Abstract
Cough and phlegm are common in COPD. Previous studies have shown conflicting evidence regarding their association with mortality and lung function. We sought to better understand how cough and phlegm impact mortality and lung function in COPD. We analyzed data from the Lung Health Study, consisting of 5,887 smokers with mild to moderate airflow obstruction followed longitudinally. We assessed the association between baseline symptoms of cough alone, phlegm alone, and cough and phlegm with 12.5-year mortality and annual lung function decline. Average age at entry was 48.5 years (± 6.8) with 63% males and 4% African Americans. Cough alone was present in 17%, phlegm alone in 12%, while 31% had both. Neither symptom alone was associated with death, but the combination was associated with increased risk of death after adjustment for age, gender, race, smoking status at year 5, pack-years smoked, randomization group, baseline FEV1 percent predicted (HR 1.27, 95% CI 1.02–1.59). Individuals with cough and phlegm together more commonly died of respiratory causes than those without. Cough with phlegm was associated with 48 mL lower baseline FEV1 (95% CI –90, –6), while neither symptom alone was associated with baseline FEV1. No symptom was associated with FEV1 longitudinally. Cough and phlegm together are associated with mortality and lung function decrement in mild-to-moderate COPD, independent of lung function and smoking status. Respiratory causes of death are common among those with cough and phlegm. Such information can help to identify subsets of individuals with COPD having higher risk for adverse outcomes.
Keywords :
Introduction
Symptoms of cough, phlegm and wheezing are common among individuals with chronic obstructive pulmonary disease (COPD) (Citation1,2). Such symptoms have been shown to have an impact on quality of life (Citation3,4) and risk for acute exacerbation of COPD (Citation4,5). Whether such symptoms are associated with mortality and lung function decline remains controversial and depends on the population studied and the definitions of symptoms used. Studies of general population cohorts have shown that symptoms of cough, phlegm, wheeze and breathlessness have been associated with all cause mortality (Citation6,7). Although some studies in individuals with COPD have suggested that these symptoms are linked to mortality (Citation8–12), others have shown no association (Citation13,14).
The association between respiratory symptoms and lung function decline is also unclear. Studies of general population cohorts (including nonsmokers, smokers and those with and without COPD) have shown that symptoms of chronic bronchitis (Citation15,16), chronic mucous hypersecretion (Citation17,18), and symptoms of cough, phlegm, wheezing or dyspnea (Citation19) have been associated with accelerated FEV1 decline. A Swiss general population study found that cough, phlegm or shortness of breath was associated with lung function decline in individuals with mild COPD, but not in individuals without COPD or those with moderate to severe COPD (Citation20).
In contrast, another European study showed that individuals with cough, phlegm or shortness of breath had greater lung function decline over a broader spectrum of COPD severity (Citation21). What remains unclear is what specific components of these respiratory symptoms are important in determining risk for mortality and lung function decline in COPD. Further, it is important to understand how symptoms of cough and phlegm contribute to mortality and lung function decline in a population of at-risk smokers with mild-to-moderate airflow obstruction, as this group is most likely to benefit from early interventions.
The Lung Health Study (LHS) provides an excellent dataset from which to determine the associations between respiratory symptoms and long-term outcomes in individuals with mild to moderate COPD (Citation22). In this study, we evaluated the association between the symptoms of cough alone, phlegm alone, and cough and phlegm with 5-year lung function decline and 12.5-year mortality.
Methods
Study population
LHS I (Citation22–24) was a multicenter randomized study of smoking cessation intervention and long-term bronchodilator use on lung function over 5 years in 5,887 active smokers aged 35 to 60 years with evidence of mild-to-moderate airflow obstruction (prebronchodilator FEV1/FVC < 0.70 and prebronchodilator FEV1 55–90% predicted). LHS III followed 98.3% of participants up to 14.5 years to determine the long-term impact of smoking cessation (Citation25,26). To minimize potential bias from loss to follow-up in LHS III, we limited lung function analyses to 5 year data. Survival analysis used LHS III data.
