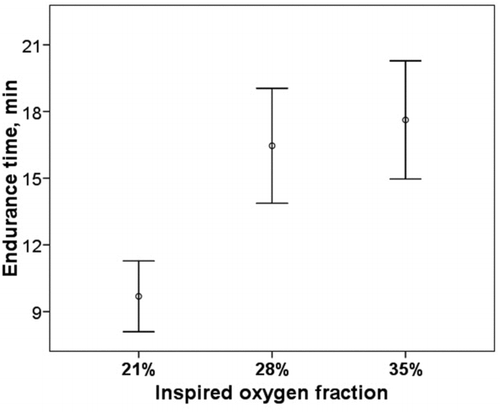Abstract
Background: At Bogota's altitude (2640 m), the lower barometric pressure (560 mmHg) causes severe hypoxemia in COPD patients, limiting their exercise capacity. The aim was to compare the effects of breathing oxygen on exercise tolerance. Methods: In a blind, crossover clinical study, 29 COPD patients (FEV1 42.9 ± 11.9%) breathed room air (RA) or oxygen (FIO2 28% and 35%) during three treadmill exercise tests at 70% of their maximal capacity in a randomized order. Endurance time (ET), inspiratory capacity (IC), arterial blood gases and lactate were compared. Results: At the end of the exercise breathing RA, the ET was 9.7 ± 4.2 min, the PaO2 46.5 ± 8.2 mmHg, the lactate increased and the IC decreased. The oxygen significantly increased the ET (p < 0.001), without differences between 28% (16.4 ± 6.8 min) and 35% (17.6 ± 7.0 min) (p = 0.22). Breathing oxygen, there was an increase in the PaO2 and SaO2, higher with FIO2 35%, and a decrease in the lactate level. At “isotime” (ET at RA), with oxygen, the SpO2, the oxygen pulse and the IC were higher and the heart rate lower than breathing RA (p < 0.05). Conclusion: Oxygen administration for COPD patients in Bogotá significantly increased ET by decreased respiratory load, improved cardiovascular performance and oxygen transport. The higher increases of the PaO2 and SaO2 with 35% FIO2 did not represent a significant advantage in the ET. This finding has important logistic and economic implications for oxygen use in rehabilitation programs of COPD patients at the altitude of Bogotá and similar altitudes.
Introduction
The dyspnea and the limitation of exercise capacity are common in patients with chronic obstructive pulmonary disease (COPD) and significantly affect their quality of life. The limitation of exercise is multifactorial and has been related to the degree of airflow obstruction, the dynamic hyperinflation, the inadequate supply of energy to the respiratory and peripheral muscles and the consequent peripheral muscle dysfunction (Citation1–3).
It has been demonstrated that oxygen therapy during exercise in COPD patients reduces the dyspnea and increases the exercise capacity by decreasing the minute ventilation, the dynamic hyperinflation, increasing the oxygen delivery to muscles, decreasing the metabolic acidosis and possibly by decreasing the “hypoxic drive” (Citation4–6).
In Bogota, a city located at 2640 meters (8660 ft) above sea level (an altitude considered as “high altitude”), the lower barometric pressure (560 mmHg) causes a decrease in the inspired and the alveolar oxygen pressures and therefore in the PaO2 in normal subjects (Citation7) and COPD patients. Compared to sea level, patients with COPD living in Bogota have more hypoxemia and desaturation at rest, which increases during physical activity, limiting their exercise capacity (Citation8,9). The correction of this hypoxemia, which can be severe during exercise, requires the administration of a very high fraction of inspired oxygen (FIO2), eventually with a Venturi mask, which is expensive and uncomfortable for the patients. In a randomized crossover clinical trial, we compared the acute effects on exercise capacity of breathing three oxygen concentrations: room air, 28 and 35%, in COPD patients long-term residents in Bogota and explored the mechanisms of improving the exercise tolerance.
