Abstract
Background: Despite the use of anatomic resection, the post-surgical recurrence rate remains high in early-stage non-small cell lung cancer (NSCLC). Chronic inflammation plays a role in the mechanism that promotes tumor initiation. This study aimed to investigate the association between recurrence outcome and chronic inflammation-related co-morbidities in early-stage resected NSCLC. Methods: A review of medical records for recurrence outcome and co-morbidities, in terms of chronic obstructive pulmonary disease (COPD), DM, asthma and cardiovascular diseases, was performed with 181 patients with stage I NSCLC that underwent anatomic resection. Results: Subjects with T descriptors as T2a disease (49.5 vs. 28.0%, p < 0.05) and the presence of COPD (42.4 vs. 20.7%, p < 0.01) had a higher risk of tumor recurrence. Univariate analysis for recurrence-free survival showed T descriptor as T2a (21.5 months vs. NR, p < 0.05) and the presence of COPD (20.5 months vs. NR, p < 0.01) as significant factors predicting reduced survival. The presence of COPD (HR: 1.98; 95% CI, 1.29–.02, p < 0.01) and T descriptor as T2a (HR: 2.01; 95% CI, 1.04–3.91, p < 0.05) remain independent predictors of reduced recurrence-free survival in the Cox regression model. Patients with COPD were at higher risk of brain recurrence (OR: 7.88; 95% CI, 1.50–41.3, p < 0.01). In contrast, patients without COPD showed a tendency toward recurrence in bone and liver (OR: 4.13; 95% CI, 1.08–15.8, p = 0.05). Conclusion: Subjects with COPD and T2a disease had a higher risk of recurrence. The role of COPD as a recurrence promoter merits further prospective investigation.
Keywords :
Introduction
Although anatomic resection is the foundation of treatment for early-stage non-small cell lung cancer (NSCLC), the post-surgical recurrence rate remains high, at around 40% (Citation1). A considerable number of factors have been investigated in an attempt to make up for the deficiency of the standard TNM staging system, with the hope of differentiating more aggressive phenotypes in order to optimize the decision making of post-surgical adjuvant chemo- or radiotherapy.
Several pathological factors, such as lymphatic vascular invasion, histology grade and type of histology, have been associated with recurrence in stage I NSCLC that underwent anatomic resection (Citation2–5). Apart from pathological factors, some studies have highlighted the implication of patient factors related to underlying co-morbidity. Diabetes mellitus (DM) and cardiovascular diseases have been reported to place patients undergoing curative resection at higher risk of local recurrence and reduced survival (Citation6, 7). In addition, patients receiving curative surgery for their NSCLC who have co-existing chronic obstructive pulmonary disease (COPD) have worse survival than their counterparts with a better pulmonary function (Citation8).
Although the underlying mechanism by which these co-morbidities drive tumor recurrence remains elusive, they share a common pathophysiological feature in chronic inflammation. A substantial number of studies have shown that a host environment with sustained inflammation nurtures the initiation and progression of tumor cells (Citation9–11). COPD, as one of the chronic morbidities featuring excessive circulating inflammatory mediators such as interleukine (IL)-6, tumor necrosis factor (TNF)-α, IL-8 and activated leukocytes, well characterizes chronic systemic inflammation (Citation12–14).
A link between COPD and lung cancer has been reported in several large-scale independent studies, revealing an increased risk of lung cancer when the severity of COPD is increased, in terms of the percentage of predicted forced expiratory volume in 1 second (FEV1) (Citation15–17). The application of semi-quantitative scoring via computed tomography (CT)-based assessment of emphysema further strengthens the relationship between the severity of air-space destruction and the incidence of lung cancer (Citation15, Citation18, Citation19). Several hypotheses point toward the genotoxic stress derived from matrix-degrading enzymes as a result of chronic inflammation that drives tumor initiation, (Citation20, 21); however, the mechanism remains poorly understood.
