Abstract
Background: Chronic obstructive pulmonary disease (COPD) is a leading cause of mortality and morbidity worldwide, charcterised by persistent airflow limitation, mucus hypersecretion, oxidative stress and airway inflammation. N-acetylcysteine (NAC) have anti-oxidant and anti-inflammatory properties, which have been shown an uncertain benefit in COPD patients. Method: Systematic searches were conducted in Cochrane, Medline and Embase electronic databases. A meta-analysis was performed to evaluate the different effect between high and low-dose NAC treatment on COPD exacerbation. Results: This review yielded 11 studies. The methodological quality of included studies were scored using the Jadad score, with a scale of 1 to 5 (score of 5 being the highest). Data showed high-dose NAC can reduce both the total number of exacerbations (RR = 0.59, 0.47 to 0.74, 95%CI, p < 0.001) and the proportion of patients with at least one exacerbation (RR = 0.76, 0.59 to 0.98, 95%CI, p = 0.03). In the low-dose group, subgroup with jadad ≤ 3 showed a significant decrease (RR = 0.69, 0.61 to 0.77, 95%CI, p < 0.001) in the proportion of patients with exacerbation, the other subgroup with Jadad score > 3 showed no significant decrease (RR = 0.98, 0.90 to 1.06, 95%CI, p = 0.59). And low-dose NAC showed no benifit in the total number of exacerbations (RR = 0.97, 0.68 to 1.37, 95%CI, p = 0.85). Neither high nor low-dose NAC treatment showed benifit in forced expiratory volume in one second(FEV1)(WMD = 1.08, −9.97 to 12.13, 95%CI, p = 0.85). Conclusion: Long-term high-dose NAC treatment may lead to a lower rate of exacerbations. But the effect of low-dose NAC treatment remains uncertain. Further researches are needed to confirm this outcome and to clarify its mechanisms.
| Abbreviations | ||
| COPD | = | Chronic obstructive pulmonary disease |
| FEV1 | = | Forced expiratory volume in 1 second |
| WMD | = | Weighted mean difference |
| 95%CI | = | 95% confidence interval |
| RR | = | Rate Ratio |
| NAC | = | N-acetylcysteine. |
Introduction
Chronic obstructive pulmonary disease (COPD) is characterised by persistent airflow limitation, mucus hypersecretion, oxidative stress and airway inflammation. It is a leading cause of mortality and morbidity worldwide, with rapid decline in pulmonary function, compromised quality of life and reduced life span. Although available treatment strategies for COPD are beneficial, they are far from satisfactory, and thus alternative treatments are urgently needed.
Oxidant/antioxidant imbalance and airway inflammation are important processes in the pathogenesis of COPD (Citation1). Increased expression of markers of oxidative and nitrosative stress, such as 4-hydroxynonenal, thiobarbituric acid reactive substances and nitrotyrosine (Citation2, 3) can aggravate the inflammation of the small airway, which is related to lung function (Citation4), exacerbation and quality of life. Reducing oxidative stress and airway inflammation is supposed to be effective in the treatment of COPD.
N-acetylcysteine (NAC) is a derivative of amino acid L-cysteine, which is most commonly used as an apophlegmatisant, with direct/indirect anti-oxidant and anti-inflammatory properties. It has been confirmed to be beneficial in some pulmonary diseases such as pulmonary oxygen toxicity, idiopathic pulmonary fibrosis (Citation5) and cystic fibrosis (Citation6).
Recent studies (Citation7, 8) showed that NAC helps to prevent COPD exacerbation and improve pulmonary function. However, results of these studies were conflicting, possibly due to different NAC doses or concomitantly used drugs. Studies (Citation8, 9) with 600 mg NAC twice daily reported that NAC could reduce COPD exacerbations versus placebo.
Conversely, other studies (Citation10, 11) with less than or equal to 600mg NAC daily failed to demonstrate the beneficial effect on exacerbation frequency. Meanwhile, some studies (Citation12, 13) pointed out that the anti-oxidant effect of NAC was dose-dependent, which only exerts its anti-oxidant effect at high doses. Thus, we hypothesized that the effect of NAC in preventing COPD exacerbations only exists at high doses, which has never been specifically investigated in previous systematic reviews (Citation14, 15).
