Abstract
Recent trends in prescriptions for medicines used to treat chronic obstructive pulmonary disease (COPD) in the United States have received little attention. Our objective was to examine trends in prescribing practices for medications used to treat COPD. We examined data from surveys of national samples of office visits to non-federal employed office-based physicians in the United States by patients aged ≥40 years with COPD recorded by the National Ambulatory Medical Care Survey from 1999 to 2010. From three diagnostic codes, office visits by patients with COPD were identified. Prescribed medications were identified from up to 8 recorded medications. The percentage of these visits during which a prescription for any medication used to treat COPD was issued increased from 27.0% in 1999 to 49.1% in 2010 (p trend < 0.001). Strong increases were noted for short-acting beta-2 agonists (17.6% in 1999 to 24.7% in 2010; p trend < 0.001), long-acting beta-2 agonists as single agents or combination products (6.2% in 1999 to 28.3% in 2010; p trend < 0.001), inhaled corticosteroids as single agents or combination products (10.9% in 1999 to 30.9% in 2010; p trend < 0.001), and tiotropium (3.8% in 2004 to 17.2% in 2010; p trend < 0.001). Since 1999, prescription patterns for medicines used to treat COPD have changed profoundly in the United States.
Keywords :
Introduction
Chronic obstructive pulmonary disease (COPD) continues to be a substantial source of morbidity and mortality in the United States (Citation1, 2). This disease, which is largely caused by noxious pulmonary irritants, is often progressive in nature and is characterized by a largely irreversible airway obstruction and an inflammatory component. Although the clinical course is variable, many patients experience a progressive loss of pulmonary function that may be a consequence of continued exposure to harmful agents as well as exacerbations of their condition. As the disease progresses, some patients may experience hypoxia necessitating the use of oxygen. Consequently, patients with COPD experience impaired health-related quality of life, excess outpatient visits, visits to the emergency room, and hospitalizations. The ultimate toll of this disease manifests itself in an elevated mortality rate.
Several decades ago, treatment options for COPD were limited and consisted chiefly of agents that led to the short-term alleviation of symptoms. Over time, however, the therapeutic arsenal at the disposition of physicians has broadened considerably especially with the advent of longer-acting inhaled beta-adrenergic agents, anticholinergic agents, and corticosteroids. Clinical trials have generated evidence of the usefulness of these longer-term medications, which have generally provided superior treatment of symptoms, reduced exacerbations, reduced mortality, and possibly reduced the loss of lung function (Citation3–7). As a result of these developments, professional societies and international organizations have developed and periodically adapted treatment guidelines for COPD (Citation8–11).
In light of this evolution in treatment paradigms for COPD, an understanding of how treatment practices by the medical community in the United States continue to evolve is valuable in gauging the impact of treatment guidelines and generating insights as to whether efforts to speed the translation of clinical guidelines into real world practice are possibly needed. Yet, little such information is available. To examine recent patterns in the pharmacologic treatment of patients with COPD, we examined trends in prescribed medications used to treat COPD among adults in the United States from 1999 to 2010 using an annual national survey of office visits implemented by the Centers for Disease Control and Prevention (CDC).
Methods
Our analyses were conducted using data from the National Ambulatory Medical Care Survey (NAMCS) from 1999 to 2010. Using a multistage sampling design, NAMCS is intended to provide national estimates about the use of ambulatory medical care services in the United States. For each year, a national probability sample of nonfederally employed office-based physicians, and, since 2006, of physicians working in Community Health Centers was selected. Master files of the American Medical Association (AMA) or the American Osteopathic Association (AOA) were used to construct the sampling frame for nonfederally employed office-based physicians, whereas information from the Health Resources Administration and the Indian Health Service was used to construct the sampling frame for physicians working in Community Health Centers. The various stages of selection included primary sampling units (PSUs) (counties, county equivalents (such as parishes and independent cities), towns, townships, minor civil divisions), physician practices within PSUs, and patient visits within practices. For that final stage of selection, physicians selected a systematic random sample of patient visits during an assigned week. Data collection is performed by physicians, their staff, and field representatives of the U.S. Census Bureau. An important consideration about NAMCS is that each data record on the data set contains information about the patient-physician encounter or visit. Thus, NAMCS yields estimates about patient-physician encounters or visits rather than patients per se as the former constitutes the basic sampling unit of the survey. To generate national estimates of the use of ambulatory medical care services, sampling weights were constructed based on the following steps: inflation of reciprocals by sampling probabilities, adjustment for nonresponse, ratio adjustment, and weight smoothing. Detailed information about the survey can be found elsewhere (Citation12).