Although a considerable number of studies have favored chronic inflammation as a driving force of lung cancer, little is known about whether it plays a part in tumor recurrence when lung cancer has been curatively resected. Furthermore, certain pro-inflammatory mediators involving ranges of chemokines and growth factors have been shown to dictate the acquisition of the organ-specific metastatic property of tumors (Citation22, 23). Whether chronic inflammation is associated with a pattern of recurrence in surgically treated NSCLC is also uncertain.
In this study, a cohort of patients with early-stage NSCLC that had undergone anatomic resection was included in an attempt to determine factors that favor tumor recurrence. The primary aim was to determine the association between recurrence outcome and the chronic inflammation-related co-morbidities. In addition, the pattern of recurrence and the survival outcome were also analyzed.
Materials and methods
Inclusion of the study population
The medical records of patients with the diagnosis of NSCLC who had undergone thoracotomy in Chang Gung Memorial Hospital between January 2001 and January 2005 were retrospectively reviewed. Patients who underwent anatomic resection which included a full mediastinal and hilar lymph nodes dissection with all level sampled on the ipsilateral side were pathologically staged, and those who were found to have stage I disease (T1a N0, T1b N0, and T2a N0) as defined by the AJCC 7th edition were included in this study. Patients with a postoperative follow-up period of less than three months, and those with tumor with a sarcomatoid or neuroendocrine histology were excluded from analysis. Other features that prompted elimination from the study included AJCC T2b, T3, T4, N1, N2, N3 diseases, absence of a tumor-free surgical margin and metastatic disease. This study was approved by the institutional review board of Chang Gung Memorial Hospital.
Determination of co-morbidities
The patients’ chronic inflammation-related co-morbidities, including COPD, DM, asthma and cardiovascular disease, were reviewed using the medical records. The global initiative for chronic obstructive lung disease (GOLD) definition, including a history of chronic symptoms (cough, sputum production or dyspnea) or a history of exposure to tobacco or noxious gas, combined with FEV1/FVC <70% without full reversibility to bronchodilator, was used for COPD assessment. Diagnosis of DM was via one of the following criteria: hemoglobin A1C ^ 6.5%, fasting plasma glucose value ^126 mg/dL, or random plasma glucose value ^ 200 mg/dL with associated symptoms of hyperglycemia (thirst, polyuria, weight loss or blurred vision). Asthma assessment was based on the global initiative for asthma (GINA) definition: a history of wheezing, cough or chest tightness combined with an increase in FEV1 > 12% and 200 mL after administration of a bronchodilator. Patients with cardiovascular diseases were defined as having a documented history of coronary heart disease (i.e., myocardial infarction or angina pectoris) or cerebrovascular disease (i.e., stroke or transient ischemic attack).
Determination of recurrence
Post-surgical follow-up was evaluated routinely by the physician's clinical assessment via imaging studies and invasive procedures using CT scans and bronchoscopy. When the patient reported a neurological symptom, a brain CT or magnetic resonance imaging (MRI) exam was arranged for evaluation of the central nervous system. CT scan of the abdomen, bone scan or positron emission tomography scan was performed based on the patient's report of symptoms and the physician's clinical assessment. Surgical biopsy, CT or bronchoscopy-guided biopsy was performed when tumor recurrence was equivocal in the imaging study. Only the first recurrence event was scored in the analysis.
Local recurrence (LR) was considered as having recurrent tumor at surgical stump, staple line, ipsilateral hilum, and/or mediastinum. Recurrence sites other than LR; including the supraclavicular fossa, were considered as distant failure (DF). In addition, the number of DF events evaluated in an organ-based manner was also recorded. Patients presenting with concurrent LR and DF as their first event were scored as DF due to the predominance of a clinically worse outcome. Second primary (SP) lung cancer was determined when a new lesion presented histology different from the index tumor or the same histology with a clinical presentation most consistent with new primary lung cancer. All recurrence events, including SP lesions, were reviewed by two chest specialists to assure the consistency.
