Abstract
Rationale: Bone marrow derived progenitor cells participate in the repair of injured vessels. The lungs of individuals with emphysema have reduced alveolar capillary density and increased endothelial apoptosis. We hypothesized that circulating levels of endothelial and hematopoietic progenitor cells would be reduced in this group of patients. Objectives: The goal of this study was to measure circulating levels of endothelial progenitor cells (EPCs) and hematopoietic progenitor cells (HPCs) in subjects with COPD and to determine if progenitor levels correlated with disease severity and the presence of emphysema. Methods: Peripheral blood mononuclear cells were isolated from 61 patients with COPD and 32 control subjects. Levels of EPCs (CD45dim CD34+) and HPCs (CD45+ CD34+ VEGF-R2+) were quantified using multi-parameter flow cytometry. Progenitor cell function was assessed using cell culture assays. All subjects were evaluated with spirometry and CT scanning. Measurements and Main Results: HPC levels were reduced in subjects with COPD compared to controls, whereas circulating EPC levels were similar between the two groups. HPC levels correlated with severity of obstruction and were lowest in subjects with severe emphysema. These associations remained after correction for factors known to affect progenitor cell levels including age, smoking status, the use of statin medications and the presence of coronary artery disease. The ability of mononuclear cells to form endothelial cell colony forming units (EC-CFU) was also reduced in subjects with COPD. Conclusions: HPC levels are reduced in subjects with COPD and correlate with emphysema phenotype and severity of obstruction. Reduction of HPCs may disrupt maintenance of the capillary endothelium, thereby contributing to the pathogenesis of COPD.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is the most prevalent respiratory disease in the world and is the 5th leading cause of death in developed nations (Citation1). It is characterized by airflow limitation that is not fully reversible and commonly affects both the airways and lung parenchyma. Importantly, growing evidence suggests that the pulmonary microvasculature is also a prominent site of involvement. Alveolar capillary density is markedly reduced in emphysema lung tissue and apoptotic endothelial cells can readily be identified in these regions (Citation2–6). Moreover, disruption of endothelial function in experimental animals leads to alveolar epithelial cell death and airspace enlargement, suggesting that maintenance of alveolar structure and function requires an intact capillary endothelium (Citation7–11).
The processes that maintain the alveolar capillary network have not been fully defined, however it is increasingly recognized that bone marrow-derived progenitor cells play important roles, particularly hematopoietic progenitor cells (HPCs) and endothelial progenitor cells (EPCs). HPCs and EPCs can be found in the peripheral blood during health and disease and are thought to function synergistically to maintain and repair the endothelium (Citation12, 13). HPCs migrate to areas of injury where they adopt positions directly adjacent to areas of active vessel growth and produce growth factors and cytokines that induce blood vessel sprouting and endothelial cell proliferation. Conversely, EPCs may incorporate directly into repairing vessels, perhaps in response to signals provided by HPCs. We hypothesized that circulating levels of both EPCs and HPCs would be reduced in patients with COPD compared to matched controls.
Studies involving progenitor cells in COPD and other disease states have been challenged by the lack of a clear consensus regarding the precise surface markers expressed by EPCs relative to HPCs. This led some authors to a misclassify HPCs as EPCs in the past and resulted in conflicting interpretations regarding EPC levels and function in disease states. This is particularly true for COPD, in which levels of circulating EPCs have been reported as being either the same as controls (Citation14) or significantly reduced (Citation15–17). A second limitation regarding studies that have measured EPCs in COPD has been the use of spirometry as the sole means of classifying subjects. This strategy does not enable distinction between chronic bronchitis and emphysema and may misclassify patients with early emphysema as healthy (Citation18–21). CT scans may be used to identify emphysema in these cases. Accordingly, a major goal of the work presented herein was to classify circulating progenitor cells in a well-defined cohort of subjects with COPD assessed by both lung physiology and high resolution CT (HRCT) scanning.
The two main methods for quantifying progenitor cells are surface immunophenotyping with flow cytometry and cell culture with secondary purification and/or expansion. Since no single cell antigen adequately identifies progenitor cells with flow cytometry, a panel of markers is required. At least one of the markers must correspond with immaturity or ìstemness,î while a second must identify cell lineage. Accordingly, we used a standardized gating strategy to identify CD34+ progenitor cells (Citation22) in concert with CD45 to distinguish cells of the hematopoietic lineage (CD45+) from those with putative endothelial properties (CD45dim) (Citation23–25). Vascular endothelial growth factor-receptor 2 (VEGF-R2) was used to further identify progenitor cells with angiogenic potential (Citation26–28), whereas CD133 was used to distinguish immature progenitor cells (Citation29, 30). Standardized cell culture methods were used as a second means of quantifying the angiogenic potential of progenitor cells in patients with COPD. Our data suggest that circulating levels of HPCs are significantly reduced in patients with COPD, whereas EPC levels remain unchanged.
Materials and Methods
Patient Selection
The study population included healthy individuals and subjects with COPD and who were local participants in the COPDGene Study (http://www.copdgene.org/), an ongoing multi-center study designed to identify the genetic factors associated with COPD (Citation31). These subjects were between the ages of 45–80 and were current or former smokers with a 10 pack-year or greater history of cigarette smoking. A group of healthy never smokers was recruited as a second control population to control for effects of tobacco smoke. All subjects completed a medical questionnaire that included tobacco use history and medical diagnoses. Subjects were excluded from our study that had lung disease other than COPD, a recent exacerbation of COPD (prior 6 months), angina pectoris, a recent history of myocardial infarction or angioplasty (prior 6 months), decompensated heart failure, active malignancy, or chronic renal failure. This research protocol was approved by the institutional review board at National Jewish Health. All subjects provided written informed consent.
Physiologic testing
Spirometry was performed using the EasyOne spirometry system (NDD, Zurich, Switzerland) before and after the administration of albuterol. Quality control was performed for all spirometric tests using both an automated system and manual review. The diagnosis of COPD was defined using criteria specified by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) (Citation32).
