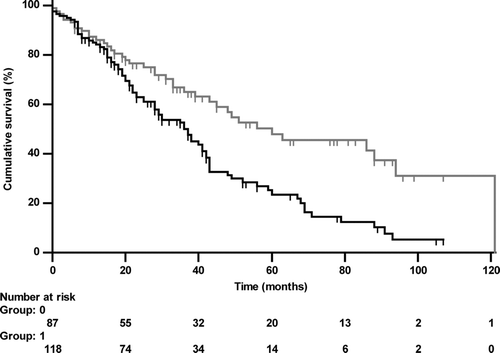
Figure 3d. Kaplan-Meier curve of the Charlson-index either < (grey line, group 0) or ≥ (black line, group 1) the median value (2.0; HR 1.852 (1.297–2.644), p < 0.001) regarding all-cause mortality.
Risk factors of death from respiratory cause: Only age and BMI, but not laboratory parameters were related to survival in univariate analyses (). The intake of diuretics (p = 0.002) or digitalis (p < 0.001) and left-heart failure were again associated with higher mortality (). COTE- and Charlson-index and the respective age-combined indices were also related to death ().
Multivariate analyses: Including factors being significant in univariate analyses regarding death from both any and respiratory cause in addition to gender, only age, RV/TLC and the COTE-index were independently associated with survival from all cause (). Similar results were obtained using the Charlson-index instead of the COTE-index. In contrast, in multivariate models for respiratory death, only BMI and age were independent risk factors (). In order to evaluate the potential additional information provided by lung hyperinflation and COTE, we calculated models without and with these additional risk factors. Using a Cox regression model to account for the censored values, COTE and hyperinflation were significant additional risk factors (p < 0.05 each) in the presence of the other risk factors (BOD, ADO, BODE) that were significant if taken separately.
Table 4a. Multivariate analysis for death from any cause using the respective median
Table 4b. Multivariate analysis for death from respiratory cause using the respective median
This was confirmed by likelihood ratio tests evaluating the gain in fit when including the additional parameters; p-values were always less than 0.001. The ADO remained statistically significant in the presence of COTE and hyperinflation (p < 0.05), whereas BOD und BODE became non-significant.
Exploratory factor analysis: When using BMI, age, Hb, leukocytes, mMRC, RV/TLC, COTE, Charlson and ADO in a factor analysis of risk factors, there remained 180 patients with complete data. Four factors explained 74.5% of total variance. The factor loadings indicated that age, ADO and RV/TLC (the latter inversely) were attributed to factor #1, mMRC and leukocytes to #2, COTE and Charlson to #3, and BMI and Hb to #4. Factor loadings were well separated and either ≥ 0.70 or < 0.30; the only exception was ADO which also loaded on #3 (loading 0.56).
Discussion
This multidimensional prognostic assessment of patients with very severe COPD indicated that co-morbidities, assessed by Charlson or COTE-index, and resting hyperinflation in terms of RV/TLC, were independently related to mortality. The prognostic power of the COPD-specific COTE-index was similar to that of the Charlson-index but in each case improved when including age. In contrast, the prognostic power of single co-morbidities or other lung function measures including FEV1 was much weaker.
The current GOLD recommendations already propose to take into account symptoms and exacerbations (Citation4). Interestingly, recent data indicate that category B COPD, which is characterized by high dyspnoea despite a relatively preserved lung function, has a higher risk for death compared to group C (Citation29), and the authors suggested that unrecognized co-morbidities may contribute to this. Accordingly, category B patients showed the highest rate (20.7%) of ischemic heart disease, highlighting that co-morbidities should be included in the staging of COPD.
In the present investigation, cardiovascular risk factors or cardiac diseases were highly prevalent. More than 50% of the patients had systemic hypertension, and 18.7% left-heart failure, 16.9% coronary heart disease, and 33.5% one of both (data not shown). This is in line with the findings from large cohorts in which patients with COPD stage III/IV showed the highest prevalence of systemic hypertension (51.2%) and cardiovascular diseases (22.1%) (Citation19). It is also reassuring that frequency and distribution of most co-morbidities were similar to those reported by Divo and coworkers (Citation17). Our data add to these findings that in very severe COPD anemia was present in 23% of patients, obesity in 20.6%, and underweight in 10.5%. A malignancy, mostly lung cancer, had been diagnosed in 11.6%, fitting to the known relationship between airway obstruction and incidence of lung cancer (Citation30).
