Abstract
Chronic Obstructive Pulmonary Disease (COPD) management represents a significant health resource use burden. Understanding of current resource use, treatment strategies and outcomes can improve future COPD management, for patient benefit and to aid efficient service delivery. This study aimed to describe exacerbation frequency, pharmacotherapy and health resource use in COPD management in routine UK primary care. A retrospective, observational study using routine clinical records of 511 patients with COPD, was undertaken in 10 General Practices in England. Up to 3 years’ patient data were collected and analysed. 75% (234/314) patients with mild-moderate COPD (≥50% predicted FEV1) received inhaled corticosteroids (ICS). 11% of patients (54/511) received ICS monotherapy. Mean (standard deviation) annual exacerbation frequency was 1.1 (1.2) in mild-moderate, 1.7 (1.6) in severe (30–49% predicted FEV1) and 2.2 (2.0) in very severe (<30% predicted FEV1) COPD. 14% patients (69/511) had a mean exacerbation frequency of ≥3/year (‘frequent-exacerbators’); 9% (27/314) of patients with mild-moderate, 19% (27/145) with severe and 29% (15/52) with very severe COPD. 14% (10/69) of frequent-exacerbators failed to receive inhaled long-acting beta agonists (LABA), 25% (17/69) inhaled long-acting muscarinic antagonists (LAMA), and 12% (`/69) ICS. Frequent-exacerbators had a median of 6.67 primary care contacts/year, 1.0 secondary care visits/year and 21% were hospitalised for COPD/year. Inhaled therapy was frequently inappropriate, with over-use of ICS in patients with mild-moderate COPD. COPD exacerbations were associated with high health resource use and occurred at all levels of disease severity. COPD management strategies should encompass risk-stratification for both exacerbation frequency and physiological impairment.
Introduction
COPD has major impacts on patients and on health systems globally. In 2010 23,870 people in England and Wales died as a result of the condition (Citation1), making it the fifth-most common cause of death in the UK (Citation2). The World Health Organisation anticipates that by 2030 it will have become the third most common cause of death worldwide (Citation3).
The management of COPD represents a significant health resource burden on the UK National Health Service (NHS), with annual costs estimated at around £982 million each year (Citation2). This is likely to increase with an ageing population and a rising prevalence, unless strategies are put in place to prevent development of COPD and improve management of those who have the disease. The UK government has recognised this growing need and has invested in smoking prevention education, supporting smoking cessation and initiatives to improve the management of patients with COPD by introducing a new Outcomes Strategy (Citation4) and national clinical guidance in the area (Citation2).
Given both the personal and economic burdens of COPD and current pressure on the UK NHS to make significant financial savings over the forthcoming years, it is important that GPs and commissioners focus on measures to maximise the quality, efficiency and cost-effectiveness of COPD management. A clear understanding of current treatment patterns and resource use will be essential to this process, to ensure that resources can be targeted effectively and potential areas for improvement identified. Little information currently exists for COPD on the ‘real-life’ resource utilisation in the UK across primary and secondary care (Citation5) and there is inadequate research investigating whether the recommended evidence-based treatment strategies are being followed in the real world (Citation6).
The primary objective of this study was to describe the NHS resource use associated with the primary care management of COPD in routine UK clinical practice. Secondary objectives were to describe the exacerbation frequency and treatment strategies received by patients with COPD during a 3-year period.
Methods
Study design
A multi-centre, retrospective observational study. The UK health system ensures a full record of diagnoses, investigations and prescriptions is collected, which follows patients if they change family doctor (GP), providing a full record of resource use. Ten centres were purposefully selected to ensure geographical representation and a mixture of urban and rural settings. All centres had a GP with an interest in describing the management of COPD and an estimated population of patients with COPD of sufficient size to allow recruitment of the required number of patients to the study. Ethical approval was given by East of England Research Ethics Committee in May 2010 (ref 10/H0311/16) and local organisations.
Study patients
Practice databases were searched to identify eligible patients (spirometry confirmed diagnosis of COPD, in 2007). Patients were excluded if receiving end of life palliative care for COPD, did not provide written informed consent or had participated in any COPD interventional clinical trial.