CT and quantitative analysis
Whole-lung volumetric multi-detector CT was performed at full inspiration using a standardized protocol (Citation31, Citation33). The extent of emphysema was determined using quantitative densitometric analysis with VIDA software (VIDA Diagnostics, Iowa City, IA). The lungs were broken into 6 lobar regions (right upper lobe, middle lobe, right lower lobe, left upper lobe, lingua, left lower lobe), and percent low attenuation area (%LAA) was defined for each region by determining the number of voxels with a CT attenuation value of less than −950 Hounsfield units (HU) (Citation31, Citation34). Mean values from all six lobes were combined to determine the total%LAA for both lungs. This number was used for data analysis. Coronary artery calcification was identified on CT scans as described previously (Citation35).
Collection and processing of samples
Venous blood was collected directly into 8ml cell preparation tubes (CPT) coated with sodium citrate (BD Diagnostics, Franklin Lakes, NJ). The volume of blood contained in each tube was recorded and specimens were placed on a rotating platform at room temperature until cells could be isolated. Peripheral blood mononuclear cells (PBMCs) were isolated from the CPT tubes using density centrifugation as specified by the manufacturer. PBMCs were gently aspirated from the tubes and washed twice with room temperature phosphate-buffered saline. Cell counts were enumerated using a hemacytometer and light microscopy.
Cell differentials were determined with light microscopy on Wright–Giemsa-stained cytospins. Cells were then divided for culture assays and flow cytometry to quantify EPCs and HPCs. In all cases greater than 95% of purified cells were mononuclear cells. In preliminary studies we determined that our assays had the greatest reproducibility when cells were isolated within 90 minutes of collection. Therefore, specimens that arrived in our laboratory more than 90 minutes after collection were discarded.
Flow cytometry
Staining for flow cytometry was performed immediately following cell isolation. Non-specific staining was minimized by incubating the cells in a 20-μl FcBlock (Miltenyi Biotec, Auburn, CA) and 80 μl of staining media (PBS, 10% fetal bovine serum, 0.1% sodium azide) for 20 minutes on ice. The samples were then incubated with the following fluorochrome conjugated antibodies: CD45-PerCP (clone 2D1), CD34-PE (clone 563), CD133-APC (clone 293C3) and VEGF-R2/KDR-FITC (clone 89106). All antibodies were obtained from BD Biosciences except for ones directed against CD133 (Miltenyi Biotec) and VEGF-R2 (R&D Systems, Minneapolis, MN). Pre-conjugated isotype antibodies were obtained from the same manufacturers and were added at equivalent antibody concentrations. After staining, cells were washed twice in staining buffer, fixed in 0.5% paraformaldehyde and then taken directly to flow cytometry.
Flow cytometry was performed using a CyAn cytometer (Dako, Carpinteria, CA). To ensure appropriate voltage compensation, single stained specimens with antibodies directed against CD45 were run for each fluorochrome for each experiment. Forward and side scatter gates were adjusted to collect live cells. Doublets were excluded using pulse width. At least 500,000 live cells were counted for each sample. Samples with fewer cells were excluded from analysis.
Because EPCs represent relatively ìrare events,î we followed a modified ISHAGE (International Society of Hematotherapy and Graft Engineering) gating protocol that has been described to isolate CD34+ cells (Citation22). Single-stain controls (positive control) and fluorescent minus one (FMO) controls (negative controls) were used to set gates. FMO controls include all of the test antigens except the antigen of interest and therefore provide optimal detection of rare events while accounting for fluorescence spillover (Citation36). Isotype IgG controls were used to rule out non-specific background staining. Data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Cell culture
Endothelial cell colony forming units (EC-CFU) were cultured using the EndoCult Liquid Medium Kit (StemCell Technologies, Vancouver, Canada) per the manufacturer's protocol. PBMCs were seeded onto 6-well fibronectin-coated tissue culture plates (BD Biosciences) at 5 × 106 cells/well. After 48 hours, non-adherent cells were collected and plated in their existing media in 24-well fibronectin-coated tissue culture plates at a concentration of 1 × 106 cells/well. On day 5, colonies were inspected with an inverted microscope (Olympus Optical, Center Valley, PA) and were identified as a central cluster of round cells surrounded by outward projecting spindle cells.
Biostatistics
Statistics were performed using JMP software (SAS Institute, Cary, NC). Normally distributed continuous data are reported as mean ± standard deviation and were assessed using the Student's t-test. Non-normally distributed data are reported as median and 25–75% quartiles. Nonparametric analyses (Wilcoxon and Kruskal–Wallis tests) were performed on these data. Pearson's test or Fisher's exact test was used to compare categorical data. Univariate analyses were initially performed to determine the clinical factors associated with HPC levels. Based on these results, multivariable logistic regression analyses were performed incorporating variables that were previously reported to be associated with circulating progenitor cell levels including age, gender, tobacco use, administration of statin medications, and evidence of coronary artery disease as assessed by CT. Interaction terms between each individual confounding variable were entered into the initial model to assess for effect modification; no interaction terms were significant (p value > 0.05). Reported p values are two-sided. An α value of 0.05 was used in all analyses.
Results
A total of 93 subjects were enrolled in the primary study (). Of these, 61 met the GOLD criteria for COPD (Citation32). The remaining 32 subjects were classified as controls. The groups were similar in terms of age, gender and smoking status. Mononuclear cell concentrations in the peripheral blood were also similar. Twenty-nine subjects in the control group and 60 in the COPD group were evaluated with HRCT. 82% of subjects in the COPD group had emphysema by CT scan. 18% had bronchial wall thickening without emphysema. Importantly, nearly one-third of the subjects in the control group also had emphysema. The presence of emphysema in smokers with normal spirometry is consistent with prior publications (Citation18–21). Circulating progenitor cell levels may be reduced in individuals with coronary artery disease (Citation37, 38). Therefore HRCT was used to identify coronary artery calcification (Citation35). Subjects with COPD had higher rates of coronary artery calcification than controls. Statin drugs may increase circulating progenitor cell levels (Citation39); however, statin use was similar between groups.
