Abstract
Background. COPD is the fourth leading cause of death in the world. In the case of exacerbations or persistent symptoms, regular treatment with long-acting bronchodilators is recommended to control the symptoms, reduce exacerbations and improve health status. Objectives. To describe patterns of drug utilization among patients diagnosed with COPD, to measure continuity with long-acting bronchodilators, to identify determinants of not receiving long-acting therapy continuously. Methods. We identified a cohort of patients discharged from hospital with diagnosis of COPD between 2006 and 2008. Patients were observed for a two-year follow-up period, starting from the day of discharge. Follow-up was segmented in six-month periods, in order to dynamically evaluate prescription patterns of Long-Acting Beta-Agonists (LABA), tiotropium, and inhaled corticosteroids. Patients with prescriptions for LABA and/or tiotropium in each of the six-month periods were defined as “continuously treated with long-acting bronchodilators.” The degree of drug treatment coverage was measured through the Medication Possession Ratio (MPR). Logistic regression was performed to identify determinants of not receiving long-acting bronchodilators continuously. Results. A total of 11,452 patients diagnosed with COPD were enrolled. Only 34.8% received long-acting bronchodilators continuously. The MPR was greater than 75% in 19.6% of cases. Among the determinants of not receiving long-acting bronchodilators continuously, older age and co-morbidities played an important role. Conclusions. In clinical practice, the COPD pharmacotherapy is not consistent with clinical guidelines. Medical education is needed to disseminate evidence-based prescribing patterns for COPD, and to raise awareness among physicians and patients on the health benefits of an appropriate pharmacological treatment.
Background
Chronic Obstructive Pulmonary Disease (COPD) is the fourth-leading cause of death in the world. By 2020, COPD is expected to become the third-leading cause of death, accounting for 5 million deaths per year, and the fifth-leading causing of disability worldwide (Citation1).
The care of patients with COPD has changed radically over the past two decades and novel therapies can improve the health status of patients (Citation2). In the case of moderate to severe COPD, exacerbations or persistent symptoms, regular treatment with long-acting bronchodilators (long-acting beta-agonists or tiotropium) is recommended to control the symptoms, to reduce the occurrence of acute exacerbations and to improve the quality of life (Citation3, 4). Symptomatic patients with severe COPD who experience repeated exacerbations are recommended to add inhaled corticosteroids (ICS) to their long-acting bronchodilator treatment (Citation5).
With regard to ICS used as a mono-component, a number of large randomized controlled trials have suggested that ICS monotherapy may not modify the natural history of COPD (Citation6–8). While some small improvements in FEV1 (Forced Expiratory Volume in One Second) have been reported, ICS monotherapy appears to have no significant effect on long-term decline in FEV1 in patients with mild to moderately severe COPD. Moreover, a recent systematic review on the efficacy and safety of inhaled steroids used as a mono-component, showed that ICS monotherapy increases the risk of local side effects, such as oropharyngeal candidiasis and hoarseness (Citation9). Therefore, no clinical guidelines recommend the use of ICS alone.
Non-adherence to treatment reduces the clinical benefit of therapy and can account for many of the observed differences between efficacy and effectiveness of the drug treatment (Citation10). The average adherence rates in clinical trials have been estimated to be around 70–90% among patients diagnosed with COPD (Citation11–13). However, the observed rates in clinical practice range between 20–60% (Citation14–16). In patients with COPD, drug discontinuation may increase the frequency of exacerbations, the number of hospitalizations (Citation17–19), and ultimately result in increased health care costs (Citation20). However, COPD undertreatment is not entirely due to the patient's behavior. In fact, survey results suggest that clinicians may have major gaps in their knowledge of COPD management. Several studies demonstrated that general practitioner's adherence to clinical guidelines is low and COPD is often misdiagnosed or treated inappropriately (Citation21–23).
Despite compliance to guidelines and drug adherence play a central role in the treatment of COPD, limited information exists on the patterns of use of COPD therapy in routine care.
Objectives
To describe patterns of drug utilization among patients diagnosed with COPD, to measure continuity and adherence to long-acting bronchodilators therapy (long-acting beta-agonists or tiotropium), and to identify determinants of not receiving long-acting bronchodilators continuously.