Symptoms
Participants completed respiratory symptom questionnaires at randomization. We defined four symptom groups: 1) no cough or phlegm, 2) cough alone (a positive response to “Do you usually have a cough?” and negative response to “Do you usually produce phlegm?”), 3) phlegm alone (a positive response to “Do you usually produce phlegm?” and a negative response to “Do you usually have a cough?”), and 4) cough and phlegm (positive responses to both “Do you usually have a cough?” and “Do you usually produce phlegm?”), with reference group being those without symptoms. We also evaluated the Fletcher-Peto definition of chronic bronchitis, which indicates the presence of cough and phlegm for at least three months out of the year for longer than a year (Citation27).
Baseline data on symptoms were used in analyses. We chose to divide patients into 4 groups based upon the presence of these symptoms given that this approach would allow us to understand if there was an interaction in those with both symptoms, which is an assessment of the presence of chronic productive cough. Because the Fletcher-Peto definition of chronic bronchitis is a different definition for chronic productive cough, requiring specific timings of symptoms, we compared the two groups to understand the differences in the associations based upon different epidemiologic definitions. We also assessed dyspnea (Citation28) and frequent wheeze as reported by the patient.
Outcome
Primary outcome was time to all-cause death over 12.5 years. Causes of death, hospitalization and morbidity were determined by a morbidity and mortality review board (Citation24). Follow-up for vital status was carried out until 2001 with a mean follow-up duration of 12.0 years and vital status ascertainment in 98.3% (Citation25). We also studied post-bronchodilator FEV1, assessed by spirometry (Spirotech 500; Spirotech, Atlanta, Georgia) at baseline and annually during the first 5 years of study (Citation26). The procedure for recording spirometry has been described previously (Citation29).
Statistical methods
Demographics and clinical characteristics were compared in the four symptom groups using χ2 tests and analysis of variance (ANOVA) to analyze differences in categorical and continuous variables, respectively.
We assessed associations between the four symptom groups (all groups in one model) with all-cause mortality using Cox proportional hazards models, adjusting for age at enrollment, gender, race (African American or other), smoking status at year 5 (with sustained quitters being those individuals who quit before year 5 and remained quit at the year 5 assessment, intermittent quitters being individuals who quit at some point during the study but restarted smoking prior to the year 5 assessment, and continuous smokers being individuals who did not attempt quitting prior to year 5, a classification scheme used in previous analyses of the Lung Health Study population (Citation26)), baseline FEV1 percent predicted, randomization group and pack-years smoked.
A separate analysis was performed using the Fletcher-Peto definition of chronic bronchitis as a subgroup of those individuals having usual cough and phlegm. We analyzed causes of death among the four symptom groups. For each cause of death, we compared case fatality rates of patients with symptoms (cough alone, phlegm alone, cough with phlegm groups separately) to asymptomatic patients (no cough or phlegm) using χ2 tests with Bonferroni correction. We also constructed separate survival models for frequent wheeze and dyspnea, adjusted as above for confounders.
For associations between symptoms and lung function, we used previously described generalized estimating equations (GEE) models (Citation30), computing differences in baseline FEV1 (in mL) as well as changes in FEV1 slope (mL/year). We adjusted for age, gender, current smoking status, previous visit smoking status, race, pack-years smoked, height, randomization group, and body mass index (BMI) including terms for slope with gender and current smoking status. We used Stata 12 (Citation31) statistical software. LHS was previously approved by the IRBs as each site, respectively.
Results
Baseline characteristics
The baseline characteristics of study participants have been previously published (Citation32). Among the study population, 17.4% reported cough alone, 11.9% reported phlegm alone, 31.2% reported both and 39.5% reported neither symptom (). We also found that among those with both symptoms together, 71% had chronic bronchitis based upon the Fletcher-Peto definition. We assessed baseline and demographic characteristics of the cohort among the 4 symptom groups (). Average age was around 48 years at recruitment for all groups, and gender distribution was similar except in the phlegm only group, which had fewer females.
Figure 1. Overlap of symptoms of cough and phlegm in the cohort. The Venn diagram depicts the overlap of symptoms of “usual cough” and “usual phlegm” in the cohort. All four symptom groups with proportion of individuals included in group depicted. (See methods section for definitions of “usual cough” and “usual phlegm”).