Consent for access to medical records was sought postally in batches of 50 taken consecutively from an alphabetical list of potentially eligible patients, aiming for 50 consenting patients at each centre (500 in total). However, some centres did not have 50 eligible, consenting patients, so oversampling was performed in centres with more eligible patients in order to maintain the overall sample size ().
Table 1. Number of patients consenting / included at each centre
Data collection
Pseudonymised data (demographics, resource use and patient pathway information) were collected manually and electronically by trained researchers experienced in extracting data from UK GP clinical records and software systems, between July and November 2010 for the previous 3 years (i.e. 2007–2010). Comorbidities and COPD exacerbations (defined as prescription of antibiotics and/or oral corticosteroids for an acute respiratory episode) were determined using information documented in the patient's medical record. Resource use parameters included in the study were COPD-related primary and secondary care contacts and hospitalisations; these parameters were chosen as they were considered to have most impact on overall cost burden.
Analysis
Data were analysed using Microsoft Excel® and SPSS® according to a prespecified analysis plan. The main endpoint was overall NHS resource use. Secondary endpoints included the number of COPD exacerbations/patient/year and the proportion of patients prescribed each drug or combination of drugs for COPD over a three year period. Analysis included stratification of endpoints by severity of disease, exacerbation frequency and presence of concomitant asthma, (as these are factors which may affect treatment decisions and healthcare resource use). Descriptive data are presented using the mean (standard deviation, SD), median (interquartile range, IQR) or percentages, as appropriate. Comparisons between groups were undertaken using appropriate parametric or non-parametric tests according to data distribution.
Chi-squared tests of consistency were performed on the distribution of the number of exacerbations for each type of treatment, to determine whether there were any significant differences between the participating centres.
Stratification for COPD severity according to FEV1 (forced expiratory volume in one second) was based on the classification system outlined in the 2004 National Institute for Health and Clinical Excellence (NICE) COPD Guideline; mild COPD –FEV1 ≥50% predicted, moderate 30–49% predicted and severe <30% predicted. These categories were up-to-date at the time of study protocol development, but have subsequently been changed in the 2010 NICE guideline revision (Citation2). To reflect current terminology, the updated (2010) classifications (outlined below) are used in this article:
Mild-moderate COPD: FEV1 ≥50% predicted (note –the NICE 2010 guideline differentiates between mild COPD (FEV1 ≥80% predicted) and moderate COPD (FEV1 50–79% predicted), however all patients in this study with FEV1 ≥50% predicted were included in a single group).
Severe COPD: FEV1 30–49% predicted
Very severe COPD: FEV1 <30% predicted
Stratification for COPD severity was based on each patient's FEV1 measurement recorded during 2007. Where a patient had more than one FEV1 measurement documented during 2007, and fell into more than one severity bracket, the least severe was used.
Results
Demographics and study sample
A total of 511 patients were included. Between 26 and 85 patients were recruited per practice (). The mean time from diagnosis to data collection was 8 years. In all, 16 patients (3%) were diagnosed with COPD less than 3 years prior to data collection so had less than a full 3-year observation period (2.67–2.99 years).
In 2007, 314 (61%) had mild-moderate, 145 (28%) had severe and 52 (10%) very severe COPD. 266 (52%) were male. Mean (SD) age at COPD diagnosis was 66 (10.23) years.
The most commonly recorded comorbidities were asthma (n = 162, 32%) and ischaemic heart disease (n = 133, 26%). Overall 396 patients (77%) had at least one recorded comorbidity ().
Table 2. Patient demographics and study sample
Exacerbation frequency
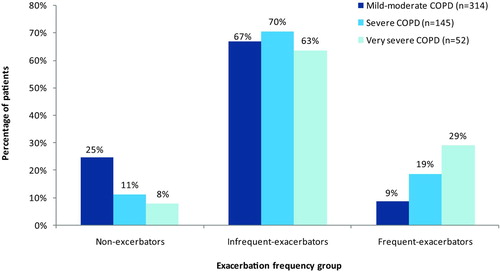
The number of exacerbations occurring during the study period was annualised and stratified by 0/year (‘non-exacerbators’), >0<3/year (‘infrequent-exacerbators’) and ≥3/year (‘frequent-exacerbators’). The mean (SD) annual exacerbation frequency was 1.1 (1.2) in patients with mild-moderate COPD, 1.7 (1.6) in severe and 2.2 (2.0) in very severe COPD (Analysis of variance (ANOVA) p < 0.0001). Median (IQR) annual exacerbation frequency was 0.7 (0.33–1.33) in mild-moderate, 1.3 (0.34–2.67) in severe and 1.7 (0.92–3.00) in very severe COPD. shows the proportion of patients in each exacerbation frequency group, stratified by disease severity. The presence of relevant co-morbidities, stratified by exacerbation frequency, is shown in .