Table 1. Subject Characteristics
Circulating EPC levels are similar in patients with COPD and controls
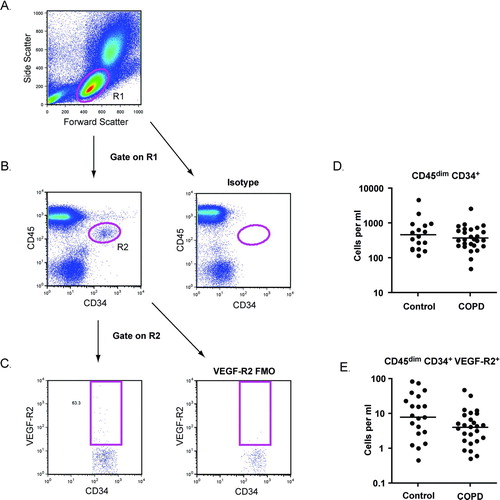
EPCs were identified with flow cytometry using the ISHAGE (International Society of Hematotherapy and Graft Engineering) gating strategy (Citation22). As shown in , cells with forward and side scatter properties similar to lymphocytes were selected (R1) and doublets were excluded (not shown). EPCs were defined as CD45dim CD34+ events (gate R2). VEGF-R2 expression was further assessed. The numbers of CD45dim CD34+ EPCs and CD45dim CD34+ VEGF-R2+ subsets were similar between subjects with COPD and control subjects with a history of tobacco use (D, E).
Figure 1. Endothelial progenitor cell (EPC) quantification. (A) Peripheral blood mononuclear cells were identified based on forward scatter and side scatter (R1). (B) Following exclusion of doublets, CD45dimCD34+ cells were selected (R2). Isotype staining was used to confirm the specificity of the CD34 antibody. (C) Cells that expressed VEGF-R2 were identified based on fluorescence minus one (FMO) controls. (D-E) Total numbers of CD45dimCD34+ EPCs and CD45dimCD34+ VEGF-R2+ cells per ml of blood for subjects with COPD and normal spirometry. Horizontal lines indicate geometric mean. N = 17 for controls. N = 31 for COPD.
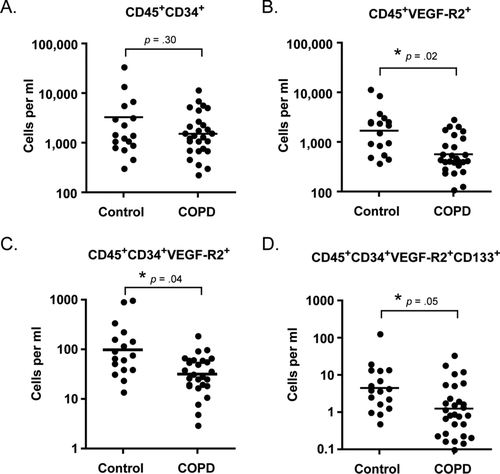
Hematopoietic Progenitor Cells (HPCs) are reduced in patients with COPD
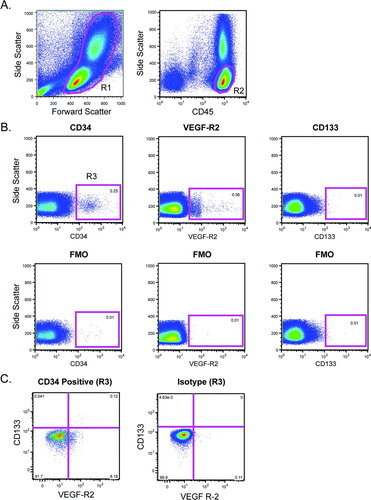
Because HPCs may also participate in angiogenesis, we next sought to determine if HPC levels were different in subjects with COPD versus matched controls. HPCs were identified based on forward and side scatter properties, high expression of CD45 and the progenitor cell markers CD34, VEGF-R2and CD133 (). As shown in , there was no significant difference in the levels of CD45+CD34+ cells between the groups (p = 0.3); however, levels of VEGF-R2 expressing HPCs, and immature HPCs (as defined by CD133 expression) were significantly reduced in subjects with COPD.
Figure 2. Quantification of hematopoietic progenitor cells (HPCs). (A) Peripheral blood mononuclear cells were identified based on forward scatter and side scatter (R1). Following doublet exclusion, CD45+ cells with low side scatter were selected (R2). (B) Cells from R2 were analyzed for expression of CD34, VEGF-R2, and CD133. Gates were based on fluorescence minus one (FMO) controls. (C) CD45+CD34+ cells (from R3) were assessed for VEGF-R2 and CD133 expression.
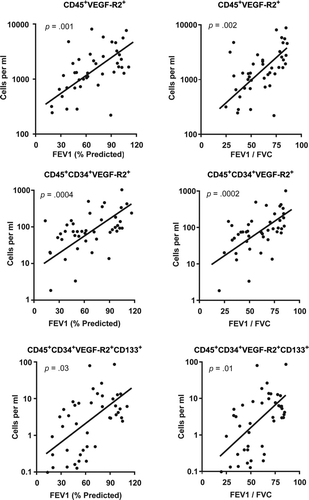
Hematopoietic progenitor levels correlate with severity of COPD
We hypothesized that HPC levels would be lowest in subjects with the greatest severity of lung disease. To test for this association, univariate analysis was performed comparing HPC levels as quantified by flow cytometry with post-bronchodilator lung function. This demonstrated a significant correlation between all HPC subsets and severity of disease (). In multivariable analyses that included age, gender, smoking status, statin use, and the presence of coronary disease, HPC levels independently correlated with airflow limitation (FEV1) and degree of obstruction (FEV1/FVC) (p < 0.05).