Materials and Methods
Data sources
The study subjects were recruited from individuals registered with the Local Health Units (LHU) of Lazio region in Italy (about Citation5 million residents), who were eligible over the study period. The LHU is a body delegated by the National Health System to provide health care to the residents of a specific geographic area. Drug information was collected from the regional Drug Claims Register, which was described in detail elsewhere (Citation24). This Register contains information on the drugs reimbursable by the National Health Service which are dispended to patients. The data available on each prescription include the patient's identification number, the prescribing physician's number, the Anatomical-Therapeutic-Chemical (ATC) code of the drug purchased, the number of packs, the number of units per pack, the dosage, the unit cost per pack and the prescription date. The drugs under study are equally available for all residents, in accordance with the universal health-care insurance coverage.
Using a unique and anonymous subject identifier, drug information was cross-linked to records of hospitalization (Hospital Information System), subject's socio-demographic characteristics and records of emergency visits (Healthcare Emergency Information System), in order to create a chronological, clinical, healthcare-related patient profile. Any date of death was derived from the Mortality Information System.
Study population
Using the Hospital Information System, we identified a cohort of patients discharged from the hospital with a recorded diagnosis of COPD between January 1st 2006 and December 31th 2008. Specific criteria for cohort inclusion were main diagnosis of COPD (ICD-9-CM codes: 490, 491, 492, 494, 496) or main diagnosis of COPD-related causes along with a secondary diagnosis of COPD. COPD-related causes included respiratory failure (ICD-9-CM: 518.81–518.84), dyspnea and other respiratory distress (786.0), cough (786.2) and abnormal sputum (786.4). The first hospitalization fulfilling the selection criteria during the enrollment period was considered as the index admission. Recorded diagnoses in the Hospital Information System had been validated in a previous evaluation of clinical records (Citation25). About 94% of the reviewed cases were confirmed as being COPD cases.
Exclusion criteria
Patients not alive at discharge, aged less than 45 years, with secondary diagnosis of major trauma (ICD-9-CM: 484–487) or major surgeries (surgical diagnosis-related group, excluding 482 and 483) during the index admission were excluded. Patients who died during the follow-up period were excluded from the primary analysis, to guarantee an equal follow-up time for measuring persistence and continuity with therapy.
Patient characteristics
Patients were characterized according to socio-demographic factors (age, gender, educational level and area of residence), COPD severity, concomitant respiratory diseases, previous use of respiratory drugs, previous use of oral corticosteroids and antibacterials, previous use of non-respiratory drugs and co-morbidities.
The following conditions were considered as a proxy of COPD severity and assessed in the 12 months preceding the index admission: hospitalizations for COPD, diagnosis of respiratory failure (ICD-9-CM: 518.81–518.84), invasive respiratory procedures (ICD-9-CM: 31.1, 31.2, 96.7, V44.0, see Appendix for details), staying in intensive care unit during a COPD hospitalization, emergency visits for COPD and use of oxygen.
The following concomitant respiratory diseases were assessed during the 24 months preceding the cohort entry including the index admission: asthma (ICD-9-CM: 493), chronic respiratory disease other than COPD (ICD-9-CM: 135, 495, 500–505, 515–517, 519, 508.1, 518.1–518.3), pulmonary infections (ICD-9-CM: 011, 480–487.0, 510, 511, 513) and acute pulmonary symptoms (ICD-9-CM: 512, 415, 786.0, 518.0). We also identified other co-morbidities including diabetes, hypertension, ischemic heart disease, heart failure, other chronic heart diseases, arrhythmia, cerebrovascular diseases, peripheral vascular diseases, obesity-dyslipidemia, liver disease, chronic digestive disease (excluding liver), chronic renal diseases, neurological and muscle disease, anemia and coagulation disorders, thyroid disease, depression, psychiatric disease, peptic ulcer and gastroesophageal reflux disease, rheumatologic diseases and connective tissue disease, HIV and disorders of the immune system, cancer (a detailed description of diagnoses and the associated ICD-9-CM codes is reported in Appendix).