Table 3. Recorded co-morbidities, stratified by exacerbation frequency
Resource use
The median (IQR) number of COPD-related primary care contacts (including GP surgery visits, home visits and telephone contacts) was 2.33 (1.45–3.33)/year for mild-moderate, 3.33 (2.33–5.00) for severe and 3.67 (2.67–6.42) for very severe COPD (Kruskal-Wallis H-test, p < 0.001) (). shows the health professionals consulted for primary care contacts occurring during the study period.
Figure 2. Distribution of personnel seen during COPD-related primary care contacts for routine or exacerbation management. aPercentage of all recorded primary care contacts occurring during the study period. Includes GP surgery visits, home visits and telephone contacts. b'Other’ is comprised predominantly of Community Respiratory Team and physiotherapist contacts.
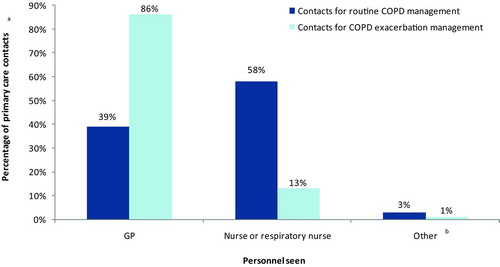
Table 4. Resource use, stratified by COPD severity
For secondary care resource use (), the median (IQR) number of COPD-related visits (including hospitalisations, accident & emergency (A&E) attendances, outpatient visits and day cases) was 0 (0.00–0.33)/patient/year in mild-moderate, 0.33 (0.00–1.00) in severe and 1.00 (0.00–2.08) in very severe COPD (Kruskal–Wallis H-test, p < 0.001). The proportion hospitalised each year for COPD increased from 4% to 10% to 16% (respectively) in sub-groups with mild-moderate, severe and very severe COPD (Chi-squared, p = 0.0034). The median (IQR) length of stay was similar in all severity groups (5 days in mild-moderate (IQR 2-10) and severe COPD (IQR 2-9), 6 (3-11) days in very severe COPD).
Health resource use and exacerbation frequency
The median (IQR) number of COPD-related primary care contacts/year increased from 1.33 (0.67–2.00) to 2.67 (2.00–3.67) to 6.67 (5.33–8.67) respectively in the non-exacerbator, infrequent-exacerbator and frequent-exacerbator groups (Kruskal-Wallis H-test, p < 0.00001). The median (IQR) number of secondary care visits/year was 0 in non-exacerbators (IQR 0.00-0.00) and infrequent-exacerbators (IQR 0.00–0.67) and 1.00 (0.33–2.67) in frequent-exacerbators (Kruskal Wallis H-test, p < 0.0001). 1% of non-exacerbators, 6% of infrequent-exacerbators and 21% of frequent-exacerbators were hospitalised each year (Chi-squared, p < 0.0001) ().
Table 5. Resource use, stratified by exacerbation frequency
Prescribing –routine COPD pharmacotherapy
and show the drug treatments prescribed for routine COPD management. The proportion of patients prescribed a particular drug/combination of drugs is the proportion of patients prescribed that treatment at any point during the study observation period, irrespective of treatment duration.
Figure 3. Proportion of patients prescribed ICS during the study period, according to COPD severity, presence of asthma and exacerbation frequency. an values show the total number of patients within each sub-group. bThe boxes below the n values show the number (and percentage) of patients within each sub-group who were prescribed ICS during the study period.