This meta-analysis aimed to evaluate via a subgroup analysis (high dose and low dose NAC groups) the effect of long-term high/low dose NAC treatment in COPD exacerbations and pulmonary function.
Materials and methods
Search strategy and inclusion criteria
We conducted a comprehensive literature search for English language articles examining the effect of long-term high/low dose NAC treatment in stable patients with COPD. High-dose NAC treatment was defined as the use of more than 600 mg daily, and low dose NAC treatment was defined as the use of less than or equal to 600 mg daily. These definitions were chosen based on published studies (Citation9, Citation16).
We searched the Cochrane database, Medline and Embase for randomized, controlled trials published up to August 1, 2013. We searched terms for disease (chronic obstructive pulmonary disease, chronic bronchitis, pulmonary emphysema, or COPD), and terms for drugs (acetylcysteine). We examined the bibliographies and reference lists of included articles and relevant reviews to identify additional relevant studies.
Studies were included if they met the following criteria: 1) Design: randomized controlled clinical trials, 2) intervention: orally-administered NAC plus standard therapy versus placebo plus standard therapy in subjects with stable COPD, more than 3 months; 3) outcome: total number of COPD exacerbations, proportion of patients with at least one COPD exacerbations, pulmonary function and adverse events. We excluded studies that were published as protocol, or written in non-English language.
Types of outcome measures
Primary outcome
The total number of exacerbations as a function of person-seasons.
Secondary outcomes
The number of patients with at least one exacerbation.
Spirometry: Forced expiratory volume in 1 second (FEV1).
Adverse events: Gastrointestinal disorders (including diarrhea, reflux esophagitis, gastric complaint).
Data collection and analysis
Selection of studies
Two reviewers independently checked the relevant studies from the literature search. Trials were selected from identified studies, based on previously agreed inclusion criteria. Study characteristics and outcomes were collected by two reviewers. Two studies (Citation17, 18) were included in this review, but we failed to get the full articles despite contacting the authors. The data of these studies were extracted from a published meta-analysis (Citation14). Any difference in opinion about eligibility was resolved by consensus.
Assessment of risk of bias in included studies
Methodological quality of included studies was assessed independently by two reviewers. The Cochrane Allocation Concealment Scale and Jadad scores Jadad et al. (Citation19) were used, with a scale of 1 to 5 (score of 5 being the highest). This scale is used to assess randomisation, double blinding and withdrawals/dropouts.
Data extraction and management
Data from included studies was extracted and rechecked independently by two reviewers. Of studies selected, a variety of specific trial characteristics were recorded including study design, Acetylcysteine dose/duration, age, smoking history, inhaled corticosteroids (ICS) treatment, COPD severity and Jadad score. The primary endpoint was the total number of COPD exacerbations as a function of person-seasons (one season include 12 weeks). Secondary endpoints included the proportion of patients with at least one COPD exacerbations, pulmonary function and adverse events.
One large trial (Citation11) reported the exacerbation rate instead of the proportion of patients with exacerbation. The count data was estimated by halving the annualized exacerbation rate and multiplying the number of patients allocated to either placebo or NAC group by this 6-month exacerbation rate. Another study (Citation20) reported number of patients with more than 2 exacerbations, so the total exacerbation number was estimated by multiplying the number of patients with more than 2 exacerbations by 2, and plus the number of patients with only 1 exacerbation.
For the forced expiratory volume in 1 second (FEV1), the standard deviation of change from baseline was inferred from the standard deviation of baseline and final score (Citation7). All formulations were converted based on the recommendations from the Cochrane handbook for systematic reviews of interventions. For dichotomous variables, withdrawals were taken as negative outcome. For continuous variables, withdrawals were not taken into calculation.
Measures of treatment effect
Trials were combined using the Review Manager. For dichotomous variables, a Mantel–Haenszel Rate Ratio with 95% confidence intervals was calculated. If significant heterogeneity was detected (p < 0.1), causes of heterogeneity were subsequently explored via subgroup analyses, otherwise a random effect model was selected. For continuous variables, we used a fixed effect weighted mean difference (WMD) for measurements and 95% confidence interval (95% CI) was calculated. Sensitivity analysis was made to assess the impact of excluding studies based on methodological quality. Statistical analysis was performed using RevMan software version 5.1.