Prevalent users of long-acting bronchodilators were defined as at least 1 prescription of long-acting beta-agonists (ATC codes: R03AC12, R03AC13, R03AC18) or tiotropium (ATC: R03BB04) filled during the 12 months preceding the index admission. Similarly, previous use of oral corticosteroids and antibacterials were defined as at least 1 prescription in the 12 months preceding the index admission (ATC: H02AB and J01, respectively). In the same period, we used number of distinct non-respiratory drugs as a crude measure of concomitant polytherapy.
Medications with the first five digits of the ATC code equal were considered as a group (Citation26); respiratory drugs (ATC: R03) as well as oral corticosteroids and antibacterials were excluded from the computation. In order to evaluate the overall use of drugs related to cardiovascular diseases, cardiovascular drugs were defined as at least 1 prescription of cardiac therapies (ATC: C01), anti-diabetic drugs (A10), antiplatelets (B01AC04, B01AC05, B01AC06), antihypertensives (C02, C03, C07, C08, C09) or statins (C10AA) in the 12 months before the index admission.
Follow-up period
Patients were observed for a 2-year follow-up period, starting from the day of discharge of the index admission. Follow-up was segmented in six-month periods, in order to dynamically evaluate prescription patterns of Long-Acting Beta-Agonists (LABA - ATC codes: R03AC12, R03AC13, R03AC18), tiotropium (ATC: R03BB04), ICS (ATC: R03BA, R03AK04 excluding fixed combination salbutamol/ipratropium) and the fixed combination LABA/ICS (ATC codes: R03AK06, R03AK07).
The drug-use patterns
Patients were classified into five mutually exclusive groups ().
Figure 1. Patient classification according to use of the study drugs during the follow-up period: the drug-use patterns.
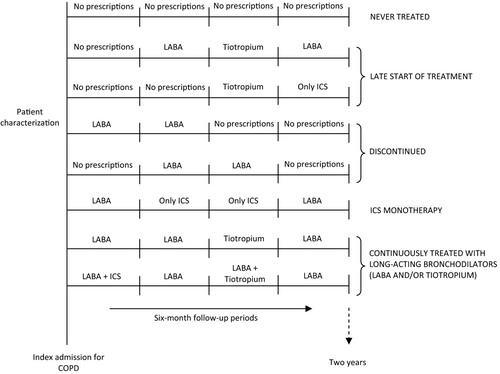
never treated: patients who never filled any study drug prescription during the follow-up period;
late start of treatment: use of any study drug began more than 6 months after discharge;
discontinued: treatment with any study drug was discontinued for at least 6 months;
ICS monotherapy: patients filling the study drugs in each of the six-month periods, who were treated for at least 6 months with ICS in monotherapy without any prescription of long-acting bronchodilators;
continuously treated with long-acting bronchodilators: patients with prescriptions for LABA and/or tiotropium in each of the six-month periods. For each six-month period, treatment was defined as at least one prescription of any study drug. In a sensitivity analysis, continuously treated patients were defined by using the same criteria but considering three-month time-windows.
The adherence measurement
A deepening was performed on adherence to treatment, in order to evaluate the degree of drug treatment coverage over the 2-year follow-up period. Adherence to long-acting treatment (LABA and/or tiotropium) was measured through the Medication Possession Ratio (MPR), calculated as the number of days of medication supplied during the study period on the basis of the Defined Daily Doses (DDD) (Citation27) divided by the number of calendar days in the study period. Patients were defined as adherent when 75% or more of their individual follow-up was covered by treatment (MPR ≥ 75%). The non-adherent patients were further classified into the following classes: “MPR < 50%”; “50% ≤ MPR< 75%”. For each patient, periods spent in hospital or rehabilitation facilities (inpatient regimen) were excluded in this computation, as during these periods drugs are dispensed by the facility and not retrievable through our databases. The MPR calculation does not require that all patients have an equal follow-up time, therefore in a secondary analysis, adherence to long-acting therapy was also measured for those patients who died during the follow-up period, in order to provide a more representative description of adherence patterns in routine care settings.