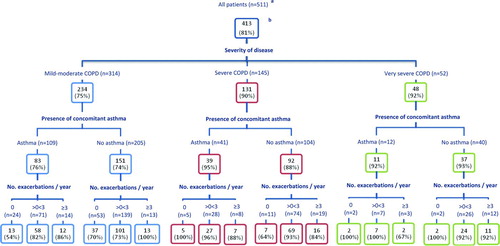
Table 6. Drug treatments prescribed for routine COPD management during the observation period –according to exacerbation frequency
Inhaled Corticosteroid (ICS) use
In all, 413 patients (81%) were prescribed ICS, as a single agent, combination inhaler with a LABA or both (). And, 54 patients (11%) were prescribed ICS monotherapy ().
ICS were prescribed for 234/314 (75%) of patients with mild-moderate COPD, including 37/53 (70%) of patients with no asthma diagnosis and no recorded exacerbations ().
LABA, LAMA and ICS use ()
In all, 403 patients (79%) were prescribed a LABA (as either a single agent, combination inhaler with ICS or both) and 295 (58%) a LAMA. And, 393 patients (77%) were prescribed a LABA and/or LAMA in combination with ICS, and 236 (46%) triple therapy (LABA+LAMA+ICS). The proportion of patients prescribed triple therapy was similar in the groups of patients with and without recorded co-morbidities (181/396 (46%) and 55/115 (48%) respectively).
Analysis of LABA and LAMA use by exacerbation frequency showed that 10/69 (14%) of frequent-exacerbators were not prescribed a LABA, 17 (25%) did not receive a LAMA and 8 (12%) received no ICS during the 3 year study period. 46/69 (67%) of frequent-exacerbators received combined treatment with all three (LABA+LAMA+ICS).
Chi-squared tests of consistency carried out on the distribution of the number of exacerbations for each type of treatment showed no significant difference between centres (i.e., all differences could be explained by the expected random variation).
Discussion
This article reports the results of a retrospective, observational research study of 511 patients with COPD, recruited from 10 primary care practices in England.
The mean time from diagnosis to data collection for patients in the study sample was 8 years, showing that the majority had well-established COPD at the time of data collection. The rate of co-morbidities was high, with 77% of patients overall having at least one co-morbidity recorded. The proportion of patients in this study with ischaemic heart disease, type II diabetes, depression and high BMI was 26%, 14%, 14% and 21% (respectively); these proportions were noted to be higher than national estimates (for England) for the prevalence of each disease (coronary heart disease –20.8% of men and 10% of women aged 65–74 (7); diabetes (type I or type II) –5.8% aged 17 or over (8); depression –11.7% aged 18 or over (Citation8); obesity –10.7% aged 16 or over) (Citation8).
Then, 32% of patients in the sample had asthma also diagnosed and recorded. However, as objective supporting evidence of asthma diagnosis was not required, this is likely to be an overestimate of the true rate of concomitant asthma (previously reported at around 13%)(Citation9) and is likely to reflect misdiagnosis in patients with non-specific respiratory symptoms. A previous diagnosis of asthma can potentially explain the early use of ICS in patients with mild COPD, although we found high use of ICS in patients with mild-moderate COPD regardless of a previous asthma diagnosis.
In line with previous research (Citation10,11), the study results confirm that exacerbation frequency increases with increasing physiological disease severity. However, our data demonstrate that patient all levels of disease severity may be non-exacerbators, infrequent-exacerbators or frequent-exacerbators; 8% of patients with mild-moderate COPD had ≥3 exacerbations/year, 9% with very severe COPD had no exacerbations during the 3-year period and the proportion of infrequent-exacerbators was similar across severities. This discrepancy between physiological severity and exacerbation frequency was also observed in the ECLIPSE study (Citation12) and is suggestive of a frequent-exacerbator ‘phenotype’, independent of disease severity.
The proportion of non-exacerbators in the current study appears to be slightly lower than in the ECLIPSE study (19% of patients in this study vs 23% of patients in the ECLIPSE study had no exacerbations over the 3-year study period). If this is a true difference, it is difficult to attribute to our study having a more complete method of identifying COPD exacerbations, since we relied on routine documentation whereas in the ECLIPSE study, exacerbation details were specifically collected at study follow-up visits by investigators, with their patients. The difference may be due to the ECLIPSE patients receiving more optimal treatment.