Results
Study characteristics
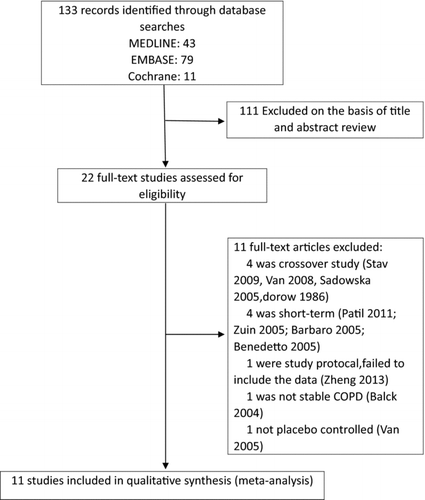
We identified 11 trials (comprising 1281 patients who received NAC and 1306 patients who received placebo) that met our inclusion criteria, of which 2 used high dose NAC (600 mg twice daily) (Citation8, 9), and 9 used low dose NAC (7, 10, 11, 17, 18, 20–23). A summary of the study selection process is shown in .
All the studies were published between 1976 and 2013, and the relevant demographic data is summarized in . The treatment periods for the studies ranged from 4 months to 3 years. Ten studies were described as randomized, double-blind, placebo controlled studies, but methods of randomization or masking were not adequately described. Jadad score ranged from 2 to 5 points. One study was described as a randomized, single-blind, placebo controlled study, with a Jadad score of 1 point.
Table 1. Major study characteristics from the 11 studies included in the meta-analysis
Study results
Effect of NAC therapy on exacerbation
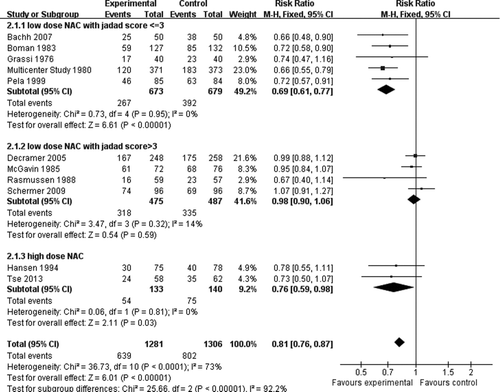
The primary endpoint was the total number of exacerbations as a function of person-seasons. High dose NAC treatment involving 2 studies and 273 patients showed a significant decrease (RR = 0.59, 0.47 to 0.74, 95%CI, , p < 0.001), and low-dose NAC treatment involving 9 studies and 2314 patients showed no significant decrease (RR = 0.97, 0.68 to 1.37, 95%CI, , p = 0.85). Overall, there was no statistical difference between NAC and placebo groups (RR = 0.86, 0.65 to 1.16, 95%CI, , p = 0.33).
Figure 2. Forest plot from meta-analysis, including 8 included RCTs, assessing relative risk (RR) of COPD exacerbations as a function of person-seasons.
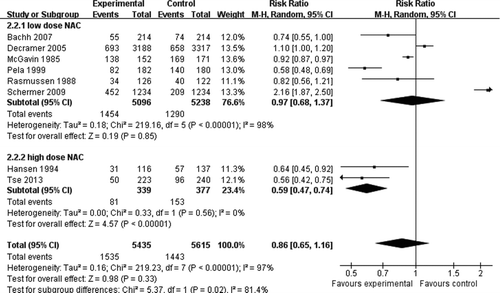
Secondary endpoint was the number of patients with at least one exacerbation. High-dose NAC treatment showed a significant decrease in the number of exacerbation (RR = 0.76, 0.59 to 0.98, 95%CI, , p = 0.03). In the analysis with low-dose NAC studies, statistical heterogeneity was detected, so subgroup was restricted to studies with Jadad score > 3. The subgroup (Citation7, Citation17, Citation18, Citation20, Citation23) with jadad ≤ 3 showed a significant decrease in the risk of exacerbation (RR = 0.69, 0.61 to 0.77, 95%CI, , p < 0.001). Subgroup analysis (Citation10, 11, Citation21, 22) restricting to studies with Jadad score > 3 showed no significant difference in the risk of exacerbation (RR = 0.98, 0.90 to 1.06, 95%CI, , p = 0.59). Overall, there was a statistical difference between high/low dose NAC treatment and placebo treatment (RR = 0.81, 0.76 to 0.87, 95%CI, , p < 0.01).