Statistical analysis
Recurrence-free survival was calculated from the time of surgical treatment to the time of the first event of LR, DF or SP. Overall survival was calculated from time of surgical treatment to the time of death from all causes. All group variables were analyzed by Fisher's exact test with contingency tables; while time-to-event variables were estimated by Kaplan–Meier analysis with the log-rank test used for analyzing the difference between curves. All reported p values were two-sided and a p value < 0.05 was considered statistically significant.
Results
Baseline characteristics of study subjects
One hundred eighty-one patients who had undergone anatomic resection and were found to have stage I disease were included (). The mean age was 63.9 years, and 120 (66.3%) of the patients were male. Twenty-six (14.4%) patients were stage IA and 155 (85.6%) were stage IB; 114 (63%) patients had adenocarcinoma, 59 (32.6%) had squamous cell carcinoma and 8 (4.4%) had NSCLC not otherwise specified (NSCLC-NOS). A history of smoking was noted in 98 (54.1%) patients, and 171 (94.5%) underwent lobectomy as the surgical treatment. A review of co-morbidities showed COPD in 59 (32.6%) patients, DM in 48 (26.5%), asthma in 17 (9.4%) and cardiovascular diseases in 32 (17.7%) patients. Of the subgroup of patients who had COPD, 38 (64.4%) had GOLD grade 1 and 21 (35.6%) had GOLD grade 2.
Table 1. Baseline patients characteristics (N = 181)
Factors associated with tumor recurrence
Tumor recurrence was noted in 99 (54.7%) patients (). Compared to the patients without recurrence, those with tumor recurrence showed significantly higher T descriptors as T2a disease (49.5% vs. 28.0%, p < 0.05) and a greater presence of COPD (42.4% vs. 20.7%, p < 0.01). Other predictive factors, including age, gender, pathological subtype, smoking status, type of surgical treatment and co-morbidities other than COPD, were not found to have a remarkable association with tumor recurrence.
Table 2. Factors associated with recurrence after anatomic resection of tumor
Predictive factors of recurrence-free survival
The median time to recurrence with respect to each predictive variable was analyzed (). In univariate analysis, factors significantly associated with reduced freedom from recurrence were T descriptor as T2a (21.5 months vs. NR, p < 0.05) and presence of COPD (20.5 months vs. NR, p < 0.01). The Kaplan–Meier curve of recurrence-free survival demonstrated a higher risk of recurrence in T2a disease (HR: 1.94; 95% CI, 1.03–3.65, p < 0.05, ) and in patients with presence of COPD (HR: 2.11; 95% CI, 1.41–3.15, p < 0.01, ). The identification of independent predictors using the Cox regression model showed that the presence of COPD (HR: 1.98; 95% CI, 1.29–3.02, p < 0.01) and T descriptor as T2a (HR: 2.01; 95% CI, 1.04∼3.91, p < 0.05) remained independent predictors of reduced freedom from recurrence ().
Figure 1. Kaplan–Meier curve of recurrence-free survival showing (A) T2a versus T1a and T1b disease and (B) presence versus absence of COPD. Patients with T2a disease (HR: 1.94; 95% CI, 1.03–3.65, p < 0.05) and COPD (HR: 2.11; 95% CI, 1.41–3.15, p < 0.01) had a higher risk of recurrence.