Using three data fields that contained International Classification of Diseases (ICD)-9 codes for the physician's diagnosis, we defined COPD if any of the fields contained one of the following ICD-9 codes: 490, 491, 492, and 496. The surveys contained 6 data fields for recording medications from 1999 to 2002 and 8 such fields from 2003 to 2010. Using the classification system of drugs by entry name developed by the National Center for Health Statistics, we identified medications used in the treatment of COPD and created several groups of medications (). We counted two combinations of medications (albuterol and atropine; albuterol and cromolyn) that were prescribed infrequently as albuterol. For some medication entries for the above corticosteroids that did not list a brand name, we counted the steroid when one of the three reasons for the office visit listed one or more of the following: shortness of breath; labored or difficult breathing (dyspnea); wheezing; breathing problems; cough; excessive sputum; bronchitis; emphysema; or other respiratory diseases (includes COPD). We did not include codes that indicated various nonspecific codes for bronchodilator, inhaler, nebulizer, beta agonist, metered dose inhaler, aerosol therapy, and asthma medication.
Table 1. Medications used in the treatment of chronic obstructive pulmonary disease and groupings of medications used in this study's analyses
We examined trends in prescriptions by presence or absence of respiratory symptoms that were included on one of the three data fields listing reasons for the office visit and included shortness of breath, labored or difficult breathing (dyspnea), wheezing, breathing problems, cough, and excessive sputum. To improve the stability of the estimates for these analyses, we grouped survey years into 4-year blocks: 1999–2002, 2003–2006, and 2007–2010.
We limited our analyses to patients who were aged ≥40 years. Because the physician-patient encounter or visit constitutes the unit of analyses, our estimates represent the percentage of visits made by patients for whom a diagnostic code for COPD was recorded and who were prescribed the medications of interest among all physician-patient encounters or visits that listed a diagnostic code for COPD. Linear trends in the percentages of these office visits during which patients were prescribed medications used to treat COPD were tested by using orthogonal polynomial contrasts. Then, t-tests were used to perform two sample tests of significance. Sampling weights were used to generate percentages. The statistical software programs SUDAAN (Release Citation11.0.0) and SAS (version Citation9.Citation3) were used to conduct the analyses.
Results
From 1999 to 2010, the raw number of office visits by patients with a diagnosis of COPD on the data files ranged from 277 to 456 representing an estimated annual number of visits in the United States ranging from 11,867,000 to 21,358,000 after weighting. Mean age increased significantly over this time, whereas the percentages of visits for COPD by men and whites showed no significant linear trends ().
Table 2. Demographic characteristics of patients with chronic obstructive pulmonary disease aged ≥40 years, National Ambulatory Care Medical Survey 1999–2010
Overall trends
Short-acting agents were commonly prescribed medications to patients with COPD, and the percentage of office visits during which one of these agents was prescribed increased strongly from 23.8% in 1999 to 31.9% in 2010 ( p linear trend ???? 0.001) (, ). Short-acting beta-2 agonists (SABA) were the most commonly prescribed short-acting medication, and the percentage of office visits by patients with COPD that were characterized by a prescription for a SABA increased significantly from 17.6% in 1999 to 24.7% in 2010 (linear trend ???? 0.001). The combination of a SABA and ipratropium was the next most commonly prescribed medication. From 1999 to 2004, the percentage of patients being prescribed such combination products increased to 10.6%, then dropped off to 8.9% by 2005, and remained relatively unchanged though 2010. Generally, fewer than 6% of patients received prescriptions for ipratropium (except in 2004) and methylxanthines. From 2001 on, prescriptions for ipratropium ( p ???????? 0.008) and methylxanthines ( p ???????? 0.001) were generally in a downward trend.
Figure 1. Unadjusted percentage (95% standard error) of patients with chronic obstructive pulmonary disease aged ≥40 years who were prescribed major groups of medications used to treat chronic obstructive pulmonary disease, by year, National Ambulatory Medical Care Survey 1999–2010.