Methods
Patients
This was a randomized, blind, crossover clinical study on COPD patients with moderate to severe airflow obstruction (FEV1 £ 60% predicted value), who had been clinically stable for at least six weeks, not receiving oxygen permanently and were long term residents in Bogotá to exclude the acute changes due to the ascent of altitude. Current smokers or patients with other respiratory diseases, chest wall disturbances and cardiovascular diseases other than cor pulmonale were excluded. The study was approved by the Research Ethics Committee of the institution and the patients signed a consent form.
Due to the absence of similar previous studies at the Bogota's altitude, a pilot study was conducted with COPD patients to measure the endurance time during three tests breathing air or FIO2 of 28% and 35%. With these results, a type I error of 0.05 and a power of 80%, a sample size of 29 patients was calculated (Citation10).
Pulmonary function test at rest
Spirometry, lung volumes by plethysmography and diffusing capacity of the lung for carbon monoxide (DLCO) were done according to the standards of the American Thoracic Society (ATS) on a V-MAX 229 and Autobox 6200 (SensorMedics Inc, Yorba Linda, CA) and using the Crapo equations (Citation11–13).
Exercise testing
All patients completed a symptom-limited incremental exercise test on a treadmill breathing room air. In a subsequent visit, subjects performed three endurance tests at 70% of the maximal exercise capacity of the incremental test, in random order and blinded for the patients, with room air, oxygen at 28% and at 35%, with a 60–90-min recovery period between the tests. Minute ventilation (VE), oxygen uptake (VO2) and carbon dioxide output (VCO2) were measured breath-by-breath with a metabolic chart (V-MAX 229 Spectra, SensorMedics). For the analysis of the data, the average of each variable during 2 minutes of rest, and in the last minute of the exercise was used. Pulse oximetry (SpO2) was measured continuously. Blood samples were collected at rest and at peak exercise, using a catheter inserted into a radial artery, for analysis of lactate (Vitros 250, Johnson & Johnson) and arterial blood gases (Rapidlab 860, Bayer). The alveolar-arterial oxygen difference (P[A-a]O2), the dead space to tidal volume ratio (VD/VT) and the arterial oxygen content were calculated.
Dyspnea with activities of daily living was assessed by the Medical Research Council scale (MRC) (Citation14). During the endurance tests, dyspnea and lower limb fatigue were evaluated by the Borg scale (Citation15). To evaluate dynamic hyperinflation, the inspiratory capacity (IC) was measured before and during exercise testing.
Data analysis
The results are presented as means and standard deviation. The normal distribution of continuous variables was evaluated using the Shapiro–Wilk test. VE, tidal volume (VT), respiratory frequency (fR), IC, gas exchange and symptoms were compared in the three endurance tests (FIO2 21, 28 and 35%) at the end of the exercise and at the “isotime” (exercise time in the endurance test while breathing air). Comparison between the responses of breathing air and oxygen were made by paired and unpaired t test. Two-tailed hypothesis were stated with a significance level ( p value) < 0.05 and the statistical software Stata 7.0 was used.
Results
We studied 6 women and 23 men with COPD. At baseline the patients had moderate to severe obstruction, hyperinflation, severe reduction of diffusion, moderate hypoxemia (PaO2 50.8 ± 6.6 mmHg) and mild hypercapnia (PaCO2 34.7 ± 4.4 mmHg) for the altitude of Bogota (). All patients completed the exercise test.
Table 1. Subjects characteristics (N = 29)
Variables at the end of exercise endurance tests
Breathing air, the patients developed severe hypoxemia and desaturation (PaO2 46.5 ± 8.2 and SaO2 77.6% ± 10.3), more hypercapnia, increased blood lactate and decreased inspiratory capacity indicative of dynamic hyperinflation. With FIO2 of 28 and 35% there was a slight increase in VO2 with no change in the VCO2, a significant increase in PaO2, SaO2 and in the arterial oxygen content, and a significant decrease in the lactate level at the end of the exercise in comparison to breathing air (). The PaO2, SaO2 and SpO2 with FIO2 35% were higher than with FIO2 28%. There was not significant increase in the PaCO2 with oxygen ().