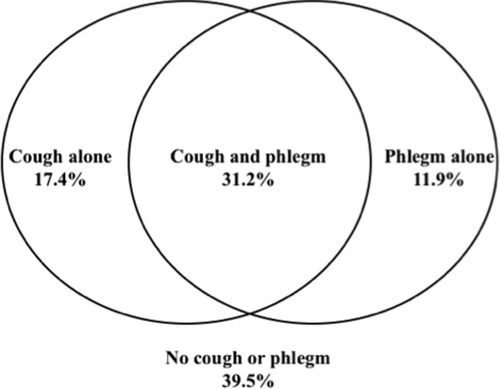
Table 1. Baseline characteristics of LHS cohort
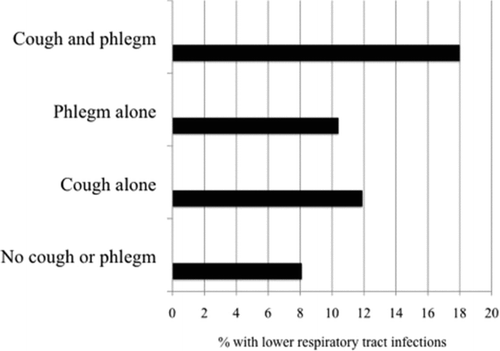
A lower percentage of African Americans complained of cough, with or without phlegm (overall p < 0.001). Pack-years of smoking were higher in patients having cough and phlegm (pack-years smoked 41.3–42.9 compared to 37.7 in those without symptoms, p < 0.001). Groups with cough or phlegm had higher dyspnea scores as measured by the Modified Medical Research council (mMRC) score (p < 0.001). They were also less likely to be sustained quitters at year 5 (overall p = 0.031). Patients who reported lower respiratory tract infections, including chest colds, bronchitis, pneumonia and flu, in the year prior to randomization had a greater proportion of those with cough and phlegm (overall p < 0.001, ).
Survival
We assessed the associations between the four symptom groups with survival over 12.5 years after adjusting for age at enrollment, gender, race (African American or other), smoking status at year 5 (sustained quitter, intermittent quitter, continuous smoker), baseline FEV1 percent predicted, randomization group and pack-years smoked. Compared to those without symptoms, neither cough alone (HR 0.90; 95% CI 0.67–1.20) nor phlegm alone (HR 0.80; 95% CI 0.57, 1.12) was associated with significantly higher risk of death ().
Figure 3. Associations between individual symptoms and mortality over 12 years. Analyses were adjusted for age, gender, race, smoking status at year 5, baseline FEV1% predicted, pack-years smoked and randomization group. Group without cough or phlegm was the comparison group for all analyses of cough and phlegm. Points are hazard ratios and lines are 95% confidence intervals.
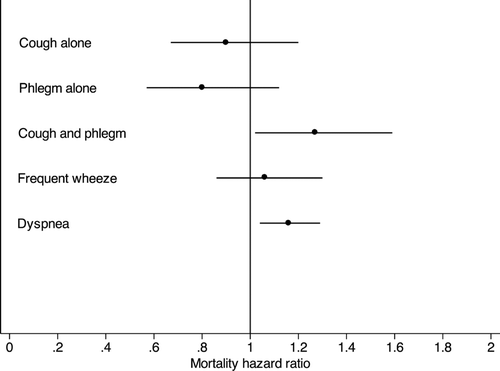
The group having symptoms of cough with phlegm had a 27% increased risk of death (HR 1.27; 95% CI 1.02–1.59) compared to those without symptoms. This is in comparison to other important respiratory symptoms such as dyspnea (HR 1.16, 95% CI 1.04–1.29) and frequent wheeze (HR 1.06, 95% CI 0.86–1.30), also depicted in . shows that over 12.5 years of follow-up, a total of 575 individuals died: 214 (9%) individuals without symptoms of cough or phlegm, 88 (9%) individuals with cough alone, 65 (9%) with phlegm alone and 208 (11%) with both. After comparing causes of death, we found no overall significant difference for cause of death between symptom groups.
Table 2. Causes of death in the LHS cohort, comparing those with and without symptoms of cough and phlegm
However when assessing individual causes of death between the four symptom groups, patients who had cough with phlegm had significantly greater respiratory mortality than those who were asymptomatic ( p = 0.009). Notably, the mean time to death among these 575 individuals was 7.7 years (standard deviation 3.4 years), and median was 8.5 years (25th percentile 4.8 years, 75th percentile 10.7 years), indicating that this mortality risk was unlikely to be related to lower respiratory tract infections experienced in the year prior to randomization.