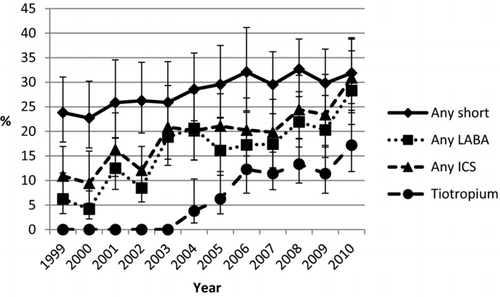
Table 3. Percent (standard error) of office visits of patients with chronic obstructive pulmonary disease aged ≥ 40 years who were prescribed a medication used to treat chronic obstructive pulmonary disease, by year, National Ambulatory Care Medical Survey 1999–2010
We examined the Food and Drug Administration's online drug database for year of approval of medications used in this analysis to provide temporal context for the trends in medications, particularly long-acting medications. For the major classes, the agents that were first approved included metaproterenol in 1974 (SABA), salmeterol in 1994 (LABA), ipratropium in 1986 (anticholinergics), aminophylline in 1940 (methylxanthines), beclomethasone in 1986 (ICS), albuterol/ipratropium in 1996 (SABA plus anticholinergic), and salmeterol/fluticasone in 2000 (LABA plus ICS). Prescriptions for long-acting agents increased strongly during the study period: the percentage of office visits during which a prescription for such an agent was issued more than tripled from 11.8% in 1999 to 37.2% in 2010 ( p linear trend ???? 0.001) (, ).
Combination products were the most commonly prescribed medication followed by tiotropium. Both classes of medications showed strong increases since they were approved by the Food and Drug Administration (salmeterol plus fluticasone in 2000; tiotropium in 2004) ( p linear trend ????0.001 for both). The percentage of office visits with a diagnosis of COPD during which a combination product containing a long-acting beta-2 agonists (LABA) and an inhaled corticosteroid (ICS) were prescribed increased from 6.2% in 2001 to 26.7% in 2010 ( p linear trend ????0.001), Furthermore, the -percentage of office visits with a diagnosis of COPD during which tiotropium was prescribed increased from 6.3% in 2005 to 17.2% in 2010 ( p linear trend ???? 0.001). In contrast, prescriptions for LABAs as single agents and ICS as single agents decreased significantly during the same period ( p linear trend ???? 0.001 for both).
Excluding patient visits listing asthma (ICD-9 code 493) on one of the three diagnosis fields had little effect on the results ().
Table 4. Percent (standard error) of office visits of patients with chronic obstructive pulmonary disease aged ????40 years who were prescribed a medication used to treat chronic obstructive pulmonary disease, by year, National Ambulatory Care Medical Survey 1999–2010. Patients with asthma were excluded
Respiratory symptoms and prescribed medications
The percentage of office visits with a recorded reason for visit of respiratory symptoms ranged from a low of 34.4% in 2007 to a high of 49.7% in 2002 (). In general, medications were as likely to be prescribed during office visits that listed respiratory symptoms as one of the reasons for the office visit as during office visits that did not (). For both patients with and without respiratory symptoms, significant increases in the percentage of office visits by patients with a diagnosis of COPD who were prescribed a medication of interest were noted for SABA, LABA plus ICS, any LABA, any ICS, tiotropium, any long-acting medication, and any medication.
Figure 2. Percentage (95% confidence interval) of office visits among patients aged ≥40 years with chronic obstructive pulmonary disease listing respiratory symptoms as one of three reasons for the visit, by gender and year, National Ambulatory Medical Care Survey 1999–2010.