The trend over time
Percentages of patients still on long-acting bronchodilators treatment (LABA and/or tiotropium) during the follow-up were reported by six-month periods. The analysis was performed in three different groups of patients with COPD: the entire study cohort; patients who were prescribed with long-acting bronchodilators in the 12 months preceding the index admission (prevalent users); patients without filled prescriptions for long-acting bronchodilators in the 12 months preceding the index admission (potentially, new users). Moreover, percentages of patients receiving ICS in different combination therapies (with or without LABA or tiotropium) were calculated separately for each of the six-month follow-up periods.
Statistical Analysis
Continuous variables are presented as mean values ± standard deviation (SD). Multivariate logistic regression was performed to identify determinants of not receiving long-acting bronchodilators continuously (drug-use patterns from 1 to 4). The model included age, gender, educational level, proxies of COPD severity, concomitant respiratory diseases, previous use of oral corticosteroids, previous use of antibacterials and patient's co-morbidities. All estimates were adjusted for area of residence. Model accuracy was measured by the area under the Receiver Operating Characteristic (ROC) curve (Citation28). All analyses were performed using SAS Statistical Software Package version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
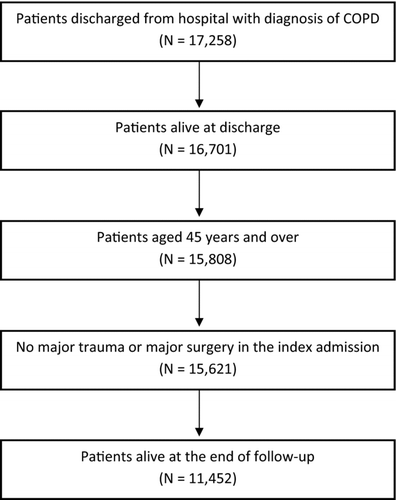
From the initial number of 17,258 patients discharged from hospital with a diagnosis of COPD, 11,452 patients were enrolled in the cohort (). Then, 55.3% of patients were males and the mean age was 73.6 ± 9.6 years. 32.7% patients received long-acting bronchodilators (LABA and/or tiotropium) in the 12 months preceding the index admission. Hypertension (53.0%), diabetes (24.2%), arrhythmia (17.0%), heart failure (15.6%) and obesity (14.4%) were the most common co-morbidities. The majority of patients (83.8%) were treated with cardiovascular drugs. The analysis on concomitant use of non-respiratory drugs showed that more than 25.9% of patients were taking 5 to 7 drugs before the index admission, 22.1% of patients 8 to 10 drugs and 21.6% were taking more than 10 drugs.
shows the patterns of filled prescriptions by patients with COPD during the 6 months immediately following the index admission. Then, 27.2% of the patients did not take any study medication. 23.9% were treated with the fixed combination LABA/ICS with the addition of tiotropium, 18.5% received only the fixed combination LABA/ICS and 15.2% of subjects filled prescription for ICS without any long-acting bronchodilators. Overall, in the first six-month period of observation, 57.6% of patients were treated with long-acting bronchodilators. Almost 29% received short-acting beta-agonists and about 26.3% had recorded utilization of oxygen canisters.
Table 1. Pharmacological treatment in the 6 months immediately after hospital discharge
shows the cross-tabulation of sociodemographic factors, proxies of COPD severity, concomitant respiratory diseases and previous pharmacological treatments by drug-use pattern. In the 2 years after hospital discharge, 15.6% of patients did not receive any study drug, 6.4% started treatment late, 31.1% discontinued treatment with any study drug for at least 6 months and 12.1% received, for at least 6 months, ICS monotherapy without any prescription of long-acting bronchodilators. A total of 34.8% of patients filled prescriptions for LABA and/or tiotropium in each of the six-month periods of follow-up. If we had applied the same criteria using three-month time-windows, this proportion would be decreased to 18.3%.