Although the relevance of co-morbidities is widely recognized (Citation31), their systematic assessment, particularly with regard to prognosis, is not yet standard in COPD. Among single co-morbidities, we found left-heart failure and atrial fibrillation to be related to all-cause mortality, though statistical significance was only reached when analyzed in combination. In line with previous data, Hb (Citation32,33), leukocyte number (Citation34), malnutrition and malignancies (3,8,35–37) were also associated with survival. However, compared to the integrative indices (Charlson or COTE), the prognostic value of single co-morbidities was clearly inferior.
Many investigators have used the Charlson-index to evaluate the co-morbidity-associated mortality risk, although this instrument originally was developed for follow-up of patients with chronic diseases after hospital discharge (Citation11) and has never been explicitly validated in COPD. In the present study, the Charlson-index was strongly linked to death from any or respiratory cause and even independently related to survival. This result resembles the findings from a historic cohort comprising 128 stable COPD patients with LTOT and FEV1 of 25.4% (Citation13), and from a prospective multicenter cohort with less severe COPD (Citation8). Similar to the present study, the patients of these two studies predominately died from respiratory cause (77% and 73.2%, respectively), suggesting that co-morbidities have a strong impact on the outcome of adverse respiratory events.
In contrast, studies addressing the prognostic value of the Charlson-index after an acute exacerbation provided conflicting results. Although in two studies (Citation16,Citation38) it was identified as predictor of survival, a larger investigation (Citation15) found only signs of ischemic heart disease or of cor pulmonale but not the Charlson-index to be predictive. In another study of similar size (Citation14), the Charlson-index was also not predictive for mortality, most likely due to the relatively low prevalence of cardiac disease (15.8% ischemic heart disease, 2.1% arrhythmia, 4.2% cor pulmonale) or lung cancer (2.2%) upon inclusion, as compared to our study (45% left-heart diseases, 11.9% malignancies).
Divo and colleagues recently identified 12 out of 79 co-morbidities as independently related to survival and proposed the COTE-index (Citation17). Notably, the prognostic value of most of the single diseases was similar to that found in the present study. The COTE-index attributes the highest risk to anxiety, but in females only. In the present investigation, the association between psychiatric disorders and survival was not statistically significant, although depression or anxiety were common and more prevalent in females, in line with previous data (Citation39,40). Moreover, none of our patients had “pulmonary fibrosis” as this was an exclusion criterion as we aimed to include only patients with severe obstructive ventilatory impairment due to COPD.
COTE- and Charlson-index have not yet been compared in the literature. We found the COTE-index to be linked to mortality from any and respiratory cause, similar to the Charlson-index, although the former comprises fewer co-morbidities. This is plausible in the light that the COTE-index has been specifically developed for COPD and includes diseases such as atrial fibrillation and depression/anxiety which are common in COPD (Citation39–41) and related to survival (39,42–44). Conversely, the Charlson-index, although more comprehensive, includes multiple diseases (connective tissue disease, dementia, mild liver disease, or hemiplegia) that probably have a low impact on survival in COPD. In line with previous data (Citation14,Citation45) we also found chronic renal failure to be common in COPD but according to the literature (Citation14,Citation38), its prognostic significance is weak. Consequently it is, in contrast to the Charlson-index, not included in the COTE-index. The association of both indices with mortality was stronger when combined with age. The combined age-Charlson-index has already been proposed to account for this important covariate (Citation20). Although age is an unmodifiable factor, co-morbidities probably gain more weight at higher age.
We also assessed the multidimensional BODE-index (Citation5). This analysis was, however, limited by the availability of the 6-MWD, which was available in only 125 patients, mostly due to refusal to perform this test and/or major disability. To circumvent this problem, we additionally built the BOD score, which was also linked to survival. In a large recent analysis, ADO and BODE were the best multicomponent indices predicting time to death (Citation46). The authors also assessed co-morbidities by the Charlson-index but unfortunately did not report its association with survival or a comparison with the BODE or ADO-index. Interestingly, in the present study, the COTE-index was also related to mortality in patients with higher BOD or ADO-scores (≥ median values as cut-off, data not shown). This suggests that the assessment of co-morbidities could further improve the prognostic evaluation even in patient at very high risk. Moreover, the results of the multivariate analysis underlined that hyperinflation and COTE are bearing additional information not covered by the other risk factors such as BODE or ADO. However, as the result for ADO showed, they do not substitute for the other factors. Thus, lung hyperinflation and the co-morbidity index seem to carry independent additional information in the severely impaired patients studied. This has, however, to be verified in prospective trials.