Lung function
As shown in , in adjusted models we found no significant associations between cough alone or phlegm alone with baseline FEV1 when compared to those without either symptom. The presence of both symptoms, however, was associated with 48 mL lower FEV1 at baseline compared to those with neither symptom ( p = 0.026). The rate of decline in FEV1 was not different among the four symptom groups ().
Table 3. Adjusted associations between individual symptoms and baseline post-bronchodilator FEV1 (mL)
Table 4. Adjusteda difference in decline of post-bronchodilator of FEV1 (mL/year) among four symptom groups over follow-up
The Fletcher-Peto definition of chronic bronchitis (present in 71% of those participants with usual cough and phlegm together) was not significantly associated with mortality (HR 0.89; 95% CI 0.63–1.24), baseline lung function (–13 mL; p = 0.577) or lung function change over follow-up (1 mL/yr; p = 0.758) in this study ().
Table 5. Associations with death, FEV1 decline and baseline FEV1 among individuals with usual cough and phlegm also meeting criteria for Fletcher-Peto definition of chronic bronchitis
Discussion
In this analysis of data from the Lung Health Study, we have shown that, though neither presence of cough alone nor phlegm alone is strongly associated with risk of death, the presence of both symptoms together is associated with a substantial risk for death when compared to individuals with neither symptom, independent of known risk factors for death in COPD such as lung function and smoking status. Further, we have shown that when comparing those individuals with cough and phlegm to those without these symptoms, those with cough and phlegm more commonly die from noncancerous respiratory causes. Finally, we have shown that individuals with cough and phlegm have significantly lower lung function than those without such symptoms, but not more accelerated loss of lung function.
Our first finding is that cough and phlegm together is associated with a 27% increase in the hazard of death compared to lack of these symptoms, independent of other well-known risk factors for death in a population with COPD. Putting this in perspective, we show that this increase in risk for mortality is even stronger than that attributed to dyspnea, measured by the MMRC score, which has been shown to be associated with mortality risk and included in well-known prediction models for mortality in COPD (Citation33,34). Previous studies showing associations between symptoms of cough and phlegm with mortality (Citation6–8) were performed in different populations, including the elderly (Citation7), and those with more severe COPD (Citation8). Our study population differed from these previous studies in that it included relatively young individuals with early COPD as well as at-risk smokers (whose FEV1/FVC ratio increased to above 0.70 after bronchodilator administration), followed over a relatively long period of time. Our study adds to findings of previous studies in that we have shown that after isolating specific symptoms of cough alone and phlegm alone, only the combination of cough and phlegm appears to appreciably impact mortality in COPD. Thus, using information about the presence of these symptoms ultimately could be added to enhance existing models that aim to predict risk for long term mortality in COPD.
Our second finding was that noncancerous respiratory causes of death were more common among those with cough and phlegm compared to those with cough alone, phlegm alone or neither symptom. Notably, at baseline assessment, our data show a substantially higher prevalence of lower respiratory tract infections in the past year for the group with cough with phlegm compared to the other groups. Chronic cough and sputum production is thought to be related not only to inflammation in the airways and chronic bronchitis but also to bronchiectasis and bacterial colonization of the airways, which has been thought to affect up to 30% of individuals with COPD (Citation35–37).
Previous studies have shown that COPD patients with cough and phlegm are at a greater risk for exacerbations (Citation4,5). Although a study of the ECLIPSE cohort showed a higher risk for exacerbations in those with cough, but not those with phlegm or chronic bronchitis (Citation38), our study clarifies these potentially conflicting results in showing the specific combination of symptoms, usual cough and usual phlegm together (but not either symptom alone nor the traditional, but unvalidated Fletcher-Peto definition of chronic bronchitis), that is associated with poor outcomes in COPD. Our study extends the findings of previous work to show that these patients are also at increased risk for death from respiratory causes, presumably lower respiratory infections, suggesting a possible mechanism for the increased mortality in the subgroup with cough and phlegm.