Table 2. Variables at the end of symptom-limited endurance tests
Endurance time
In the test breathing RA (FIO2 21%) the endurance time was 9.7 ± 4.2 minutes. The oxygen administration significantly increased the duration of the constant load exercise: 16.4 ± 6.8 min with FIO2 of 28% ( p < 0.001) and 17.6 ± 7.0 min with FIO2 of 35% ( p < 0.001), without significant difference between the two tests with oxygen (p = 0.22) ().
Variables at the “isotime”
Compared with the values observed while breathing air, there was a significant increase in SpO2 and oxygen pulse while breathing oxygen and a significant decrease in heart rate, respiratory rate and dynamic hyperinflation. The only significant difference between tests with oxygen was a greater SpO2 with 35% FIO2 ().
Table 3. Variables at the endurance exercise isotime
Dyspnea and fatigue of the lower limbs
At the end of the constant load exercise and at the isotime, dyspnea and fatigue of the lower limbs were similar in the three exercise test (Tables and ).
Discussion
This study demonstrates that in COPD patients living at the altitude of Bogota with moderate to severe hypoxemia, supplemental oxygen (FIO2 28% and 35%) significantly increases the duration of exercise. Although PaO2 and SaO2 were higher with the FIO2 of 35% than 28%, this increase did not offer an additional benefit to the endurance time.
Exercise time
The improvement of endurance time with oxygen found in this study was greater than that considered clinically significant for an intervention, which should be at least 33% above baseline (Citation16). Although in other studies there were non-responder patients to oxygen, in this study all were responders (Citation17). It's very relevant that there was no difference in the endurance time between tests with 28% and 35%, a finding contrary to the results reported by Somfay, who found that in COPD patients, without baseline desaturation and saturation close to 100%, while receiving oxygen, the improvement in exercise time was dose dependent up to FIO2 of 50% (Citation18).
The fact that our study was done at an altitude of 2640 m above sea level, with a barometric pressure of 560 mmHg, placed our patients’ SaO2 and PaO2 during exercise in the inclined section of the dissociation curve of hemoglobin; thereby a significant increase in SaO2 with the comparatively less increase in PaO2. In our patients with severe exercise desaturation, the FIO2 of 35%, doubled the PaO2 (46.5 ± 8.2 to 94.5 ± 17.3 mmHg) and achieved a 23% improvement in SaO2 (77.6 ± 10.3 to 95.8 ± 3.4%), which is not much larger than the 18% increase obtained with the FIO2 28% (77.6 ± 10.3 to 91.4 ± 6.5%). With these similar saturations, there was no significant difference in arterial oxygen content, which may explain in part why there was no difference in exercise duration with the two FIO2 studied.
Mechanisms associated with the improvement of the endurance time
Previous studies have shown that the administration of oxygen improves exercise tolerance and alleviates dyspnea in COPD patients (Citation19–21), a physiological response attributed to several mechanisms: slower breathing pattern that, in turn reduces minute ventilation and dynamic hyperinflation (Citation6,Citation18,Citation22), decreased metabolic acidosis by the improved oxygen delivery to muscles (Citation4,Citation23), improvement in peripheral muscle activity (Citation23,24), decreased pulmonary hypertension (Citation25) and inhibition of peripheral chemoreceptors (Citation5).
The increase in exercise duration found in our patients may be attributed to different mechanisms: 1. Decreased respiratory load, shown by a significant decrease in respiratory rate and in dynamic hyperinflation at the isotime. 2. Improvement in cardiovascular performance and oxygen delivery to tissues which is suggested by the decrease in the heart rate, increased oxygen pulse, increased oxygen content and the reduced lactate production. 3. Possible decreased ventilation, by decreased hypoxic stimulation of peripheral chemoreceptors, a phenomenon that occurs even in normoxic subjects (Citation26). We consider that the complete abolition of this stimulus, described in other studies, is a very unlikely mechanism given the level of FIO2 administered and the not very high oxygen saturation values reached in our patients.