Effect of NAC on spirometry and adverse effect
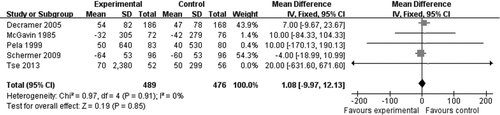
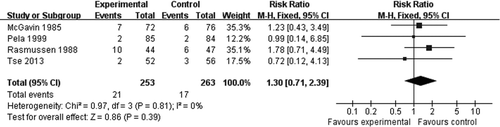
A spirometry evaluation of patients between high/low dose NAC treatment and placebo treatment showed no significant increase in FEV1 (MD = 1.08, −9.97 to 12.13, 95%CI, , p = 0.85). Gastrointestinal disorders including diarrhea, reflux esophagitis, and gastric complaint were reported in some studies, but NAC did not significantly increase the risk of such adverse reactions (RR = 1.30, 0.71 to 2.39, 95%CI, , p = 0.39).
Discussion
NAC is an effective mucolytic agent with direct/indirect anti-oxidative and anti-inflammation properties that can reduce oxidative stress and inflammation in the lung tissue by replenishing glutathione levels.
Some clinical studies (Citation9, Citation22, Citation23) have suggested a protective effect of NAC on the risk of exacerbations in COPD patients. However, conflicting results were reported, especially in low dose NAC treatment. Published meta-analyses (Citation14, Citation24) indicated that NAC could reduce COPD exacerbations versus placebo, in which they pooled the high-dose and low-dose NAC treatment together and used the proportion of COPD patients with at least one exacerbation rather than the actual number of exacerbations as an endpoint.
This review is distinguished from others by making a subgroup analysis of the high-dose and low-dose NAC treatment. Furthermore, most of the included studies included patients with moderate to severe COPD, and they are more likely to have more than one exacerbation during the long-term treatment course. So the number of exacerbations was chosen as the primary end point, and the number of patients with at least one exacerbation was the secondary outcome.
The analysis involving high-dose NAC showed a statistically significant reduction in both the number of exacerbations and the proportion of patients with at least one exacerbation. In contrast, the subgroup analysis restricting to low-dose NAC showed a negative outcome in the number of exacerbations.
According to conclusions of some studies (Citation12, 13), this can be explained by the fact that the anti-oxidant effect of NAC is dose-dependent. It is well known that NAC exerts its mucolytic effect at low dose while the anti-oxidant effect appears only at higher doses (1200–1800 mg daily). Another review (Citation16) pointed out that NAC was able to inhibit oxidative stress at both high and low concentrations. The anti-inflammatory property of NAC is highly dose-dependent and can only be seen at high concentration as demonstrated in an in vitro experimental study. Another study (Citation25) demonstrates that high dose NAC (1200mg daily) treatment shows no additional benefit on reducing oxidative stress, but it improves the symptom of COPD that is thought to be associated with its immunomodulating property.
Meanwhile, 9 included studies with low dose NAC reported the total number of patients with exacerbations as an important outcome of the treatment, but the results remain conflicting. Significant heterogeneity (p < 0.0001); I≤ = 77%) among component studies was identified and was addressed by using subgroup analysis. A Jadad score of 3 was employed as a boundary to define subgroups. The heterogeneity of each group became insignificant (I≤ = 0%,14%). The subgroup analysis (Citation7, Citation17, Citation18, Citation20, Citation23) restricting to studies with Jadad ≤ 3 showed a significant decrease in the number of patients with exacerbations, but the other subgroup analysis (Citation10, 11, Citation21, 22) restricting to studies with jadad score > 3 showed no significant decrease. Such results are inconsistent with most of the formerly published studies (Citation7, Citation17, Citation18, Citation20) and reviews (Citation14, Citation24). This maybe attributable to the fact that these studies were published fifteen years ago, and are not properly designed. These reviews did not separate the high-dose from low-dose NAC treatment, and used a random effect model when the heterogeneity was significant.