Table 2. Patient characteristics by drug-use patterns
A sub-analysis of the 1,782 patients who have not received any study drug, showed that they were treated with alternative therapies, such as systemic corticosteroids (43.8%), xanthine derivatives (26.6%), short-acting anticholinergic drugs (15.8%), short-acting beta-agonists (12.0%) and oxygen (8.9%).
shows the trend of patients who are still on long-acting bronchodilators treatment (LABA and/or tiotropium) during the four six-month periods of follow-up. For each of the six-month follow-up periods, the first bar refers to all patients diagnosed with COPD, the second bar refers to those patients who were prescribed with long-acting bronchodilators in the 12 months preceding the index admission (prevalent users) whereas the third bar refers to patients without filled prescriptions for long-acting bronchodilators in the 12 months preceding the index admission (potentially, new users).
Figure 3. Patients who are still on long-acting bronchodilators treatment (LABA and/or tiotropium) at each of the six-month follow-up period.
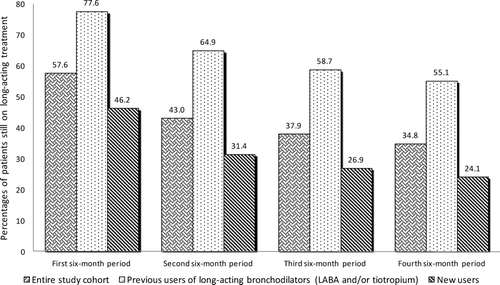
With regard to the entire COPD cohort, the proportion of subjects still on treatment with long-acting bronchodilators, which in the first six-month period amounted to 57.6%, dropped to 43.0% in the second six-month period, further down to 37.9% in the third six-month period and to 34.8% in the last six-month period. When analysing prevalent users and new users the trend had a similar shape but, with regard to prevalent users, the proportions of patients still on treatment always were substantially higher (from 77.6% in the first six-month period to 55.1% in the last six-month period) than those calculated on the whole cohort of patients while, with regard to new users, the proportions always were lower (from 46.2% in the first six-month period to 24.1% in the last six-month period).
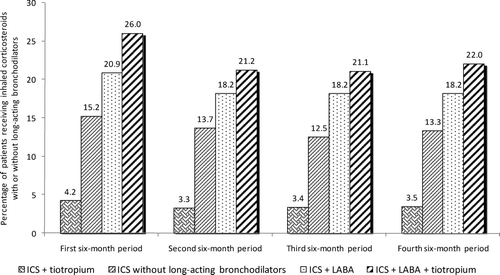
shows the percentages of patients receiving ICS with or without long-acting bronchodilators (LABA and/or tiotropium) at each of the six-month follow-up period. In each portion of the follow-up, the triple combination was the most prescribed, followed by ICS plus LABA, ICS without long-acting bronchodilators and ICS plus tiotropium. For all regimens, there was a decrease of prescriptions moving from the first six-month period to the second one. From the second six-month period onwards, drug use prevalences remained fairly stable.
Figure 4. Patients receiving inhaled corticosteroids (ICS) with or without long-acting bronchodilators (LABA and/or tiotropium) at each of the six-month follow-up period.
Adherence to long-acting treatment was also analyzed in terms of treatment coverage over the 2-years follow-up period. In subjects classified as “continuously treated with long-acting bronchodilators” (3,987 patients), the MPR was lower than 50% in 32.6% of cases; between 50% and 75% in 14.3% of cases; greater than 75% in 53.1% of cases. Overall, 2,114 pa tients out of 11,452 (18.5%) was found to be both continuously treated and adherent to long-acting therapy.
When analyzing adherence to long-acting treatment for the entire cohort of enrolled subjects (11,452 patients), the MPR was lower than 50% in 73.3% of cases; between 50% and 75% in 7.1% of cases; greater than 75% in 19.6% of cases. The secondary analysis of adherence to long-acting treatment in subjects who had been excluded from the main analysis because they died during the observation period (4,169 patients), showed that the MPR was lower than 50% in 78.9% of cases; between 50% and 75% in 5.0% of cases; greater than 75% in 16.1% of cases.