This is, to our knowledge, the first study which used body plethysmography to evaluate the prognostic impact of lung function impairment exclusively in patients with very severe COPD, i.e. a FEV1 < 30% predicted. Resting hyperinflation in terms of RV/TLC was a strong and independent predictor, much better than FEV1. These findings add to previous data from patients exclusively receiving NPPV (Citation9) and to a study that used inspiratory capacity for the description of hyperinflation (Citation8). Resting hyperinflation and dynamic hyperinflation during exertion appear to reduce the patients’ activity independently of FEV1 (Citation47). Moreover, hyperinflation negatively influences cardiac function (Citation48), leading to reductions in left ventricular filling, stroke volume and cardiac output (Citation49,50). The mechanical disadvantage contributed to the fact that hyperinflation limits physical activity and exercise performance and enhances dyspnoea (Citation51), all of which are known to be related to survival (Citation5,Citation52). These findings correspond to the beneficial effects of surgical lung volume reduction (Citation53). Moreover, left ventricular mass, a predictor of heart failure and cardiovascular mortality measured by cardiac magnetic resonance, is associated with RV and RV/TLC (Citation54). Taken together, these findings highlight the role of measuring hyperinflation, to extend the prognostic panel in patients with severe COPD.
To better understand the relationship between risk factors themselves, we performed a factor analysis in a subset of these variables. The subset included those found as significant in univariate analyses; however, some of them were omitted to avoid redundancies that could bias the results. COTE- and Charlson-index were found to be associated with each other but none of the other risk factors. This probably reflects the fact that these indices directly quantify co-morbidities. Age and ADO were positively associated with each other and negatively with RV/TLC. We interpret this as a hint that in our population ADO was dominated by age; still there was some loading on the mMRC factor.
The inverse association with RV/TLC possibly reflects a selection effect, as patients with both high age and RV/TLC may have a high probability of not having entered our study; this interpretation would be consistent with the survival data. mMRC was associated with leukocyte counts, and hemoglobin with BMI, suggesting that systemic inflammation is linked to dyspnoea, and that low BMI is related to low Hb, both of which are established predictors of increased mortality. Although the complexity of very severe COPD renders it likely that associations of some kind can be found between many predictors, these specific results underline that the characteristics of our population were consistent with the known clinical characteristics of the disease.
Limitations
Patients with COPD were selected primarily on spirometric criteria, suggesting that we analyzed a “real-life” cohort with typical co-morbidities. On the other hand, this could lead to a selection bias by excluding patients who were not able to perform spirometry. Moreover, as it was a major goal to explore risk factors exclusively in patients with very severe COPD, the tight range of FEV1 may have led to an underestimation of the prognostic value of FEV1 within this group. Therefore, its weak performance does not invalidate its overall usefulness in COPD. Unfortunately, co-morbidities acquired during follow-up could not be recorded. Their inclusion might even have enhanced the prognostic value of co-morbidities. Co-morbidities were carefully reviewed with regard to medical history and medication, but patients did not undergo non-pulmonary diagnostic procedures. Therefore the prevalence of diseases such as liver cirrhosis or gastric/duodenal ulcers, being relevant for prognosis (Citation17), might have been underestimated.
The same may apply to dementia which is known to be highly prevalent in COPD (Citation55, 56), but could not be assessed in a representative manner according to the study design. Moreover, in a large proportion of patients, 6-MWD was not available. There are, however, already many data on the clinical and prognostic significance of 6-MWD and the BODE-index (Citation5); we determined these parameters primarily for the purpose of comparison. Not all patients were in a stable state and some had a mild exacerbation but this was not significantly associated with survival. It also would affect only a subset of the measures evaluated, and, in view of the severe airflow obstruction given, the potential improvement after recovery may be rather modest and does not invalidate our findings. Based on our inclusion criteria we excluded patients with severe exacerbations including those with acute respiratory failure, although the definition used was a pragmatic approach and not fully congruent with that proposed in the most recent guidelines (Citation4).
In conclusion, our findings indicate that in very severe COPD co-morbidities and resting hyperinflation were independent risk factors of mortality. Among single co-morbidities, cardiac diseases, malnutrition and malignancies were linked to survival. The prognostic value of the COTE-index was at least equal to that covered by the more complex Charlson-index, and even higher when combined with age. Considering the poor prognosis in very severe COPD, the COTE-index provides a valuable, comprehensive, systematic assessment of relevant co-morbidities also in these patients. This can be supplemented by the measurement of hyperinflation via body plethysmography, providing additional information not covered by spirometry.