Contrary to previous similar works, in this study the increase of the PaCO2 breathing oxygen was not greater than in the exercise tests breathing air and we also did not observe any significant change in the VCO2 during exercise. One possible explanation was that we didn't use higher oxygen concentrations. An unexpected result was that despite the decrease in dynamic hyperinflation during exercise test with oxygen, a physiological mechanism associated with diminished perception of the intensity of symptoms (Citation4,Citation6,Citation27), there was no decrease in dyspnea either at the end of the constant load endurance exercise or at the isotime. This suggests the involvement of other mechanisms in the relief of dyspnea during exercise, which will be studied in subsequent studies.
Implications of the administration of oxygen in altitudes
Most authors recommend the administration of oxygen in patients with hypoxemia during exercise and pulmonary rehabilitation, a recommendation that is not endorsed for patients without hypoxemia in exercise (Citation28). There is no agreement, however, in the level of correction of hypoxemia to be pursued and therefore in the amount of FIO2 to be administered. In Bogota, where the altitude above sea level (2640 m or 8,660 feet) causes a very significant hypoxemia during exercise in patients with moderate to severe COPD (Citation8,9) the use of a FIO2 to completely correct hypoxemia during pulmonary rehabilitation programs often requires the use of very high flows or Venturi masks that, besides being uncomfortable, increases the cost and makes it almost impossible to use in daily life. This study shows that with an easily achievable FIO2 of 28%, it is possible to obtain a partial correction of hypoxemia that is very effective in improving the exercise duration and correcting the variables involved in the limitation for exercise and to meet the objectives of rehabilitation programs.
The strengths of this study were that is the first one to evaluate the response to exercise with supplemental oxygen in patients with COPD at this high altitude and that it was a crossover clinical trial with patients who were blind to the intervention. The limitations include not having measured other variables involved in exercise limitation such as pulmonary hypertension, which may be important at this altitude and could decrease with the administration of oxygen.
Because our aim was to evaluate the effect in exercise with a relatively low FIO2 (28 and 35%), used in clinical practice during pulmonary rehabilitation, we didn´t evaluate the role of peripheral chemoreceptors in the relief of dyspnea or the improvement of exercise duration with the administration of higher levels of FIO2. In future studies of COPD patients at Bogotá's altitude, we expect to fully assess the role of oxygen during exercise in relieving dyspnea, its effect on pulmonary hypertension and the results from combining with other interventions such as administration of bronchodilators and pulmonary rehabilitation.
Conclusion
Administration of supplemental oxygen in patients with moderate-to-severe COPD in Bogota, a high altitude city, significantly increased exercise time. The mechanisms associated with this response were decreased respiratory load, improved cardiovascular performance and oxygen transport to the periphery. Although with the administration of 35% FIO2 there was a greater increase in PaO2 and oxygen saturation than that achieved with a 28% FIO2, this increase did not represent a significant advantage in terms of duration of exercise. The demonstration of the beneficial effects of oxygen administration with an FIO2 of 28% has significant logistic and economic implications in the long-term management and rehabilitation programs of patients with COPD at the altitude of Bogota and other similar altitudes.
Declaration of Interest Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgment
This study was registered in COLCIENCIAS (National Administrative Department of Science, Technology and Innovation –Bogota, Colombia) and they supported us with a grant.
References
- O'Donnell DE, Webb KA. The major limitation to exercise performance in COPD is dynamic hyperinflation. J Appl Physiol 2008; 105(2):753–755.
- Aliverti A, Macklem PT. The major limitation to exercise performance in COPD is inadequate energy supply to the respiratory and locomotor muscles. J Appl Physiol 2008; 105(2):749–751.
- Debigare R, Maltais F. The major limitation to exercise performance in COPD is lower limb muscle dysfunction. J Appl Physiol 2008; 105(2):751–753.
- O'Donnell DE, Bain DJ, Webb KA. Factors contributing to relief of exertional breathlessness during hyperoxia in chronic airflow limitation. Am J Respir Crit Care Med 1997; 155(2):530–535.
- Somfay A, Porszasz J, Lee SM, Casaburi R. Effect of hyperoxia on gas exchange and lactate kinetics following exercise onset in nonhypoxemic COPD Patients. Chest 2002; 121(2):393–400.