Endothelial cell colony forming units (EC-CFU) are reduced in patients with COPD
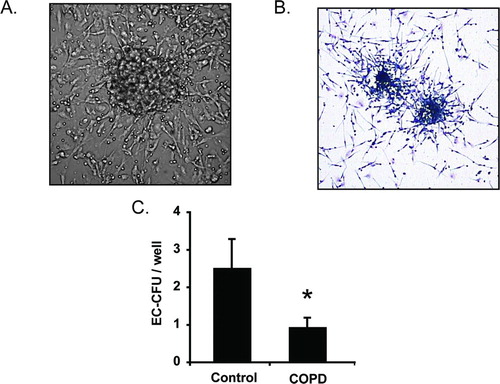
Endothelial cell colony forming units are comprised of a central rounded cluster of cells (primarily lymphocytes and CD45+CD34+VEGF-R2+ HPCs) surrounded by spindle-shaped cells (monocytes) that radiate outward from the center (Citation40–44). Formation of the colonies requires cytokine and growth factor-mediated crosstalk between the HPCs and leukocytes and therefore may reflect functional status of HPCs as well as absolute numbers. Accordingly, we assessed the colony forming potential of hematopoietic cells by quantifying EC-CFUs that grew from mononuclear cells cultured on fibronectin (A, B). As anticipated, EC-CFU formation was significantly reduced in subjects with COPD versus controls (C). This association was maintained after correction for covariates including subject age, smoking status, coronary calcification and statin use and corresponded with our flow cytometry data that show diminished numbers of CD45+CD34+VEGF-R2+ cells in the circulation of patients with COPD.
Figure 5. Endothelial cell colony forming units (EC-CFU) are reduced in subjects with COPD. (A) Phase contrast photomicrograph of an EC-CFU in culture (40×). EC-CFUs consist of a central rounded core that is surrounded by spindle shaped cells projecting outward from the center. (B) Light micrograph of Wright–Giemsa stained EC-CFUs (20×). (C) EC-CFU counts from non-adherent mononuclear cells cultured in 24-well plates. Bars represent mean, SEM. N = 22 control subjects and 48 COPD subjects. *p = 0.05.
Angiogenic progenitor cells are reduced in individuals with emphysema
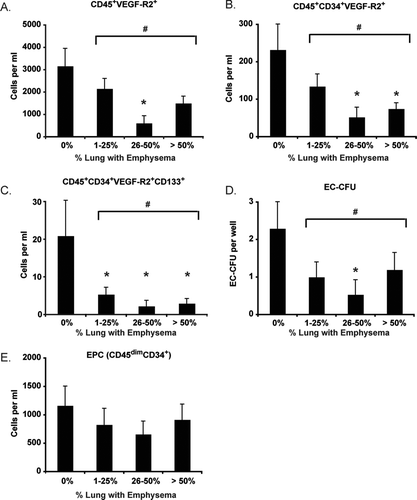
It is well recognized that the small airways are the major site of airflow limitation in COPD and that emphysema contributes to a variable extent due to loss of elastic recoil pressure (Citation45–47). Hence, some patients with emphysema may have relatively little airflow limitation whereas others with moderate or severe obstruction may have very little emphysema. Since alveolar capillary loss is more closely linked to destruction of the alveoli (i.e., emphysema) than airways disease, we sought to determine if angiogenic HPC levels correlated with emphysema severity by HRCT. Accordingly, subjects were stratified into four groups based on the percent of lung tissue in which emphysema was present (% low attenuation area (%LAA)). As shown in A-C, circulating levels of angiogenic HPCs (including CD45+VEGF-R2+, CD45+CD34+VEGF-R2+, and CD45+CD34+VEGF-R2+CD133+subsets) were reduced in subjects with moderate (26–50% LAA) and severe emphysema (> 50% LAA) but not in individuals with mild disease (LAA ≤ 25%) disease. Similar relationships existed between EC-CFU levels and emphysema severity (D). EPC levels were similar between control subjects and individuals with emphysema (E).
Figure 6. Progenitor cell levels in emphysema. Emphysema severity was determined using CT scanning lung attenuation area. (A–C) Angiogenic hematopoietic progenitor cell subsets; (D) Endothelial cell-colony forming units (EC-CFU); (E) Endothelial progenitor cell (EPC) levels. Bars represent mean, SEM. *p < .05 versus control. #p < .05 for all subjects with emphysema versus subjects without emphysema.
Discussion
Taken as a whole, our data suggest that circulating levels of hematopoietic progenitor cells are reduced patients with COPD compared to subjects with normal lung function. This association was maintained when covariates such as age, gender, smoking status, statin use and cardiac disease were considered. Moreover, HPC levels were lowest in patients with the greatest degrees of airflow limitation and those with emphysema predominant phenotypes. In striking contrast, there was no difference in EPC levels between patients with COPD and controls.
Current evidence suggests that HPCs and EPCs play important roles in angiogenesis and that they work in concert to repair and maintain the capillary endothelium—although their precise roles have yet to be determined. It has long been recognized that pulmonary capillary density is reduced in patients with emphysema (Citation3). However, only recently has it been suggested that failure to maintain the capillary endothelium may be an important cause of emphysema (Citation48, 49). In this regard, elegant studies have shown that blockade of VEGF receptor signaling in rodents leads to alveolar capillary dropout with secondary death of alveolar epithelial cells and resultant emphysema (Citation8, Citation11). Unfortunately, these studies did not explore whether VEGF receptor blockade affected circulating progenitor cells. VEGF is a critical chemotactic factor for both HPCs and EPCs, and signaling through VEGF receptors is critical to their function (Citation50, 51). It is therefore enticing to speculate that blockade of VEGF receptors led to impaired progenitor cell mobilization, function, and homing which contributed to the failed maintenance of the pulmonary capillaries in these model systems. In support of this concept, our study suggests that not only are HPC levels lower in subjects with COPD, but that elements of their function may also be impaired, as demonstrated by reduced EC-CFU formation in ex-vivo cultures from subjects with COPD.