Declaration of Interest Statement
None of the authors has any financial interest in the issue covered by the manuscript. The authors alone are responsible for the content and writing of the paper.
References
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370:765–73.
- Anthonisen NR, Wright EC, Hodgkin JE. Prognosis in chronic obstructive pulmonary disease. Am Rev Respir Dis 1986; 133:14–20.
- Celli BR, Cote CG, Lareau SC, Meek PM. Predictors of survival in COPD: more than just the FEV1. Respir Med 2008; 102 Suppl 1:S27–35.
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187:347–365.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes Oca M de, Mendez RA, The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350:1005–1012.
- Puhan MA, Garcia-Aymerich J, Frey M, ter Riet G, Anto JM, Agusti AG, Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet 2009; 374:704–711.
- Jones RC, Donaldson GC, Chavannes NH, Kida K, Dickson-Spillmann M, Harding S, Derivation and validation of a composite index of severity in chronic obstructive pulmonary disease: the DOSE Index. Am J Respir Crit Care Med 2009; 180:1189–1195.
- Casanova C, Cote C, Torres JP de, Aguirre-Jaime A, Marin JM, Pinto-Plata V, Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171:591–597.
- Budweiser S, Jorres RA, Riedl T, Heinemann F, Hitzl AP, Windisch W, Predictors of survival in COPD patients with chronic hypercapnic respiratory failure receiving noninvasive home ventilation. Chest 2007; 131:1650–1658.
- Corsonello A, Antonelli Incalzi R, Pistelli R, Pedone C, Bustacchini S, Lattanzio F. Co-morbidities of chronic obstructive pulmonary disease. Curr Opin Pulm Med 2011; 17 Suppl 1:S21–28.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic co-morbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383.
- Almagro P, Cabrera FJ, Diez J, Boixeda R, Alonso Ortiz MB, Murio C, Co-morbidities and short-term prognosis in patients hospitalized for acute exacerbation of COPD: the EPOC en Servicios de medicina interna (ESMI) study. Chest 2012; 142:1126–1133.
- Marti S, Munoz X, Rios J, Morell F, Ferrer J. Body weight and co-morbidity predict mortality in COPD patients treated with oxygen therapy. Eur Respir J 2006; 27:689–696.
- Terzano C, Conti V, Di Stefano F, Petroianni A, Ceccarelli D, Graziani E, Co-morbidity, hospitalization, and mortality in COPD: results from a longitudinal study. Lung 2010; 188:321–329.
- Antonelli Incalzi R, Fuso L, Rosa M de, Forastiere F, Rapiti E, Nardecchia B, Co-morbidity contributes to predict mortality of patients with chronic obstructive pulmonary disease. Eur Respir J 1997; 10:2794–800.
- Groenewegen KH, Schols AMWJ, Wouters EFM. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest 2003; 124:459–467.
- Divo M, Cote C, Torres JP de, Casanova C, Marin JM, Pinto-Plata V, Co-morbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186:155–161.
- Celli BR. Predictors of mortality in COPD. Respir Med 2010; 104:773–779.
- Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 2008; 32:962–969.
- Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined co-morbidity index. J Clin Epidemiol 1994; 47:1245–1251.
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988; 93:580–586.
- American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med 1995; 152:1107–1136.
- Sterk PJ, Fabbri LM, Quanjer PH, Cockcroft DW, O'Byrne PM, Anderson SD, Airway responsiveness. Standardized challenge testing with pharmacological, physical and sensitizing stimuli in adults. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993; 16:53–83.
- American Thoracic Society (ATS) statement. Guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166:111–117.
- Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 1998; 158:1384–1387.
- Keaney NP, Ansari K, Munby J, Price M, Kay A, Taylor IK. P219 Ten year mortality in a primary care COPD cohort: multidimensional index BOD more discriminant than GOLD staging. Thorax 2011; 66:A157.
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF3, Feldman HI, A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612.
- McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax 2007; 62:411–415.
- Lange P, Marott JL, Vestbo J, Olsen KR, Ingebrigtsen TS, Dahl M, Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med 2012;186:975–81.
- Mannino DM, Aguayo SM, Petty TL, Redd SC. Low lung function and incident lung cancer in the United States: data From the First National Health and Nutrition Examination Survey follow-up. Arch Intern Med 2003; 163:1475–1480.
- Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: Role of co-morbidities. Eur Respir J 2006; 28:1245–1257.