The logistic regression model showed that the probability of not receiving long-acting bronchodilators continuously was strongly influenced by patient's characteristics. The factors included in the model can be grouped in four categories: socio-demographic features, proxies of COPD severity, concomitant respiratory diseases and co-morbidities. With regard to socio-demographic factors, female gender was associated with an increased risk of not receiving treatment continuously (Odds Ratio “OR” = 1.55, p < 0.001); the effect of age was not linear: with respect to the reference category (age less than or equal to 59 years), the probability of not receiving long-acting therapy significantly (p < 0.001) decreased in the age classes (60–74) and (75–84) years (OR = 0.65 and 0.71, respectively) and substantially increased in the older age group (age greater than or equal to 85 years, OR = 1.47, p = 0.001); the effect of the educational level was not significant. With regard to proxies of COPD severity, diagnosis of respiratory failure (OR = 0.62, p < 0.001), previous emergency visits for COPD (OR = 0.63, p < 0.001) and use of oxygen (OR = 0.77, p < 0.001) were associated with a lower risk of not receiving treatment continuously. Among the concomitant respiratory diseases, asthma strongly reduced the probability of not receiving treatment continuously (OR = 0.52, p < 0.001). Finally, with regard to co-morbidities, diabetes (OR = 1.36), ischemic heart disease (OR = 1.17), heart failure (OR = 1.19), arrhythmia (OR = 1.22), cerebrovascular disease (OR = 1.26), obesity (OR = 1.20), liver disease (OR = 1.26), chronic renal diseases (OR = 1.20), neurological diseases (OR = 1.61), psychiatric diseases (OR = 1.62) and rheumatologic diseases (OR = 2.06) were significantly (p ≤ 0.05) associated with a higher risk of not receiving long-acting bronchodilators continuously. A similar effect was observed for cancer (OR = 1.17), on borderline statistical significance (p = 0.067). The area under the ROC curve was 0.746.
Discussion
In a study of 11,452 patients, we found that after a hospital discharge for COPD, only 34.8% of patients received long-acting bronchodilators continuously in the following two years. This pattern is immediately evident in the first 6 months after hospitalization with only 57.6% of patients filling prescriptions for long-acting bronchodilators (LABA or tiotropium). Over the 2-year observation period, this proportion further decreased for both prevalent and new users. The most critical moment in which the probability of interrupting respiratory treatments is higher was between the first and second six-month period, when the acute symptoms related to the hospitalization for COPD were distant and attenuated. Overall, 12.1% of patients received, for at least 6 months, ICS monotherapy without any prescription of long-acting bronchodilators.
Furthermore, among the patients who had not received any study drug of interest (long-acting bronchodilators or ICS), we found that “second-line” therapies, such as systemic corticosteroids, xanthine derivatives, and short-acting anticholinergic drugs were frequently used. When we restricted the analysis to the 3,987 patients who were classified as “continuously treated with long-acting bronchodilators”, we observed that only 2,114 patients were also adherent to the treatment. Thus, with respect to the entire cohort of patients diagnosed with COPD, only 18.5% of subjects were both continuously treated and adherent to long-acting agents.
Finally, among the determinants of not receiving long-acting bronchodilators continuously we found that older age (greater than or equal to 85 years) and concomitant co-morbidities, such as diabetes, heart failure, arrhythmia, cerebrovascular disease, rheumatologic diseases and psychiatric diseases, played an important role. A hypothesis for this latter finding may be related to the cumbersomeness of therapy, which increases with age and number of co-morbidities. The longer and more complex is the list of drugs prescribed, the lower is the adherence of the patient (Citation29). Clinicians may also be less likely to prescribe chronic COPD pharmacotherapy to older patients who may have psychiatric conditions and difficulty in using inhalers.
The present study shows a misuse of respiratory medications in patients with COPD and an evident under-utilization of long-acting bronchodilators. However, our data do not allow quantifying how much of this “deviation from guidelines” is attributable to the patient behavior, to the therapeutic approach recommended at hospital discharge, to specialist advice after discharge or to indications from the general practitioner.
A study conducted in Switzerland demonstrated that general practitioner's adherence to GOLD guideline is low and that COPD is often misdiagnosed or treated inappropriately (Citation21). Survey results suggest that practitioners may have major gaps in their knowledge of COPD management. Rutschmann et al. showed at least 50% of clinicians reported to be unaware of guidelines for COPD diagnosis and treatment (Citation22). Similarly, a study conducted on a random sample of US primary care physicians showed that, although 55% of practitioners were aware of major COPD guidelines, only 25% used them to guide decision-making (Citation23).