- O'Donnell DE, D'Arsigny C, Webb KA. Effects of hyperoxia on ventilatory limitation during exercise in advanced chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163(4):892–898.
- Maldonado D, Gonzalez-Garcia M, Barrero M, Torres-Duque C, Casas A. Reference values for arterial blood gases at an altitude of 2640 meters. Am J Respir Crit Care Med 2013; 187:A4852.
- Gonzalez-Garcia M, Barrero M, Maldonado D. Exercise limitation in patients with chronic obstructive pulmonary disease at the altitude of Bogota (2640 m). Breathing pattern and arterial gases at rest and peak exercise. Arch Bronconeumol 2004; 40(2):54–61.
- Gonzalez M, Barrero M, Maldonado D. The major limitation to exercise performance in COPD patients at the altitude of Bogotá (2640m) is hypoxemia. Chest 2009; 136(4):62S–62f.
- Machin D, Campbell MJ, Fayers P, Pinol A. Sample Size Tables for Clinical Studies. Second Edition. Oxford: Blackwell Science, 1997.
- Miller MR, Hankinson J, Brusasco V Standardisation of spirometry. Eur Respir J 2005; 26(2):319–338.
- MacIntyre N, Crapo RO, Viegi G Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005; 26(4):720–735.
- Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis 1981; 123(6):659–664.
- Surveillance for respiratory hazards in the occupational setting [American Thoracic Society] Am Rev Respir Dis 1982; 126(5):952–956.
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14(5):377–381.
- Puente-Maestu L, Villar F, de Miguel J, Clinical relevance of constant power exercise duration changes in COPD. Eur Respir J 2009; 34(2):340–345.
- Heraud N, Prefaut C, Durand F, Varray A. Does correction of exercise-induced desaturation by O(2) always improve exercise tolerance in COPD? A preliminary study. Respir Med 2008; 102(9):1276–1286.
- Somfay A, Porszasz J, Lee SM, Casaburi R. Dose-response effect of oxygen on hyperinflation and exercise endurance in nonhypoxaemic COPD patients. Eur Respir J 2001; 18(1):77–84.
- Snider GL. Enhancement of exercise performance in COPD patients by hyperoxia. Chest 2002; 122(5):1830–1836.
- McDonald CF, Blyth CM, Lazarus MD, Marschner I, Barter CE. Exertional oxygen of limited benefit in patients with chronic obstructive pulmonary disease and mild hypoxemia. Am J Respir Crit Care Med 1995; 152(5):1616–1619.
- Jolly EC, Di Boscio V, Aguirre L, Effects of supplemental oxygen during activity in patients with advanced COPD without severe resting hypoxemia. Chest 2001; 120(2):437–443.
- Stein DA, Bradley BL, Miller WC. Mechanisms of oxygen effects on exercise in patients with chronic obstructive pulmonary disease. Chest 1982; 81(1):6–10.
- Maltais F, Simon M, Jobin J, Effects of oxygen on lower limb blood flow and O2 uptake during exercise in COPD. Med Sci Sports Exerc 2001; 33(6):916–922.
- Gosselin N, Durand F, Poulain M, Effect of acute hyperoxia during exercise on quadriceps electrical activity in active COPD patients. Acta Physiol Scand 2004; 181(3):333–343.
- Fujimoto K, Matsuzawa Y, Yamaguchi S, Koizumi T, Kubo K. Benefits of oxygen on exercise performance and pulmonary hemodynamics in patients With COPD with mild hypoxemia. Chest 2002; 122(2):457–463.
- Whipp BJ. Carotid bodies and breathing in humans. Thorax 1994; 49(11):1081–1084.
- O'Donnell DE, D'Arsigny C, Fitzpatrick M, Webb KA. Exercise hypercapnia in advanced chronic obstructive pulmonary disease: The role of lung hyperinflation. Am J Respir Crit Care Med 2002; 166(5):663–668.
- Ries AL, Bauldoff GS, Carlin BW, Pulmonary rehabilitation: Joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest 2007; 131(5 suppl):4S–42S.