We found that a higher proportion of ‘frequent-exacerbators’ than ‘non-exacerbators’ had at least one recorded co-morbidity and more patients in this group suffered from depression. Frequent exacerbators had high levels of resource use in all parameters measured, even when lung function was well maintained, and so accrued high direct health costs. An increase in the median number of COPD-related primary care contacts/year (from 1.33 to 2.67 to 6.67) was observed in the non-exacerbator, infrequent-exacerbator and frequent-exacerbator groups (respectively); an increase was also observed in the proportion of patients hospitalised/year (1%, 6%, 21%, respectively). This observation has considerable economic importance, as a high proportion of medical expenditure for COPD occurs as a direct consequence of exacerbations (Citation13–17). Unsurprisingly, we also found higher resource use in those with more impaired lung function, consistent with the findings of previous research (Citation5, Citation13, Citation18–19). Given the economic impact of COPD exacerbations and their impact on patient outcomes, health status and quality of life (Citation20–23), reducing the frequency and severity of exacerbations across all levels of disease severity, should be a key focus of COPD management, and management strategies may need to encompass risk-stratification for exacerbation frequency as well as physiological impairment.
In terms of primary care resource use, we found that in UK practice, most routine COPD-related primary care contacts (58%) were with a practice nurse or respiratory nurse, although exacerbations were generally managed by GPs (86% of all contacts for exacerbation management). This suggests that nurse-led management occurs for routine but not for acute care.
This study indicates an apparent over-use of inhaled corticosteroids (ICS) in patients with mild-moderate COPD, with 75% of patients in this sub-group prescribed ICS during the study period. The UK 2004 NICE guidelines which were current at the time of the study recommended that patients with an FEV1 ≥50% who are uncontrolled by a short-acting bronchodilator alone, should receive maintenance monotherapy with a LABA or LAMA. Add-on ICS were only recommended in those with FEV1<50% and/or exacerbations. This high use of ICS in patients with mild-moderate COPD cannot be explained by the presence of concomitant asthma or frequent exacerbations; ICS were prescribed for 70% of patients (37/53) with mild-moderate COPD, without recorded asthma and with no exacerbations. Recent evidence indicates that some patients with an FEV1 of 50–60% may benefit from the use of ICS (Citation24–25), and more recent UK guidance has supported the use of LABA+ICS in patients with FEV1 ≥50% predicted for whom LABA monotherapy has failed (Citation2).
Nevertheless, the use of ICS in almost 3/4 of those with mild-moderate COPD is likely to have been inappropriate for most, particularly in view of concerns over increased pneumonia risk associated with ICS use in COPD (Citation26, 27). The over-use of ICS found in this study, is consistent with the findings of a recent audit of COPD prescribing conducted in a single region of the UK (Citation6); at the time of the audit 60% of all patients were on ICS and only 23% of these met guideline criteria. The inappropriate overuse of ICS has also been reported in a number of other studies conducted outside the UK and in the UK prior to the introduction of NICE guidance (Citation28–33).
In addition to the high use of ICS overall, we also found that 11% of all patients (54/511), including 32 without recorded concomitant asthma, were prescribed ICS monotherapy. None of the ICS mono-preparations currently available are licensed in COPD. The reasons for the overuse of ICS in patients with COPD are not clear. Difficulties in differentiating COPD from asthma, extrapolation of the effects of ICS in asthma or during COPD exacerbations,(Citation2) uncertainties over the effects of withdrawing long-term ICS in COPD patients,(Citation34) or lack of knowledge regarding guideline recommendations (Citation35), may be potential contributing factors which would bear further investigation.
Analysis of prescribing by exacerbation frequency showed that 14% of frequent-exacerbators did not receive any LABA, 25% any LAMA and 12% any ICS medication during the study period. Only 67% received triple therapy with all three classes of medication. However, all of these treatments have been proven to reduce exacerbation risk (Citation2), suggesting under-treatment in a high-risk group.
Strengths and limitations
In a time of financial pressure in the NHS and other health systems, this study provides evidence of a high-risk, high-expense group of patients and of inefficient, untargeted use of expensive inhaled therapies. There are a however a number of limitations. Firstly, data were collected retrospectively and therefore rely on the quality and level of detail routinely recorded in clinical records.