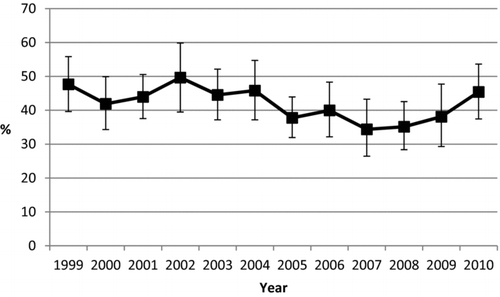
Table 5. Unadjusted percent (standard error) of office visits of patients with chronic obstructive pulmonary disease aged ≥40 years who were prescribed a medication used to treat chronic obstructive pulmonary disease, by respiratory symptom status and survey period, National Ambulatory Care Medical Survey 1999–2010
Discussion
Our analyses of trends in prescribing practices show that the period from 1999 to 2010 was a dynamic time that saw large shifts in prescribing practices for patients with COPD. Prescriptions for SABAs, combinations of LABAs and ICSs, and tiotropium increased steadily whereas prescriptions for ipratropium, methylxanthines, LABAs as single agents, and ICSs as single agents decreased. Although at the onset of the study period short-acting agents were prescribed more frequently than long-acting agents, by the end of the study period long-acting agents were prescribed about as often as short-acting agents. In 2010, approximately 50% of patients with COPD walked out of the office with a prescription for a COPD medication.
Limited research has examined trends in prescribing practices for medications used in the treatment of COPD in the United States. In an analysis of data from 1995 to 2004, prescriptions for oral corticosteroids in men and methylxanthines in men and women decreased but prescriptions for anticholinergic agents increased (Citation13).
Prior to 2000, mostly short-acting agents were available to treat COPD including SABAs, ipratropium, and methylxanthines. Longer acting agents included salmeterol, approved by the Food and Drug Administration in 1994, and ICS. Starting in 2000, long-acting therapeutic options increased with the approval of the combination of salmeterol and fluticasone in 2000, formoterol in 2001, tiotropium in 2004, arformoterol in 2006, and the combinations of formoterol and budesonide in 2006. Subsequently, tiotropium and medications containing combinations of LABAs and ICS enjoyed strong growth in prescriptions. The sizeable jump in LABAs and ICSs in 2010 should be cautiously interpreted as there can be considerable year-to-year variation in prescriptions for some classes of medications. The rates of increase in the percentage of office visits by patients with COPD during which a prescription for major groups of medications used to treat COPD was issued were reasonably similar as suggested by .
Guidelines for the pharmacologic treatment of COPD have evolved as new medications were developed and approved and more has been learned about the benefits and harms of various therapeutic options. During the study period from 1999 to 2010, various guidelines that contained recommendations for the pharmacologic treatment of patients with COPD were released that may have influenced practice patterns (Citation8, 9, Citation14–20). In the 1995 guidelines from the American Thoracic Society, long-acting beta-2 agonists were not discussed and the use of inhaled corticosteroids received little attention (Citation8). Recommendations regarding pharmacologic treatment were largely limited to short-acting anticholinergic agents and short-acting beta2-agonists. In the initial release of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines in 2001, short-acting bronchodilators were recommended for regular treatment of patients with mild COPD, one or more bronchodilators were recommended for regular treatment of those with moderate COPD, and one or more bronchodilators were recommended for regular treatment of those with severe COPD along with the use of inhaled corticosteroids in the presence of significant respiratory symptoms, lung function response, or repeated exacerbations (Citation14).
Our findings suggest that the recommendations from American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society (ACP/ACCP/ATS/ERS) and the Global Initiative for Chronic Obstructive Lung Disease are progressively being adopted. However, we were unable to establish how well the guidelines were being implemented in clinical practice because the guidelines incorporate the presence of respiratory symptoms and results from pulmonary function testing, and results from pulmonary function testing were not available in the data sets. Possibly, the development, introduction, and promotion of new medications, advertising of medications used to treat COPD to the public, continuing medical education, and evolving education of medical students may also have influenced prescribing practices.
The findings of the present study are subject to several limitations. First, sample size, although fair, was nevertheless too small to allow stratification by various covariates by single year. This necessitated pooling of data for several years. Second, because the unit of analysis was an office visit not an individual patient, it is possible that a patient could have been resampled. Third, results from spirometry even if performed were not available, and, therefore, we were unable to stratify patients by severity of their disease. Fourth, we were unable to assess whether patients were being inappropriately treated because to do so would require a comprehensive review of prescriptions received by patients or perhaps information directly obtained from patients about the medications they use. Because the sampling unit of NAMCS is the patient-physician encounter or visit at a single point of time, the survey does not provide information about the use of all medications at the patient level.