The lack of a universal consensus for defining EPCs and HPCs presents a major challenge to progenitor cell research and is a limitation of our work. For the purpose of distinguishing EPCs and HPCs in our study we used multi-detector flow cytometry with fluorochrome-conjugated antibodies directed at CD34, VEGF-R2, CD133 and CD45. CD34 is a transmembrane sialomucin that is highly expressed on hematopoietic stem cells and progenitor cells and undergoes rapid down regulation during cellular differentiation (Citation52). It is present on both EPCs and HPCs as well as some microvascular endothelial cells but not on large vessels (Citation53). Accordingly, we used CD34 as the main means of identifying progenitor cells. CD45, the common leukocyte antigen, is expressed on all leukocytes including HPCs but not on endothelial cells or EPCs (Citation54). Based on the work of Ingram and colleagues (Citation23–25) we relied on CD45 expression to distinguish EPCs (CD34+CD45dim) from HPCs (CD34+CD45high). VEGF-R2, also known as kinase-insert domain receptor (KDR) in humans, is highly expressed on endothelial cells and is the primary receptor that transmits VEGF signals in the vasculature (Citation55, 56).
VEGF-R2 is also expressed on non-committed stem cells and hematopoietic progenitor cells and thus serves as a marker for EPCs and a subset of HPCs with putative angiogenic potential (Citation26–28). CD133 is a cholesterol-binding glycoprotein that is expressed on hematopoietic stem cell and immature progenitor cells, but not on mature endothelial cells (Citation29, 30). This renders CD133 useful in identifying immature EPCs and HPCs. Accordingly, we defined EPCs as CD45dimCD34+ cells whereas HPCs were defined as CD45+CD34+VEGF-R2+ cells. The levels of EPCs and HPCs documented in our study are consistent with other publications in the field and highlight the rare frequency of these cells in the peripheral circulation of adults. As such, we were unable to perform functional assays on cells identified with flow cytometry.
Cell culture methods provide an alternative method for isolation and expansion of progenitor cells. Endothelial cell colony forming units (EC-CFU) were originally considered to represent EPCs (Citation37), however it is now recognized that this is not the case. Rather, the cells that comprise the central core of the EC-CFU consist of T lymphocytes and CD45+CD34+VEGF-R2+ cells (presumably HPCs), whereas the surrounding spindle shaped cells are derived from monocytes (Citation40–44). Formation of EC-CFU therefore reflects both the relative frequency of these cell types in the peripheral blood as well as their functional capacity. EC-CFUs are reduced in patients with high cardiovascular risk, and it has been suggested the formation of EC-CFU in culture reflects the angiogenic capacity of mononuclear cells in the circulation. In this light, the results of our study suggest that individuals with COPD may have impaired ability to maintain or repair injured vessels.
The control groups in our study warrant special mention. Cigarette smoke has been shown to transiently reduce circulating progenitor cell numbers (Citation57, 58). Tobacco smoke may also induce epigenetic changes in progenitor cells that adversely impair their function (Citation59). We therefore selected a control group that had a smoking history that was similar to that of subjects with COPD. As an additional control, we quantified progenitor cells in a cohort of 12 healthy patients with no history of tobacco exposure. Surprisingly, there were no significant differences in CD45dimCD34+ cells between healthy non-smokers and tobacco smoke exposed individuals with normal spirometry (1139 ± 199 cells/ml versus 1142 ± 382 cells/ml, p = .94). Concentrations of CD45+CD34+VEGF-R2+ cells were also similar (117 ± 57 cells/ml versus 164 34 cells/ml, p = .29). Additional factors that may impact circulating progenitor cells levels include the presence of cardiovascular disease, diabetes, hypertension, renal disease, gender and age. In this regard, subjects with COPD and control subjects with cigarette smoke-exposure were similar in regard to co-morbidities, sex and age.
A large proportion of COPD subjects had prescriptions for inhaled corticosteroids, whereas none of the control subjects used them. It is therefore possible that differences between groups were driven, in part, by inhaled medications. Similarly, we are unable to account for potential effects of hypoxemia. All subjects in the control group were normoxic. In comparison, over one third of subjects in the COPD group used supplemental oxygen. The oxygen saturations of these subjects were normal when venipuncture was performed, however this does not exclude the possibility that COPD subjects experienced transient hypoxia at some point during the preceding hours or days. A final limitation of our study is the use of density centrifugation to purify peripheral blood mononuclear cells prior to flow cytometry staining. The possibility that this commonly used method could induce selective loss of progenitors cannot be ruled out. Performing flow cytometry on whole blood could potentially minimize such effects.
The findings of our study are largely concordant with those of other groups. Fadini and colleagues reported that CD34+VEGF-R2+ and CD34+VEGF-R2+CD133+ cells were reduced in patients with COPD, however these cells were interpreted to represent EPCs (Citation15). Since greater than 95% of CD34+VEGF-R2+ cells express CD45, it is most likely that the cells identified by Fadini et al. were truly HPCs rather than EPCs (Citation15). Palange et al. reported that CD34+ cells, CD133+ cells and EC-CFUs were decreased in patients with COPD (Citation17). The former were considered to represent HPCs, and were shown to correlate inversely with disease severity. In follow-up studies, this group confirmed their previous findings and further demonstrated that CD34+VEGF-R2+ and CD133+VEGF-R2+ cells were lowest in COPD patients with severe lung disease and low body weight (Citation16). Only 3 subjects in our cohort were underweight (BMI < 18.5) and therefore we were unable to test for this association. Taken as a whole, the body of evidence suggests that circulating levels of HPCs are reduced in patients with COPD and that the function of circulating progenitor cells is impaired.