Only exacerbations that were documented in the patient's primary care records were recorded and it is possible that further exacerbations (self-managed by the patient at home and not reported to a healthcare professional or those managed solely in secondary care) may have occurred during the study period leading to an under-estimate of the exacerbation frequency. In addition, the rate of co-morbidities may not reflect the real rate of concomitant disease as only documented co-morbidities were collected in the study and these were not rigorously verified.
The conduct of the study in only 10 centres in England means that results are dependent on the practice of individual GPs and practices, which may not be representative of UK practice as a whole. However, centres were selected to ensure good geographic representation (representation from the North East, North West, Yorkshire and the Humber, East and West Midlands, South East and South West regions of England) and a mixture of urban and rural practices.
Resource use is possibly underestimated, particularly in the most severe groups of patients, as deceased patients and those judged as nearing the end of life (potentially the most resource intensive) were excluded.
The majority of patients observed in the study had long-standing COPD at the time of data collection and hence the study provides only a cross-section of 3 years of treatment at varying points in the course of disease progression. It was therefore not possible in this study to determine why treatments were initiated, if this occurred prior to the observation period.
In this study ‘treatment’ with a particular drug or combination of drugs was defined as any prescription for that medication(s) during the observation period, irrespective of treatment duration. We do not have information on whether the medications prescribed were collected and used by patients, or on how long the patient continued to use each treatment. However, this does not undermine the clinician's ‘intention-to-treat’ shown by prescribing patterns. Our analyses do not assess the efficacy of any drug or combination but rather describe how treatments are distributed among different groups of patients.
Finally, the progressive nature of the disease means that although patients were categorised as mild-moderate, severe and very severe in 2007, disease severity may have changed during the observation period and may well have changed at different rates for different patients.
Conclusions
This study confirms that there is high NHS resource use associated with the management of COPD and particularly with the management of COPD exacerbations, which occur at all levels of physiological disease severity. Inhaled therapy is frequently inappropriate, with over-use of ICS in patients with mild-moderate COPD and under-treatment in patients with frequent exacerbations. This suggests that there is a need to improve COPD management by appropriately utilising and targeting current therapies and to reduce the use of expensive medication in patients who will not benefit from their use. COPD management strategies may need to encompass risk-stratification for exacerbation frequency as well as physiological impairment.
Acknowledgments
The authors wish to thank the following medical practices for their contribution to the study: Minchinhampton Surgery, Stroud; Carterknowle & Dore Medical Practice, Sheffield; Park Surgery, Chandlers Ford, Southampton; Burbage Surgery, Burbage, Hinckley; Dr Pearston & Partners, Newcastle; Albany House Medical Centre, Wellingborough; Ash Vale Health Centre, Ashvale, Aldershot; Sherbourne Medical Centre, Leamington Spa; The Surgery, Litherland, Liverpool; Dr I D Caldwell & Partners, Daubhill, Bolton.
Declaration of Interest Statement
Neither Prof. Mike Thomas nor any member of his close family has any shares in pharmaceutical companies. In the last 3 years he has received speaker's honoraria for speaking at sponsored meetings from the following companies marketing respiratory and allergy products: Astra Zeneca, Boehringer Ingelheim, GSK, MSD, Napp, Schering-Plough, Teva. He has received honoraria for attending advisory panels with; Almirall, Astra Zeneca, BI, Chiesi, GSK, MSD, Merck Respiratory, Schering-Plough, Teva, Novartis. He has received sponsorship to attend international scientific meetings from: GSK, MSD, Astra Zeneca, Mundipharma. He has received funding for research projects from: GSK, Almirall. He is Chief Medical Advisor to the charity Asthma UK, a member of the BTS SIGN Asthma guideline group. He is a member of the EPOS Rhinosinusitis guideline group.
Dr Amr Radwan was employed by Novartis Pharmaceuticals UK and owns shares in the company.
Carol Stonham has received payment for lecturing and advisory work on an occasional basis, from the following companies: Astra Zeneca, GSK, MSD, Chiesi, Napp and Novartis. She has received payment from MSD for the development of educational presentations relating to the ASCEND asthma education programme. She is PCRS-UK Executive Committee member and Nurse Chair.