- Cote C, Zilberberg MD, Mody SH, Dordelly LJ, Celli B. Haemoglobin level and its clinical impact in a cohort of patients with COPD. Eur Respir J 2007; 29:923–929.
- Kollert F, Tippelt A, Muller C, Jorres RA, Porzelius C, Pfeifer M Hemoglobin levels above anemia thresholds are maximally predictive for long-term survival in COPD with chronic respiratory failure. Respir Care 2013;58(7):1204–1212.
- Celli BR, Locantore N, Yates J, Tal-Singer R, Miller BE, Bakke P, Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 185:1065–1072.
- Chailleux E, Laaban J, Veale D. Prognostic value of nutritional depletion in patients with COPD treated by long-term oxygen therapy: data from the ANTADIR observatory. Chest 2003; 123:1460–1466.
- Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 160:1856–1861.
- Mannino DM, Doherty DE, Sonia Buist A. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir Med 2006; 100:115–122.
- Almagro P, Calbo E, Ochoa Echaguen A de, Barreiro B, Quintana S, Heredia JL, Mortality after hospitalization for COPD. Chest 2002; 121:1441–1448.
- Laurin C, Lavoie KL, Bacon SL, Dupuis G, Lacoste G, Cartier A, Sex differences in the prevalence of psychiatric disorders and psychological distress in patients with COPD. Chest 2007; 132:148–155.
- van Manen JG, Bindels PJE, Dekker FW, IJzermans CJ, van der Zee JS, Schade E. Risk of depression in patients with chronic obstructive pulmonary disease and its determinants. Thorax 2002; 57:412–416.
- Buch P, Friberg J, Scharling H, Lange P, Prescott E. Reduced lung function and risk of atrial fibrillation in the Copenhagen City Heart Study. Eur Respir J 2003; 21:1012–1016.
- Ng T, Niti M, Tan W, Cao Z, Ong K, Eng P. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med 2007; 167:60–67.
- Steer J, Gibson J, Bourke SC. The DECAF Score: predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax 2012; 67:970–976.
- Voogd JN de, Wempe JB, Koeter GH, Postema K, van Sonderen E, Ranchor AV, Depressive symptoms as predictors of mortality in patients with COPD. Chest 2009; 135:619–625.
- Incalzi RA, Corsonello A, Pedone C, Battaglia S, Paglino G, Bellia V. Chronic renal failure: a neglected co-morbidity of COPD. Chest 2010; 137:831–837.
- Marin JM, Alfageme I, Almagro P, Casanova C, Esteban C, Soler-Cataluna JJ, Multicomponent indices to predict survival in COPD: The COllaborative COhorts to assess multicomponent indices of COPD in Spain-COCOMICS study. Eur Respir J 2013;42(2):323–332.
- Lahaije AJMC, van Helvoort HAC, Dekhuijzen PNR, Vercoulen JH, Heijdra YF. Resting and ADL-induced dynamic hyperinflation explain physical inactivity in COPD better than FEV1. Respir Med 2013; 107:834–840.
- Vassaux C, Torre-Bouscoulet L, Zeineldine S, Cortopassi F, Paz-Diaz H, Celli BR, Effects of hyperinflation on the oxygen pulse as a marker of cardiac performance in COPD. Eur Respir J 2008; 32:1275–1282.
- Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med 2010; 362:217–227.
- Watz H, Waschki B, Meyer T, Kretschmar G, Kirsten A, Claussen M, Decreasing cardiac chamber sizes and associated heart dysfunction in COPD: role of hyperinflation. Chest 2010; 138:32–38.
- O'Donnell DE, Laveneziana P. The clinical importance of dynamic lung hyperinflation in COPD. COPD 2006; 3:219–232.
- Waschki B, Kirsten A, Holz O, Muller K, Meyer T, Watz H, Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest 2011; 140:331–342.
- Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003; 348:2059–2073.
- Smith BM, Kawut SM, Bluemke DA, Basner RC, Gomes AS, Hoffman E, Pulmonary hyperinflation and left ventricular mass: The multi-ethnic study of atherosclerosis COPD study. Circulation 2013; 127:1503–1511.
- Antonelli-Incalzi R, Corsonello A, Pedone C, Trojano L, Acanfora D, Spada A, Drawing impairment predicts mortality in severe COPD. Chest 2006; 130:1687–1694.
- Grant I, Heaton RK, McSweeny AJ, Adams KM, Timms RM. Neuropsychologic findings in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med 1982; 142:1470–1476.