The mechanisms that underlie decreased levels of circulating progenitors in the peripheral blood of patients with COPD are unclear. One potential mechanism is decreased mobilization of progenitors from the bone marrow. In healthy individuals, HPC mobilization and homing is largely regulated by stromal-cell derived factor-1α (SDF-1α) (Citation60, 61). Interestingly, expression of CXCR4, the receptor for SDF-1α, is significantly down regulated on CD34+ progenitors in patients with COPD (Citation62). This may explain the finding that patients with COPD mobilize significantly fewer CD45+CD34+VEGF-R2+CD133+ cells into the peripheral blood during thoracic surgery than matched controls (Citation63). A second potential mechanism for reduced progenitor cell levels in COPD is decreased cell survival. Indeed, Fadini and colleagues showed that cell surface exposure of phosphatidylserine (an indicator of apoptosis) was increased on HPCs from patients with COPD (Citation15). In this regard, pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) may play a role. TNF-α is a potent inducer of progenitor cell apoptosis in vitro (Citation64-66), and elevated levels of TNF-α have been documented in individuals with COPD (Citation67–70).
A third mechanism that could decrease progenitor cell levels in patients with COPD is retention of these cells in injured vessels. To this point, a careful stereologic assessment of progenitor cells in the pulmonary microvasculature has not been performed. However, increased numbers of CD45+CD133+ cells have been documented on the endothelial surfaces and intimal layers of the pulmonary arteries in smokers with COPD (Citation71).
Although other studies have explored relationships between circulating progenitor cell levels and lung disease, ours is the first that attempts to distinguish putative endothelial progenitor cells from hematopoietic progenitor cells. This is important since EPCs and HPCs may play different roles in maintaining the pulmonary microcirculation and the mechanisms that regulate their mobilization, homing and retention in the tissues may be disparate. For example, the SDF-1α-CXCR4 axis serves a critical role in regulating proliferation, migration and homing of HPCs but not EPCs (Citation61, Citation72, Citation73). Along similar lines, the fractalkine receptor (CX3CR1) is expressed on both angiogenic HPCs and EPCs, but its ligation induces differential effects. On HPCs, binding of fractalkine to the receptor stimulates cell migration and adhesion, whereas on EPCs it promotes cell death (Citation61, Citation74). Accordingly, we suggest that distinguishing between HPCs and EPCs is of paramount importance for studies that investigate microvascular function in COPD. Studies that correlate progenitor cell function with cell surface markers are desperately needed to advance the field.
It is enticing to speculate that strategies to augment the number or function of circulating angiogenic progenitor cells may be beneficial in COPD. In this regard, transfusion of autologous mononuclear cells and progenitor cells has been used to enhance angiogenesis in ischemic limbs and acutely injured myocardium (Citation75, 76). Although the results of these studies have been generally favorable, it has been suggested that efficacy of cell-based therapies may be improved if the appropriate progenitor cells can be targeted (Citation77). The results of our study suggest that circulating HPCs are reduced in patients with COPD and that HPC function is impaired. Further studies are required to determine if these abnormalities contribute to the development and progression of COPD and could be a target for future therapies.
Declaration of Interest Statement
Work was supported by grants from the National Institutes of Health (HL88138, HL095432, HL089856, HL089897) and from the American Heart Association (#0675040N).
Authorship contribution: WJ and PH are responsible for experimental design; WJ, AM, LB, RB, DB are responsible for performing experiments; ZY, WJ, MK, and AK are responsible for data analysis and interpretation; and WJ, ZY, MK, RB, and PH are responsible for manuscript preparation.
Acknowledgments
The authors would like to thank Christina Schnell for coordinating the clinical research required for this manuscript.
References
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Measuring the global burden of disease and risk factors. Lancet 2006; 367:1747–1757.
- Yokohori N, Aoshiba K, Nagai A. Increased levels of cell death and proliferation in alveolar wall cells in patients with pulmonary emphysema. Chest 2004; 125:626–632.
- Liebow AA. Pulmonary emphysema with special reference to vascular changes. Am Rev Respir Dis 1959; 80:67–93.
- Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med 2001; 163:737–744.
- Imai K, Mercer BA, Schulman LL, Sonett JR, D’Armiento JM. Correlation of lung surface area to apoptosis and proliferation in human emphysema. Eur Respir J 2005; 25:250–258.
- Segura-Valdez L, Pardo A, Gaxiola M, Uhal BD, Becerril C, Selman M. Upregulation of gelatinases a and b, collagenases 1 and 2, and increased parenchymal cell death in copd. Chest 2000; 117:684–694.
- Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Moore M, Sullivan A, Nicolls MR, Fontenot AP, Tuder RM, Voelkel NF. An animal model of autoimmune emphysema. Am J Respir Crit Care Med 2005; 171:734–742.
- Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of vegf receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000; 106:1311–1319.
- Tuder RM, Zhen L, Cho CY, Taraseviciene-Stewart L, Kasahara Y, Salvemini D, Voelkel NF, Flores SC. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol 2003; 29:88–97.
- Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. Vegf is required for growth and survival in neonatal mice. Development 1999; 126:1149–1159.
- Giordano RJ, Lahdenranta J, Zhen L, Chukwueke U, Petrache I, Langley RR, Fidler IJ, Pasqualini R, Tuder RM, Arap W. Targeted induction of lung endothelial cell apoptosis causes emphysema-like changes in the mouse. J Biol Chem 2008; 283:29447–29460.
- Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood 2005;106:1525–1531.
- Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004; 104:2752–2760.
- Caramori G, Rigolin GM, Mazzoni F, Leprotti S, Campioni P, Papi A. Circulating endothelial stem cells are not decreased in pulmonary emphysema or copd. Thorax 2010; 65:554–555.