These data also show that individuals with both cough and phlegm have a significantly lower FEV1 at entry into the cohort, but not an accelerated rate of FEV1 decline. This finding differs from prior studies. One cross-sectional study showed no difference between lung function in those with and without chronic bronchitis symptoms (Citation4). Other studies have shown an association between the presence of cough, phlegm or dyspnea with FEV1 decline (Citation20,21), particularly in those with mild COPD(Citation20). It is possible that the high rate of smoking cessation achieved during the aggressive smoking cessation program of LHS may have attenuated lung function decline over follow-up for individuals reporting chronic productive cough at baseline.
Further, it is also possible that 5 years of follow-up is an insufficient amount of time in order to detect a difference in lung function decline in this study, as the previous studies have assessed these symptoms over longer periods of follow-up. In the Lung Health Study, spirometry data were available on participants for the first 5 years and then again measured in most participants at year 10–11. For this analysis, we only analyzed spirometric data over the first five years because of concerns that including the incomplete 10-year datapoint would introduce bias. However, sensitivity analyses including 10-year data showed little difference from those results we have obtained using only the first five years.
Further, we have shown that cough alone is prevalent in COPD; however it does not confer the same risk for death as well as increased airflow obstruction as chronic productive cough. It is possible that this heightened risk could be related to airways inflammation which is more likely to result in a cough that is productive of phlegm (Citation39). This phenotype is in contrast to that of individuals with cough alone, who could potentially have less inflammation but more airway reactivity or upper airway inflammation due to common causes such as reflux disease or postnasal drip. The group with phlegm only is more difficult to explain as one may find it hard to understand how phlegm is produced in the absence of a cough. It is possible that these individuals have less severe cough, and view their cough as a means for producing phlegm, which predominates their clinical picture.
Better understanding of the different clinical phenotypes that these groups represent may help to elucidate their differences in risks for death and low lung function. Ultimately, these findings can help clinicians understand which subset of the population is at higher risk for death and worse airflow obstruction based upon the presence of symptoms which are easily assessed in the clinical setting.
Of note, we found that the Fletcher-Peto definition of Chronic Bronchitis was not additionally predictive of mortality or lung function compared to questions about usual cough and usual phlegm. Though asking patients about usual cough and usual phlegm isolates a subgroup of individuals with chronic productive cough, it appears that this definition is different than one which includes the time constraints of having these symptoms for at least 3 months for more than 1 year, as in the case of the Fletcher-Peto definition. We did not find that the group of individuals with Fletcher-Peto chronic cough had worse mortality or lung function, possibly because this group comprised a small number of individuals and power was less to detect differences. However, its also possible that asking about cough and phlegm with simpler questions (“Do you usually have…”) is more predictive than the more complicated questions included in the Fletcher-Peto definition.
Our study is limited by the small number of non-Caucasian individuals limiting the generalizability of our findings. Further, the exclusion of individuals with significant comorbidities at enrollment does also limit the generalizability of our findings. However, because of the long follow-up, many individuals developed significant comorbidities while being followed, somewhat attenuating this limitation. It is also possible that we were unable to detect significant differences in outcomes for groups with cough alone and phlegm alone due to the small number of deaths in these groups given that overall this cohort was made up of relatively healthy individuals. We were limited by the lack of an accurate assessment of exacerbations in the full cohort to allow for a rigorous evaluation of how symptoms impact this important outcome. Finally, we are also limited by the lack of longitudinal data on respiratory symptoms, which could have helped us to understand whether improvement of symptoms would be associated with a change in risk for death or change in lung function.
Conclusions
Respiratory symptoms such as cough and phlegm are common in individuals with COPD. We have shown that not only do individuals with cough and phlegm have worse baseline lung function, they also have a higher risk of death, and disproportionately die due to noncancerous respiratory causes.
Given the recent focus upon identifying clinical phenotypes at risk for adverse outcomes in COPD, our findings suggest that information about common respiratory symptoms in COPD can be used to help identify a subgroup of individuals with chronic productive cough who are at higher risk of death and have worse lung function. Future efforts should attempt to elucidate the mechanism for these adverse outcomes in the subgroup with chronic productive cough, and such a mechanism is likely related to a heightened risk for respiratory complications such as lower respiratory tract infections. Ultimately, understanding this subgroup will be important as we look to study the value of therapeutic strategies in the various subphenotypes of COPD.