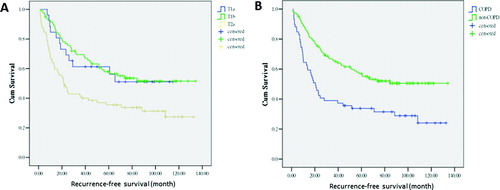
Table 3. Predicting factors of recurrence-free survival by univariate analysis
Table 4. Cox regression model of factors associated with recurrence-free survival
Relationship of COPD and pattern of recurrence
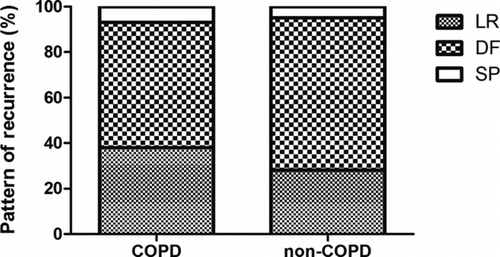
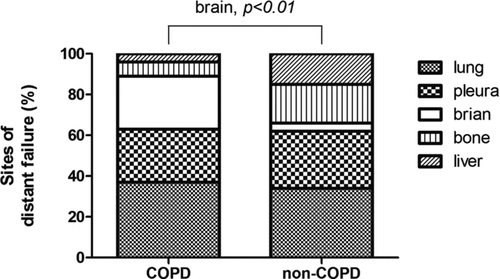
The pattern of tumor recurrence among the 42 (42.4%) patients that had a presence of COPD and the 57 (57.6%) that had an absence of COPD was analyzed. The pattern of recurrence in terms of LR, DF and SP was 38%, 55% and 7% in patients with COPD, compared to 28%, 67% and 5% (p = 0.22) in those without COPD. The stacked bar in shows the distribution of the pattern of recurrence. Sixty-one patients experienced DF, and 74 events of organ involvement, including the lung, pleura, brain, bone and liver, were documented. An organ-based evaluation of the risks of DF in patients with COPD versus non-COPD showed that patients with COPD had a higher risk of brain recurrence (OR: 7.88; 95% CI, 1.50–41.3, p < 0.01) (). In contrast, patients without COPD showed a tendency toward bone and liver recurrence (OR: 4.13; 95% CI, 1.08–15.8, p = 0.05).
Outcome of overall survival
Median overall survival was reduced with the presence of COPD (39.0 vs. 125.4 months, p < 0.01). The Cox regression model revealed the hazard ratios (HRs) were (HR: 1.88; 95% CI, 1.20–2.94, p = 0.006) for the presence of COPD, (HR: 1.84; 95% CI, 0.92–3.69, p = 0.08) for T descriptor as T2a and (HR: 2.94; 95% CI, 1.21–7.17, p = 0.02) for pathology as NSCLC-NOS.
Discussion
This study showed that in stage I NSCLC patients undergoing curative resection, those with T2a disease remained at a higher risk of tumor recurrence. This was a finding in accordance with earlier reports; however, we further demonstrated a risk of recurrence as prominent as T descriptors: i.e., patients who had COPD. As a whole, both T2a disease and COPD were independently associated with reduced recurrence-free survival.
Although curative surgical resection offers a potential cure for stage I NSCLC, the reported 5-year survival rate of 55–80% reflects the unmet need in clinical practice of improving long-term survival (Citation24–26). However, it is still uncertain as to whether the efficacy of adjuvant chemotherapy as demonstrated in large-scale randomized trials involving stage I disease can support the unmet requirement (Citation27, 28). Tumor size greater than 4 cm, therefore, has been suggested as a possible indication for adjuvant chemotherapy in this situation (Citation28).
This seeming lack of efficacy might because the factor that truly benefits from adjuvant therapy remains poorly identified. In the present study, we showed that COPD is a recurrence-promoting factor that might confound efficacy in the setting of adjuvant therapy. Therefore, this co-morbidity merits consideration as a variable that needs to be balanced in later randomized studies. Apart from T descriptor and COPD, the association between histology of NSCLC-NOS and survival outcome is marginally significant for recurrence-free survival, but significant for overall survival. This finding recapitulates the previous report which involved 19,702 stage I resected NSCLC showing the poor histologic differentiation is significantly associated with increased mortality risk (Citation4).
On the other hand, the mechanistic explanation for the development of recurrence foci in early-stage resected tumor remains poorly understood. T descriptor, as a part of the TNM system, might be a pathological feature that reflects the capability to incubate tumor niches within host organs when the tumor burden increases (Citation23, Citation29), and thereafter, could develop despite removal of the primary tumor (Citation30, 31). In addition, minimal residual disease that remains after curative resection; in the form of circulating tumor cells (CTCs), is also prominent for tumor recurrence (Citation32).