- Fadini GP, Schiavon M, Cantini M, Baesso I, Facco M, Miorin M, Tassinato M, de Kreutzenberg SV, Avogaro A, Agostini C. Circulating progenitor cells are reduced in patients with severe lung disease. Stem Cells 2006;24:1806–1813.
- Huertas A, Testa U, Riccioni R, Petrucci E, Riti V, Savi D, Serra P, Bonsignore MR, Palange P. Bone marrow-derived progenitors are greatly reduced in patients with severe copd and low-bmi. Respir Physiol Neurobiol 2010; 170:23–31.
- Palange P, Testa U, Huertas A, Calabro L, Antonucci R, Petrucci E, Pelosi E, Pasquini L, Satta A, Morici G, Circulating haemopoietic and endothelial progenitor cells are decreased in copd. Eur Respir J 2006; 27:529–541.
- Bastarrika G, Wisnivesky JP, Pueyo JC, Diaz L, Arraiza M, Villanueva A, Alcaide AB, Campo A, Seijo L, de Torres JP, Low-dose volumetric computed tomography for quantification of emphysema in asymptomatic smokers participating in an early lung cancer detection trial. J Thor Imag 2009; 24:206–211.
- Spaggiari E, Zompatori M, Verduri A, Chetta A, Bna C, Ormitti F, Sverzellati N, Rabaiotti E. Early smoking-induced lung lesions in asymptomatic subjects. Correlations between high resolution dynamic ct and pulmonary function testing. La Radiol Med 2005; 109:27–39.
- Tylen U, Boijsen M, Ekberg-Jansson A, Bake B, Lofdahl CG. Emphysematous lesions and lung function in healthy smokers 60 years of age. Respir Med 2000; 94:38–43.
- Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, Wilson J, Leader JK, Siegfried JM, Shapiro SD, Association of radiographic emphysema and airflow obstruction with lung cancer. Amer J Respir Crit Care Med 2008; 178:738–744.
- Schmidt-Lucke C, Fichtlscherer S, Aicher A, Tschope C, Schultheiss HP, Zeiher AM, Dimmeler S. Quantification of circulating endothelial progenitor cells using the modified ishage protocol. PLoS One 2010; 5:e13790.
- Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia 2007; 21:1141–1149.
- Timmermans F, Plum J, Yoder MC, Ingram DA, Vandekerckhove B, Case J. Endothelial progenitor cells: Identity defined? J Cell Mol Med 2009; 13:87–102.
- Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 2008; 28:1584–1595.
- Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N. Vegf regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature 2002; 417:954–958.
- Larrivee B, Lane DR, Pollet I, Olive PL, Humphries RK, Karsan A. Vascular endothelial growth factor receptor-2 induces survival of hematopoietic progenitor cells. J Biol Chem 2003; 278:22006–22013.
- Ziegler BL, Valtieri M, Porada GA, De Maria R, Muller R, Masella B, Gabbianelli M, Casella I, Pelosi E, Bock T, Kdr receptor: A key marker defining hematopoietic stem cells. Science 1999; 285:1553–1558.
- Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Expression of vegfr-2 and ac133 by circulating human cd34(+) cells identifies a population of functional endothelial precursors. Blood 2000; 95:952–958.
- Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. Ac133, a novel marker for human hematopoietic stem and progenitor cells. Blood 1997; 90:5002–5012.
- Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of copd (copdgene) study design. COPD 2010; 7:32–43.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: Gold executive summary. Amer J Respir Crit Care Med 2007; 176:532–555.
- Han MK, Kazerooni EA, Lynch DA, Liu LX, Murray S, Curtis JL, Criner GJ, Kim V, Bowler RP, Hanania NA, Chronic obstructive pulmonary disease exacerbations in the copdgene study: Associated radiologic phenotypes. Radiology 2011; 261:274–282.
- Kim YI, Schroeder J, Lynch D, Newell J, Make B, Friedlander A, Estepar RS, Hanania NA, Washko G, Murphy JR, Gender differences of airway dimensions in anatomically matched sites on ct in smokers. COPD 2010;8:285–292.
- Budoff MJ, Nasir K, Kinney GL, Hokanson JE, Barr RG, Steiner R, Nath H, Lopez-Garcia C, Black-Shinn J, Casaburi R. Coronary artery and thoracic calcium on noncontrast thoracic CT scans: Comparison of ungated and gated examinations in patients from the copd gene cohort. J Cardiovasc Comp Tomogr 2011; 5:113–118.
- Maecker HT, Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry A 2006; 69:1037–1042.
- Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. New Engl J Med 2003; 348:593–600.
- Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: Proof of concept for the clinical importance of endogenous vascular repair. Circulation 2005; 111:2981–2987.
- Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rutten H, Fichtlscherer S, Martin H, Zeiher AM. Hmg-coa reductase inhibitors (statins) increase endothelial progenitor cells via the pi 3-kinase/akt pathway. J Clin Invest 2001; 108:391–397.
- Rohde E, Malischnik C, Thaler D, Maierhofer T, Linkesch W, Lanzer G, Guelly C, Strunk D. Blood monocytes mimic endothelial progenitor cells. Stem Cells 2006; 24:357–367.
- Romagnani P, Annunziato F, Liotta F, Lazzeri E, Mazzinghi B, Frosali F, Cosmi L, Maggi L, Lasagni L, Scheffold A, Cd14+cd34low cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ Res 2005; 97:314–322.
- Zhang SJ, Zhang H, Wei YJ, Su WJ, Liao ZK, Hou M, Zhou JY, Hu SS. Adult endothelial progenitor cells from human peripheral blood maintain monocyte/macrophage function throughout in vitro culture. Cell Res 2006; 16:577–584.
- Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007; 109:1801–1809.
- Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: The role of angiogenic cytokines and matrix metalloproteinases. Circulation 2005; 112:1618–1627.