Declaration of Interest Statement
NP is supported by the institutional training grant funded by NHLBI (T32HL007534). MBD is supported by a K23 award from NHLBI. JEC reports grants funded by NHLBI. PDS reports fees paid to the institution on his behalf for consultancy by private entities, grants to the institution on his behalf paid by NIH and private entities, and royalties paid by Lippincott Williams & Wilkins. DPT reports consultancy fees paid to him by private entities, grants to the institution on his behalf paid by NIH and private entities, and payment for lectures paid to him by private entities. NNH reports grants funded by NIH. RAW reports money paid to him for consultancy by private entities, grants paid to the institution on his behalf by private entities, and stock options paid to him by a private entity.
NP takes responsibility for the content of this manuscript, including the data and analysis. She was also responsible for manuscript concept, data analysis, drafting of the manuscript and revisions. MBD was responsible for manuscript concept, data analysis, and revisions for intellectual content. NNH was responsible for study design, data analysis and revisions for intellectual content. JEC, PDS, DPT and RAW were responsible for data collection, manuscript concept, study design, data analysis and revisions for intellectual content. All authors approved the final manuscript.
References
- Miravitlles M. Cough and sputum production as risk factors for poor outcomes in patients with COPD. Respir Med 2011; 105(8):1118–1128.
- Lundbäck B, Lindberg A, Lindström M, Rönmark E, Jonsson AC, Jönsson E, Larsson LG, Andersson S, Sandström T, Larsson K. Obstructive Lung Disease in Northern Sweden Studies.: Not 15 but 50% of smokers develop COPD?—Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med 2003; 97(2):115–122.
- Voll-Aanerud M, Eagan TM, Plana E, Omenaas ER, Bakke PS, Svanes C, Siroux V, Pin I, Antó JM, Leynaert B. Respiratory symptoms in adults are related to impaired quality of life, regardless of asthma and COPD: results from the European community respiratory health survey. Health Qual Life Outcomes 2010; 8:107.
- Kim V, Han MK, Vance GB, Make BJ, Newell JD, Hokanson JE, Hersh CP, Stinson D, Silverman EK, Criner GJ. COPDGene Investigators: The chronic bronchitic phenotype of COPD: an analysis of the COPDGene study. Chest 2011; 140(3):626–633.
- Burgel PR, Nesme-Meyer P, Chanez P, Caillaud D, Carré P, Perez T, Roche N. Initiatives Bronchopneumopathie Chronique Obstructive Scientific Committee: Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest 2009; 135(4):975–982.
- Vollmer WM, McCamant LE, Johnson LR, Buist AS. Respiratory symptoms, lung function, and mortality in a screening center cohort. Am J Epidemiol 1989; 129(6):1157–1169.
- Hewitt J, Smeeth L, Bulpitt CJ, Tulloch AJ, Fletcher AE. Respiratory symptoms in older people and their association with mortality. Thorax 2005; 60(4):331–334.
- Prescott E, Lange P, Vestbo J. Chronic mucus hypersecretion in COPD and death from pulmonary infection. Eur Respir J 1995; 8(8):1333–1338.
- Lange P, Nyboe J, Appleyard M, Jensen G, Schnohr P. Relation of ventilatory impairment and of chronic mucus hypersecretion to mortality from obstructive lung disease and from all causes. Thorax 1990; 45(8):579–585.
- Hogg JC, Chu FS, Tan WC, Sin DD, Patel SA, Pare PD, Martinez FJ, Rogers RM, Make BJ, Criner GJ, Cherniack RM, Sharafkhaneh A, Luketich JD, Coxson HO, Elliott WM, Sciurba FC. Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med 2007; 176(5):454–459.
- Speizer FE, Fay ME, Dockery DW, Ferris BG Jr. Chronic obstructive pulmonary disease mortality in six US cities. Am Rev Respir Dis 1989; 140:S49–S55.
- Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax 2003; 58:388–393.
- Fletcher C, Peto R, Tinker C, Speizer FE. The natural history of chronic bronchitis and emphysema. New York/Toronto: Oxford University Press; 1976.