The increased number of CTCs in different clinical situation have been associated with the increased tumor burden (Citation33) and the higher risk of post-surgical relapse (Citation34). In the present study, the role of T descriptor as being closely associated with the development of recurrence was confirmed. However, the result also highlights the role of COPD as a promoter independent from T descriptor. This suggests that COPD plays a prominent role in promoting the process from tumor niches and CTCs to clinically detectable recurrence foci.
Previous studies on the development of tumor metastasis have shown that various inflammatory cytokines and growth factors are the keys regarding the step from pre-metastatic niche formation and CTCs engraftment to tumor proliferation (Citation23, Citation29, Citation35). This theory of metastasis has merit because it gives insight into the importance of tumor-derived inflammatory mediators, and shows that it is as prominent as CTCs or tumor stem cells.
However, this does not appropriately explain the cause of recurrence in a tumor-free situation in resected early-stage disease. Nevertheless, the chronic and sustained inflammatory condition in COPD provides an appropriate substitute host environment to support the progression of tumor niches after the primary tumor has been removed. Hence, our results serve to piece together some of the puzzles of tumor recurrence in early-stage resected NSCLC.
Furthermore, the analysis in this study shows the pattern of recurrence is alike between COPD and non-COPD subjects, in terms of LR, DF and SP. However, a DF with a significant preference for the brain in COPD patients and a tendency toward the liver and bone in non-COPD patients was found. The organ-specific metastatic property of tumors has been reported in several studies (Citation22, 23). Kaplan et al. showed that the acquisition of this property could be attributed to different profiles of tumor-derived chemokine (Citation23). However, the median overall survival was 39 months in the COPD cohort undergoing tumor resection, which is as short as the historical reports (around 40 months) of COPD with flow limitation in GOLD grade 4 (Citation36, 37). This suggests a detailed investigation of post-surgical follow-up for lung condition in GOLD grade 1 and 2 subjects who receive anatomic resection for NSCLC.
The limitation of this study is the inherent bias due to its retrospective nature that the identification of each co-morbidity is not as standardized as that in a prospective backdrop. This limitation also possibly contributes to the inconsistency of the co-morbidity's effect on recurrence across different studies. In addition, the profile of chronic inflammatory cytokines with regard to the presence or absence of COPD was not available in the study, which also restricts a deeper analysis. In conclusion, patients with early-stage NSCLC who have undergone anatomic resection and have the features of COPD and T2a disease have a higher risk of recurrence. The role of COPD as a recurrence promoter in this study merits further prospective investigation.
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jemal A, Siegel R, Xu J, Cancer statistics, 2010. CA Cancer J Clin, 2010; 60: 277–300.
- Hung JJ, Jeng WJ, Hsu WH, Predictors of death, local recurrence, and distant metastasis in completely resected pathological stage-I non-small-cell lung cancer. J Thorac Oncol 2012; 7:1115–1123.
- Ost D, Goldberg J, Rolnitzky L, , Survival after surgery in stage IA and IB non-small cell lung cancer. Am J Respir Crit Care Med 2008; 177:516–523.
- Ou SH, Zell JA, Ziogas A, Prognostic factors for survival of stage I nonsmall cell lung cancer patients: a population-based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer 2007; 110:1532–1541.
- Sawyer TE, Bonner JA, Gould PM, , Patients with stage I non-small cell lung carcinoma at postoperative risk for local recurrence, distant metastasis, and death: implications related to the design of clinical trials. Int J Radiat Oncol Biol Phys 1999; 45:315–321.
- Varlotto JM, Recht A, Flickinger JC, Factors associated with local and distant recurrence and survival in patients with resected nonsmall cell lung cancer. Cancer 2009; 115:1059–1069.
- Varlotto JM, Recht A, Flickinger JC, Varying recurrence rates and risk factors associated with different definitions of local recurrence in patients with surgically resected, stage I nonsmall cell lung cancer. Cancer 2010; 116:2390–2400.
- Nakajima T, Sekine Y, Yamada Y, Long-term surgical outcome in patients with lung cancer and coexisting severe COPD. Thorac Cardiovasc Surg 2009; 57:339–342.