- Gelb AF, Schein M, Kuei J, Tashkin DP, Muller NL, Hogg JC, Epstein JD, Zamel N. Limited contribution of emphysema in advanced chronic obstructive pulmonary disease. Am Rev Respir Dis 1993; 147:1157–1161.
- Thurlbeck WM. The pathology of small airways in chronic airflow limitation. Eur J Respir Dis Suppl 1982; 121:9–18.
- Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med 1968; 278:1355–1360.
- Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of copd and pulmonary emphysema. Respir Res 2006; 7:53.
- Voelkel NF, Cool C, Taraceviene-Stewart L, Geraci MW, Yeager M, Bull T, Kasper M, Tuder RM. Janus face of vascular endothelial growth factor: The obligatory survival factor for lung vascular endothelium controls precapillary artery remodeling in severe pulmonary hypertension. Crit Care Med 2002; 30:S251–256.
- Csaky KG, Baffi JZ, Byrnes GA, Wolfe JD, Hilmer SC, Flippin J, Cousins SW. Recruitment of marrow-derived endothelial cells to experimental choroidal neovascularization by local expression of vascular endothelial growth factor. Exper Eye Res 2004; 78:1107–1116.
- Zentilin L, Tafuro S, Zacchigna S, Arsic N, Pattarini L, Sinigaglia M, Giacca M. Bone marrow mononuclear cells are recruited to the sites of vegf-induced neovascularization but are not incorporated into the newly formed vessels. Blood 2006; 107:3546–3554.
- Steen R, Egeland T. Cd34 molecule epitope distribution on cells of haematopoietic origin. Leukemia Lymphoma 1998; 30:23–30.
- Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland DR, Baker MA, Greaves MF. Expression of the cd34 gene in vascular endothelial cells. Blood 1990; 75:2417–2426.
- Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA. Human cd34+ac133+vegfr-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol 2007; 35:1109–1118.
- Ferrara N, Gerber HP, LeCouter J. The biology of vegf and its receptors. Nat Med 2003; 9:669–676.
- Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol 2006; 39:469–478.
- Puls M, Schroeter MR, Steier J, Stijohann L, Hasenfuss G, Konstantinides S, Schafer K. Effect of smoking cessation on the number and adhesive properties of early outgrowth endothelial progenitor cells. Int J Cardiol 2011; 152:61–69.
- Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, Inden Y, Murohara T. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol 2004; 24:1442–1447.
- Paschalaki KE, Starke RD, Hu Y, Mercado N, Margariti A, Gorgoulis VG, Randi AM, Barnes PJ. Dysfunction of endothelial progenitor cells from smokers and copd patients due to increased DNA damage and senescence. Stem Cells 2013, in press.
- Dimmeler S. Regulation of bone marrow-derived vascular progenitor cell mobilization and maintenance. Arterioscler Thromb Vasc Biol 2010; 30:1088–1093.
- Walenta KL, Bettink S, Bohm M, Friedrich EB. Differential chemokine receptor expression regulates functional specialization of endothelial progenitor cell subpopulations. Basic Res Cardiol 2011; 106:299–305.
- Liu X, Xie C. Human endothelial progenitor cells isolated from copd patients are dysfunctional. Mol Cell Biochem 2011; 363:53–63.
- Takahashi T, Suzuki S, Kubo H, Yamaya M, Kurosawa S, Kato M. Impaired endothelial progenitor cell mobilization and colony-forming capacity in chronic obstructive pulmonary disease. Respirology 2011; 16:680–687.
- Henrich D, Seebach C, Wilhelm K, Marzi I. High dosage of simvastatin reduces tnf-alpha-induced apoptosis of endothelial progenitor cells but fails to prevent apoptosis induced by il-1beta in vitro. J Surg Res 2007; 142:13–19.
- Xu S, Zhao Y, Yu L, Shen X, Ding F, Fu G. Rosiglitazone attenuates endothelial progenitor cell apoptosis induced by tnf-alpha via erk/mapk and nf-kappab signal pathways. J Pharmacol Sci 2011; 117:265–274.
- Zhang Y, Herbert BS, Rajashekhar G, Ingram DA, Yoder MC, Clauss M, Rehman J. Premature senescence of highly proliferative endothelial progenitor cells is induced by tumor necrosis factor-alpha via the p38 mitogen-activated protein kinase pathway. FASEB J 2009; 23:1358–1365.
- Di Francia M, Barbier D, Mege JL, Orehek J. Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary disease. Amer J Respir Crit Care Med 1994; 150:1453–1455.
- Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax 2004; 59:574–580.
- Tanni SE, Pelegrino NR, Angeleli AY, Correa C, Godoy I. Smoking status and tumor necrosis factor-alpha mediated systemic inflammation in copd patients. J Inflamm 2010; 7:29.
- Liu SF, Chin CH, Wang CC, Lin MC. Correlation between serum biomarkers and bode index in patients with stable COPD. Respirology 2009; 14:999–1004.
- Peinado VI, Ramirez J, Roca J, Rodriguez-Roisin R, Barbera JA. Identification of vascular progenitor cells in pulmonary arteries of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2006; 34:257–263.
- Chute JP. Stem cell homing. Current opinion in hematology 2006;13:399–406.
- Faber A, Roderburg C, Wein F, Saffrich R, Seckinger A, Horsch K, Diehlmann A, Wong D, Bridger G, Eckstein V, The many facets of sdf-1alpha, cxcr4 agonists and antagonists on hematopoietic progenitor cells. J Biomed Biotechnol 2007; 260:65–75.
- Todorova D, Sabatier F, Doria E, Lyonnet L, Vacher Coponat H, Robert S, Despoix N, Legris T, Moal V, Loundou A, Fractalkine expression induces endothelial progenitor cell lysis by natural killer cells. PloS one 2011;6:e26663.
- Fadini GP, Agostini C, Avogaro A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis 2010; 209:10–17.
- Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE, Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: A collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol 2007; 50:1761–1767.
- Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res 2012; 110:624–637.