The commitment of the patient in adhering to treatment is also an important factor to consider. Among the strategies to reinforce this commitment, the education of the patient on relevant aspects of the disease, including repeated instructions on how to use inhalation devices, may increase the adherence to therapeutic regimens (Citation30).
It is worth noting that the frequencies of used respiratory medications in the present study are somewhat different than the published results in other large COPD cohorts. However, it may be difficult to compare drug use patterns among different studies. In fact, prescription patterns may vary according to the patients’ inclusion criteria, the period in which the study was carried out and the methods used to define and measure drug use.
In our study, we found that 48.5% of patients received long-acting beta-agonists, 66.3% received inhaled corticosteroids and 35.7% of patients received tiotropium in the first 6 months after COPD hospitalization. In a comparable cohort of patients identified using hospital discharge diagnoses (Citation31), we found that the corresponding percentages were 33.8%, 52.0% and 27.1%, respectively. When comparing with the ECLIPSE study (Citation32), in our cohort we found lower drug use proportions. However, in the ECLIPSE study recruitment criteria were very different and medication use was self-reported.
There are some study limitations to be considered. First, data on lung function that would allow for a spirometric staging of the disease were not available. However, we can assume that patients hospitalized for an acute exacerbation of COPD are probably affected by moderate or more severe stages of the disease. In this regard, recorded diagnoses in the Hospital Information System had been validated in a previous re-abstract study: the majority (94%) of reviewed cases were confirmed as being cases of COPD (Citation25). Second, if analyzing only data from patients discharged from hospital can be considered a strength of the study since it increases the reliability of the COPD diagnosis, on the other hand it also restricts the generalizability of results.
Moreover, the results come from a single region in Italy and may be not generalizable to other geographic areas. However, our findings are in line with results of other studies carried out in different healthcare settings (i.e. in the general population setting) and in other parts of Italy (Citation33). Third, our pharmaceutical database does not contain information on the prescribed daily doses and adherence to drug treatment was estimated on the basis of the DDDs. Although this is a useful instrument for comparing the results from different studies (Citation27), misclassification of drug utilization may have occurred. Finally, to allow for equal follow-up time in measuring persistence and continuity with therapy, we excluded patients who died during the observation period.
By excluding patients with a less favorable clinical situation, this criterion may have introduced a bias in the estimated proportion of adherent patients. For this reason, a deepening was carried out on excluded patients using the MPR calculation, which does not require an equal follow-up time per subject. When compared with patients included in the study, those excluded were less adherent to treatment (MPR ≥ 75%: 16.1% versus 19.6%). Therefore, the very low proportions of adherent patients that we found in this study may be slightly overestimated.
Conclusions
In clinical practice, the COPD pharmacotherapy is not consistent with clinical guidelines. Continuous medical education is needed to disseminate evidence-based prescribing patterns for COPD, and to raise awareness among physicians and patients on the health benefits of an appropriate pharmacological treatment.
Declaration of Interest Statement
This work was partially funded by the Italian Agency of Medicines; research project code: FARM8ZBT93. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997; 349(9064):1498–1504.
- Hanania NA. The impact of inhaled corticosteroid and long-acting beta-agonist combination therapy on outcomes in COPD. Pulm Pharmacol Ther 2008; 21:540–550.
- O'Donnell DE, Aaron S, Bourbeau J, Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease - 2007 update. Can Respir J 2007; 14(Suppl B):5B–32B.
- Rabe KF, Hurd S, Anzueto A, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176(6):532–555.
- Vestbo J, Hurd SS, Agustí AG, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187(4):347–65.
- Pauwels RA, Lofdahl CG, Laitinen LA, Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society study on chronic obstructive pulmonary disease. N Engl J Med 1999; 340:1948–1953.
- Vestbo J, Sorensen T, Lange P, Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 1999; 353:1819–1823.
- Burge PS, Calverley PM, Jones PW, Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. Br Med J 2000; 320:1297–1303.
- Yang IA, Clarke MS, Sim EH, Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012 Jul; 7:CD002991.