- Tockman MS, Comstock GW. Respiratory risk factors and mortality: longitudinal studies in Washington County, Maryland. Am Rev Respir Dis 1989; 140:S56–S63.
- Pelkonen M, Notkola IL, Nissinen A, Tukiainen H, Koskela H. Thirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: a follow-up in middle-aged rural men. Chest 2006; 130(4):1129–1137.
- Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187(3):228–237.
- Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med 1996; 153(5):1530–1535.
- Guerra S, Sherrill DL, Verker C, Ceccato CM, Halonen M, Martinez FD. Chronic bronchitis before age 50 years predicts incident airflow limitation and mortality risk. Thorax 2009; 64:894–900.
- Sherman CB, Xu X, Speizer FE, Ferris BG Jr, Weiss ST, Dockery DW. Longitudinal lung function decline in subjects with respiratory symptoms. Am Rev Respir Dis 1992; 146(4):855–859.
- Bridevaux PO, Gerbase MW, Probst-Hensch NM, Schindler C, Gaspoz JM, Rochat T. Long-term decline in lung function, utilisation of care and quality of life in modified GOLD stage 1 COPD. Thorax 2008; 63(9):768–774.
- de Marco R, Accordini S, Anto JM, Gislason T, Heinrich J, Janson C, : Long-term outcomes in mild/moderate chronic obstructive pulmonary disease in the European community respiratory health survey. Am J Respir Crit Care Med 2009; 180:956–963.
- Anthonisen NR. Lung Health Study. Am Rev Respir Dis 1989; 140:871–872.
- Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, Conway WA, Jr., Enright PL, Kanner RE, O'Hara P et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 1994, 272:1497–1505.
- Connett JE, Kusek JW, Bailey WC, O'Hara P, Wu M. Design of the Lung Health Study: a randomized clinical trial of early intervention for chronic obstructive pulmonary disease. Control Clin Trials 1993; 14:3S–19S.
- Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med 2005; 142:233–239.
- Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med 2002; 166:675–679.
- Fletcher CM, Peto R, Tinker C. A comparison of the assessment of simple bronchitis (chronic mucus hypersecretion) by measurements of sputum volume and by standardized questions on phlegm production. Int J Epidemiol 1974 Dec; 3(4):315–319.
- Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54:581–586.
- Enright PL, Johnson LR, Connett JE, Voelker H, Buist AS. Spirometry in the Lung Health Study. 1. Methods and quality control. Am Rev Respir Dis 1991; 143:1215–1223.
- Drummond MB, Hansel NN, Connett JE, Scanlon PD, Tashkin DP, Wise RA. Spirometric predictors of lung function decline and mortality in early chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 185(12):1301–1306.
- StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP, 2011.
- Buist AS, Connett JE, Miller RD, Kanner RE, Owens GR, Voelker HT. Chronic Obstructive Pulmonary Disease Early Intervention Trial (Lung Health Study). Baseline characteristics of randomized participants. Chest 1993; 103(6):1863–1872.
- Puhan MA, Garcia-Aymerich J, Frey M, ter Riet G, Antó JM, Agustí AG, Gómez FP, Rodríguez-Roisín R, Moons KG, Kessels AG, Held U. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet 2009 Aug; 374(9691):704–711.
- Celli B, Cote C, Marin J, Casanova C, Montes de Oca M, Mendez R, Pinto-Plata V, Cabral H. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Eng J Med 2004; 350:1005–1012.
- O'Brien C, Guest PJ, Hill SL, Stockley RA. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax 2000; 55:635–642.
- Patel IS, Vlahos I, Wilkinson TM, Lloyd-Owen SJ, Donaldson GC, Wilks M, Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 170:400–407.
- Smith IE, Jurriaans E, Diederich S, Ali N, Shneerson JM, Flower CD. Chronic sputum production: correlations between clinical features and findings on high resolution computed tomographic scanning of the chest. Thorax 1996; 51:914–918.
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, Calverley P, Rennard S, Wouters EF, Wedzicha JA. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363(12):1128–1138.
- Stănescu D, Sanna A, Veriter C, Kostianev S, Calcagni PG, Fabbri LM, Maestrelli P. Airways obstruction, chronic expectoration, and rapid decline of FEV1 in smokers are associated with increased levels of sputum neutrophils. Thorax 1996; 51(3):267–271.