Samantha Marshall is an employee of pH Associates, a professional consultancy specialising in the design, implementation and analysis of real world data projects. pH Associates received payment from Novartis to undertake this work.
This study was funded by Novartis Pharmaceuticals UK Ltd. Novartis personnel contributed to the study design and provided support with data collection, data analysis and manuscript preparation, via the contracted services of pH Associates.
References
- Office for National Statistics. Mortality Statistics: Deaths registered in England and Wales 2010 (Series DR). Office for National Statistics [Internet]. Available from http://www.ons.gov.uk/ons/rel/vsob1/mortality-statistics–deaths-registered-in-england-and-wales–series-dr-/2010/index.html. Accessed 13/09/2012.
- National Clinical Guideline Centre. (2010) Chronic Obstructive Pulmonary Disease –Management of chronic obstructive pulmonary disease in adults in primary and secondary care. London: National Clinical Guideline Centre. Available from: http://guidance.nice.org.uk/CG101/Guidance/pdf/English. Accessed 16/01/2013
- World Health Organization (WHO) 2006. Burden of COPD. World Health Organization [Internet]. Available from http://www.who.int/respiratory/copd/burden/en/index.html. Accessed 13/09/2012
- Department of Health 2011. An Outcomes Strategy for Chronic Obstructive Pulmonary Disease (COPD) and Asthma in England. Department of Health [Internet]. Available from: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_127974. Accessed 13/09/2012.
- Britton M. The burden of COPD in the U.K.: results from the Confronting COPD survey. Respir Med 2003 Mar; 97 Suppl C:S71–79.
- Jones RC, Dickson-Spillman M, Mather MJ, Marks D, Shackell BS. Accuracy of diagnostic registers and management of chronic obstructive pulmonary disease: the Devon primary care audit. Respir Res 2008 Aug; 9(1):62.
- British Heart Foundation statistics. Prevalence of CHD, stroke, myocardial infarction and angina, by sex and age, England 2006. Available from http://www.bhf.org.uk/research/heart-statistics/morbidity/prevalence.aspx. Accessed 07/08/2013.
- The NHS Information Centre for Health and Social Care. Disease Prevalence –Quality and Outcomes Framework (QOF) for April 2011-March 2012, England. Available from http://www.diabetes.org.uk/Professionals/Publications-reports-and-resources/Reports-statistics-and-case-studies/Reports/Diabetes-prevalence-2012-March-2013/. Accessed 07/08/2013
- Hardin M, Silverman EK, Barr RG, Hansel NN, Schroeder JD, Make BJ, Crapo J, Hersh CP and the COPD Gene Investigators. The clinical features of the overlap between COPD and asthma. Respir Res 2011 Sep; 12:127.
- Donaldson GC, Seemungal TA, Patel IS, Lloyd-Owen SJ, Wilkinson TM, Wedzicha JA. Longitudinal changes in the nature, severity and frequency of COPD exacerbations. Eur Respir J 2003 Dec; 22(6):931–936.
- Jones PW, Willits LR, Burge PS and on behalf of the Inhaled Steroids in Obstructive Lung Disease in Europe study investigators. Disease severity and the effect of fluticasone propionate on chronic obstructive pulmonary disease exacerbations. Eur Respir J 2003 Jan; 21(1):68–73.
- Hurst JR, Vestbo J, Anzueto A, Locantore N, MÜllerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, MacNee W, Calverley P, Rennard S, Wouters EF, Wedzicha JA for the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010 Sep; 363:1128–1138.
- Miravitlles M, Murio C, Guerrero T, Gisbert R. Costs of chronic bronchitis and COPD: a 1-year follow-up study. Chest 2003 Mar; 123(3):784–791.
- Halpern MT, Stanford RH, Borker R. The burden of COPD in the U.S.A.: results from the confronting COPD survey. Respir Med 2003 Mar; 97 Suppl C:S81–89.
- Miller JD, Foster T, Boulanger L, Chace M, Russell MW, Marton JP, Menzin J. Direct costs of COPD in the U.S.: an analysis of Medical Expenditure Panel Survey (MEPS) data. COPD 2005 Jan; 2(3):311–318.