- Revicki DA, Frank L. Pharmacoeconomic evaluation in the real world: effectiveness versus efficacy studies. Pharmacoeconomics 1999; 15:423–434.
- Rand CS, Nides M, Cowles MK, Long-term metered-dose inhaler adherence in a clinical trial. The Lung Health Study Research Group. Am J Respir Crit Care Med 1995; 152:580–588.
- Van Grunsven PM, van Schayck CP, van Deuveren M, Compliance during long-term treatment with fluticasone propionate in subjects with early signs of asthma or chronic obstructive pulmonary disease (COPD): results of the Detection, Intervention, and Monitoring Program of COPD and Asthma (DIMCA) Study. J Asthma 2000; 37:225–234.
- Kesten S, Flanders J, Serby CW, Compliance with tiotropium, a once daily dry powder inhaled bronchodilator, in one year COPD trials. Chest 2000; 118:191S–192S.
- Krigsman K, Nilsson JL, Ring L. Refill adherence for patients with asthma and COPD: comparison of a pharmacy record database with manually collected repeat prescriptions. Pharmacoepidemiol Drug Saf 2007; 16:441–448.
- Dolce JJ, Crisp C, Manzella B, Medication adherence patterns in chronic obstructive pulmonary disease. Chest 1991; 99:837–841.
- Bosley CM, Parry DT, Cochrane GM. Patient compliance with inhaled medication. Does combining beta agonists with corticosteroids improve compliance? Eur Respir J 1994; 7:504–509.
- Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax 2008; 63:831–838.
- Regueiro CR, Hamel MB, Davis RB, A comparison of generalist and pulmonologist care for patients hospitalized with severe chronic obstructive pulmonary disease: resource intensity, hospital costs, and survival. Am J Med 1998; 105:366–372.
- Osthoff M, Leuppi JD. Management of chronic obstructive pulmonary disease patients after hospitalization for acute exacerbation. Respiration 2010; 79:255–261.
- World Health Organization. Adherence to Long-Term Therapies: Evidence for Action. Geneva, World Health Organization, 2003; pp 1–45.
- Jochmann A, Neubauer F, Miedinger D, General practitioner's adherence to the COPD GOLD guidelines: baseline data of the Swiss COPD Cohort Study. Swiss Med Wkly 2010; 140:w13053:1–8; online version: www.smw.ch.
- Rutschmann OT, Janssens JP, Vermeulen B, Knowledge of guidelines for the management of COPD: a survey of primary care physicians. Respir Med 2004; 98(10):932–937.
- Foster JA, Yawn BP, Maziar A, Enhancing COPD management in primary care settings. Med Gen Med 2007; 9(3):24.
- Kirchmayer U, Agabiti N, Belleudi V, Socio-demographic differences in adherence to evidence-based drug therapy after hospital discharge from acute myocardial infarction: a population-based cohort study in Rome, Italy. J Clin Pharm Ther 2012; 37(1):37–44.
- Fano V, D'Ovidio M, Del Zio K, The role of the quality of Hospital Discharge Records on the comparative evaluation of outcomes: the example of Chronic Obstructive Pulmonary Disease. Epidemiol Prev 2012; 36(3–4):172–179.
- Schneeweiss S, Seeger JD, Maclure M, Performance of co-morbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol 2001;154(9):854–864.
- World Health Organization Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC Classification and DDD Assignment. Oslo, 2012.
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982; 143(1):29–36.
- Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother 2004; 38(2):303–312.
- Takemura M, Mitsui K, Itotani R, Relationships between repeated instruction on inhalation therapy, medication adherence, and health status in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2011; 6:97–104.
- de Luise C, Lanes SF, Jacobsen J, Cardiovascular and respiratory hospitalizations and mortality among users of tiotropium in Denmark. Eur J Epidemiol 2007; 22(4):267–72.
- Hurst JR, Vestbo J, Anzueto A, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363(12):1128–1138.
- Simoni M, Carrozzi L, Baldacci S, Respiratory symptoms/diseases, impaired lung function, and drug use in two Italian general population samples. Respir Med 2008; 102(1):82–91.