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001; 357:539–545.
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21:309–322.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646–674.
- Gan WQ, Man SF, Senthilselvan A, Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004; 59:574–580.
- Lee KY, Ho SC, Chan SY, Reduced nuclear factor-kappaB repressing factor: a link toward systemic inflammation in COPD. Eur Respir J 2012; 40:863–873.
- Walter RE, Wilk JB, Larson MG, Systemic inflammation and COPD: The Framingham Heart Study. Chest 2008; 133:19–25.
- de Torres JP, Bastarrika G, Wisnivesky JP, Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest 2007; 132:1932–1938.
- Maldonado F, Bartholmai BJ, Swensen SJ, , Are airflow obstruction and radiographic evidence of emphysema risk factors for lung cancer? A nested case-control study using quantitative emphysema analysis. Chest 2010; 138: 1295–1302.
- Wilson DO, Weissfeld JL, Balkan A, , Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med 2008; 178:738–744.
- Li Y, Swensen SJ, Karabekmez LG, Effect of emphysema on lung cancer risk in smokers: a computed tomography-based assessment. Cancer Prev Res (Phila) 2011; 4:43–50.
- Zulueta JJ, Wisnivesky JP, Henschke CI, Emphysema scores predict death from COPD and lung cancer. Chest 2012; 141:1216–1223.
- Haqqani, A.S., J.K. Sandhu, and H.C. Birnboim, Expression of interleukin-8 promotes neutrophil infiltration and genetic instability in mutatect tumors. Neoplasia 2000; 2:561–568.
- Takahashi H, Ogata H, Nishigaki R, , Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell 2010; 17:89–97.
- Mauri FA, Pinato DJ, Trivedi P, Isogeneic comparison of primary and metastatic lung cancer identifies CX3CR1 as a molecular determinant of site-specific metastatic diffusion. Oncol Rep 2012; 28:647–653.
- Kaplan, R.N., R.D. Riba, S. Zacharoulis, , VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005; 438:820–827.
- Egermann U, Jaeggi K, Habicht JM, Regular follow-up after curative resection of nonsmall cell lung cancer: a real benefit for patients? Eur Respir J 2002; 19:464–468.
- Mollberg NM, Ferguson NM. Postoperative surveillance for non-small cell lung cancer resected with curative intent: developing a patient-centered approach. Ann Thorac Surg 2013; 95:1112–1121.
- Rubins J, Unger M, Colice GL. Follow-up and surveillance of the lung cancer patient following curative intent therapy: ACCP evidence-based clinical practice guideline (2nd edition). Chest 2007; 132:355S–367S.
- Butts CA, Ding K, Seymour L, Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol 2010; 28:29–34.
- Strauss GM, Herndon JE 2nd, Maddaus MA, Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008; 26:5043–5051.
- Hiratsuka S, Watanabe A, Aburatani H, Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol 2006; 8:1369–1375.
- Demicheli R, Fornili M, Ambrogi F, Recurrence dynamics for non-small-cell lung cancer: effect of surgery on the development of metastases. J Thorac Oncol 2012; 7:723–730.
- Demicheli R, Retsky MW, Hrushesky WJ, Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: learning from failures. Nat Clin Pract Oncol 2007; 4:699–710.
- Mocellin S, Keilholz U, Rossi CR, Circulating tumor cells: the ‘leukemic phase’ of solid cancers. Trends Mol Med 2006; 12:130–139.
- Tanaka F, Yoneda K, Kondo N, Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res 2009; 15:6980–6986.
- Rolle A, Gunzel R, Pachmann U, Increase in number of circulating disseminated epithelial cells after surgery for non-small cell lung cancer monitored by MAINTRAC(R) is a predictor for relapse: A preliminary report. World J Surg Oncol 2005; 3:18.
- Hiratsuka S, Nakamura K, Iwai S, MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2002; 2:289–300.
- Celli BR, Cote CG, Marin JM, The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350:1005–1012.
- Martinez FJ, Foster G, Curtis JL, Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 2006; 173:1326–1334.