- Andersson F, Borg S, Jansson SA, Jonsson AC, Ericsson S, PrÜtz C. The costs of exacerbations in chronic obstructive pulmonary disease (COPD). Respir Med 2002 Sep; 96(9):700–708.
- Strassels SA, Smith DH, Sullivan SD, Mahajan PS. The costs of treating COPD in the United States. Chest 2001 Feb; 119(2):344–352.
- Gerdtham UG, Andersson LF, Ericsson A, Borg S, Jansson SA, Rönmark E, Lundbäck B. Factors affecting chronic obstructive pulmonary disease (COPD)-related costs: a multivariate analysis of a Swedish COPD cohort. Eur J Health Econ 2009 May; 10(2):217–226.
- Jansson SA, Andersson F, Borg S, Ericsson A, Jönssen E, Lundbäck B. Costs of COPD in Sweden according to disease severity. Chest 2002 Dec; 122(6):1994–2002.
- Miravitlles M, Ferrer M, Pont A, Zalacain R, Alvarez-Sala JL, Masa F, Verea H, Murio C, Ros F, Vidal R. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax 2004 May; 59:387–395.
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998 May; 157(5 Pt 1):1418–1422.
- Cote CG, Dordelly LJ, Celli BR. Impact of COPD exacerbations on patient-centred outcomes. Chest 2007 Mar; 131(3):696–704.
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. The relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002 Oct; 57(10):847–852.
- Jenkins CR, Jones PW, Calverley PM, Celli B, Anderson JA, Ferguson GT, Yates JC, Willits LR, Vestbo J. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH study. Respir Res 2009 Jun; 10:59.
- Calverley P, Pauwels RA, Jones PW, Anderson JA, Vestbos J. The severity of airways obstruction as a determinant of treatment response in COPD. Int J Chron Obstruct Pulmon Dis 2006 May; 1(3):209-218.
- Crim C, Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Willits LR, Yates JC, Vestbo J. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J 2009 Sep; 34(3):641–647.
- Singh S, Loke YK. Risk of pneumonia associated with long-term use of inhaled corticosteroids in COPD: A critical review and update. Curr Opin Pulm Med 2010 Mar; 16(2):118–122.
- Decramer M, Bartsch P, Pauwels R, Yernault JC. Management of COPD according to guidelines. A national survey among Belgian physicians. Monaldi Arch Chest Dis 2003 Jan–Mar; 59(1):62–80.
- Asche CV, Brixner DI, Conoscenti CS, Young DC, Shah H, Amy P. Assessment of physician prescribing for primary care patients with chronic obstructive pulmonary disease (COPD) in a national electronic medical record (EMR) research database [Abstract]. Chest 2006 Oct; 130(4):175S-b–175S.
- Van Andel AE, Reisner C, Menjoge SS, Witek TJ. Analysis of inhaled corticosteroid and oral theophylline use among patients with stable COPD from 1987–1995. Chest 1999 Mar; 115(3):703–707.
- Peperell K, Rudolf M, Pearson M, Diggle J. General practitioner prescribing habits in asthma/COPD. Asthma Gen Practice 1997 Dec; 5(2):29–30.
- De Miguel-Diez J, Carrasco-Garrido P, Rejas-Gutierrez J, Martin-Centeno A, Gobartt-Vázquez E, Hernandez-Barrera V, Gil de Miguel A, Jimenez-Garcia R. Inappropriate overuse of inhaled corticosteroids for COPD patients: impact on health costs and health status. Lung 2011 Jun; 189(3):199–206.
- Roche N, Lepage T, Bourcereau J, Terrioux P. Guidelines versus clinical practice in the treatment of chronic obstructive pulmonary disease. Eur Respir J 2001 Dec; 18(6):903–908.
- Nadeem NJ, Taylor SJ, Eldridge SM. Withdrawal of inhaled corticosteroids in individuals with COPD –a systematic review and comment on trial methodology. Respir Res 2011 Aug; 12:107.
- Rutschmann OT, Janssens JP, Vermeulen B, Sarasin FP. Knowledge of guidelines for the management of COPD: a survey of primary care physicians. Respir Med 2004 Oct; 98(10):932–937.