It is tempting to speculate that some of the observed trends in prescribing practices such as the increasing trend for medications that combine a LABA and an ICS have translated into improved care for patients with COPD. However, the NAMCS surveys are not designed to examine the question of whether the evolution in prescribing practices lead to improvements in care for patients with COPD. To adequately address the issue of improved care would require quite different studies. In addition, studies that have information about the presence of respiratory symptoms and results from pulmonary function testing are needed to assess the concordance between current clinical guidelines and clinical practice.
In conclusion, the percentage of office visits by patients with COPD that resulted in a prescription for medications used to treat COPD almost doubled from 1999 to 2010. This growth in the rate of prescriptions was led by increases in prescriptions for SABAs, LABAs plus ICSs, and tiotropium. Our results suggest that recommendations regarding pharmacologic management of patients with COPD are increasingly being adopted by physicians.
Declaration of Interest Statement
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Dr. Mannino has received honoraria/consulting fees and served on speaker bureaus for GlaxoSmithKline plc, Novartis Pharmaceuticals, Pfizer Inc., AstraZeneca PLC, Forest Laboratories Inc., and Creative Educational Concepts. Furthermore, he has received royalties from Up-to-Date. The other authors report no conflicts of interest. The authors alone are responsible for the -content and writing of the paper.
References
- Mannino DM, Braman S. The epidemiology and economics of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2007; 4(7):502–506.
- Soriano JB, Rodriguez-Roisin R. Chronic obstructive pulmonary disease overview: epidemiology, risk factors, and clinical presentation. Proc Am Thorac Soc 2011; 8(4):363–367.
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356(8):775–789.
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359(15):1543–1554.
- Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med 2008; 177(1):19–26.
- Celli B, Decramer M, Kesten S, Liu D, Mehra S, Tashkin DP. Mortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009; 180(10):948–955.
- Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten-van Molken MP, Beeh KM, Rabe KF, Fabbri LM. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011; 364(12):1093–1103.
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995; 152(5 Pt 2):S77–121.
- Qaseem A, Snow V, Shekelle P, Sherif K, Wilt TJ, Weinberger S, Owens DK. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2007; 147(9):633–638.
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, Marciniuk DD, Denberg T, Schunemann H, Wedzicha W, MacDonald R, Shekelle P. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011; 155(3):179–191.
- Global Initiative for Chronic Obstructive Lung Disease: Global Strategy for the Diagnosis, Management and Prevention of COPD. Revised 2011. 2011. Available from: http://www.goldcopd.org./ Accessed 3 February 2012.
- Centers for Disease Control and Prevention: Ambulatory Health Care Data. 2012. Available from: http://www.cdc.gov/nchs/ahcd/ahcd_questionnaires.htm. Accessed 21 August 2012.
- Suh DC, Lau H, Pokras SM, Choi IS, Valiyeva E. Sex differences in ambulatory visits for chronic obstructive pulmonary disease, based on the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey from 1995 to 2004. Respir Care 2008; 53(11):1461–1469.
- Global Initiative for Chronic Obstructive Lung Disease: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2001. Accessed 24 August 2012. Available from:
- Global Initiative for Chronic Obstructive Lung Disease: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (updated 2003). 2003. Available from: http://www.goldcopd.org/uploads/users/files/GOLDWkshp2003Clean.pdf. Accessed 24 August 2012.
- Global Initiative for Chronic Obstructive Lung Disease: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (updated 2004). 2004. Available from: http://www.goldcopd.org/uploads/users/files/GOLDWkshp2004Clean.pdf. Accessed 24 August 2012.
- Global Initiative for Chronic Obstructive Lung Disease: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (updated 2005). 2005. Available from: http://www.goldcopd.org/uploads/users/files/GOLDWkshp05Clean.pdf. Accessed 24 August 2012.
- Global Initiative for Chronic Obstructive Lung Disease: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (updated 2006). 2006. Available from: http://www.goldcopd.org/uploads/users/files/GOLDReport2006_0122.pdf. Accessed 24 August 2012.
- Global Initiative for Chronic Obstructive Lung Disease: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (updated 2007). 2007. Available from: http://www.goldcopd.org/uploads/users/files/GOLDReport07_0108.pdf. Accessed 24 August 2012.
- Global Initiative for Chronic Obstructive Lung Disease: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (updated 2009). 2009. Available from: http://www.goldcopd.com/Guidelineitem.asp?l1 = 2&l2 = 1&intId = 2003. Accessed 7 January 2011.