Our analysis has several limitations. First, publication bias was seen in this review, which is attributable to the exclusion of non-English language studies. Second, we failed to get full text articles of 2 studies published in the years 1983 and 1980. However, we tried to abstract data from another meta-analysis. Third, most of the included studies used inhaled corticosteroids as a standard treatment (Citation7, Citation9, Citation11, Citation20, Citation22), and the heterogeneity was significant. Furthermore, in another 4 studies, the concomitant drugs were not explicitly described, so subgroup analysis restricting to studies without ICS was not made to confirm if ICS treatment would attenuate the effect of NAC treatment that has been reported in other studies (Citation11, Citation14).
Although prior meta-analyses have evaluated the use of NAC in general for prevention of COPD exacerbations, none have specifically separated the high dose from low-dose NAC treatment. This review suggests substantial benefit from high-dose NAC treatment for COPD patients, but it is worth noting that only 2 studies were included in this subgroup analysis. More investigations with high/low dose NAC are needed to confirm this result.
Declaration of Interest Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hillas G, Nikolakopoulou S, Hussain S, Vassilakopoulos T. Antioxidants and mucolytics in COPD management: When (if ever) and in whom? Curr Drug Targ 2013; 14(2):225–234.
- Pinho RA, Chiesa D, Mezzomo KM, Andrades ME, Bonatto F, Gelain D, Oxidative stress in chronic obstructive pulmonary disease patients submitted to a rehabilitation program. Respir Med 2007; 101(8):1830–1835.
- Ochs-Balcom HM, Grant BJ, Muti P, Sempos CT, Freudenheim JL, Browne RW, Antioxidants, oxidative stress, and pulmonary function in individuals diagnosed with asthma or COPD. Euro J Clin Nutr 2006; 60(8):991–999.
- Wilkinson TM, Patel IS, Wilks M, Donaldson GC, Wedzicha JA. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003; 167(8):1090–1095.
- Adamali HI, Maher TM. Current and novel drug therapies for idiopathic pulmonary fibrosis. Drug Des, Develop Ther 2012; 6:261–272.
- Dauletbaev N, Fischer P, Aulbach B, Gross J, Kusche W, Thyroff-Friesinger U, A phase II study on safety and efficacy of high-dose N-acetylcysteine in patients with cystic fibrosis. Euro J Med Res 2009; 14(8):352–358.
- Pela R, Calcagni AM, Subiaco S, Isidori P, Tubaldi A, Sanguinetti CM. N-acetylcysteine reduces the exacerbation rate in patients with moderate to severe COPD. Respir Inter Rev Thor Dis 1999; 66(6):495–500.
- Hansen NC, Skriver A, Brorsen-Riis L, Balslov S, Evald T, Maltbaek N, Orally administered N-acetylcysteine may improve general well-being in patients with mild chronic bronchitis. Respir Med 1994; 88(7):531–535.
- Tse HN, Raiteri L, Wong KY, Yee KS, Ng LY, Wai KY, High-Dose N-acetylcysteine in stable chronic obstructive pulmonary disease: the 1-Year, double-blind, randomized, placebo-controlled HIACE study. Chest 2013; 144(1):106–118.
- Schermer T, Chavannes N, Dekhuijzen R, Wouters E, Muris J, Akkermans R, Fluticasone and N-acetylcysteine in primary care patients with COPD or chronic bronchitis. Respir Med 2009; 103(4):542–551.
- Decramer M, Rutten-van Molken M, Dekhuijzen PN, Troosters T, van Herwaarden C, Pellegrino R, Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): A randomised placebo-controlled trial. Lancet 2005; 365(9470):1552–1560.
- Stolarek R, Bialasiewicz P, Nowak D. N-acetylcysteine effect on the luminol-dependent chemiluminescence pattern of reactive oxygen species generation by human polymorphonuclear leukocytes. Pulmon Pharmacol Therap 2002; 15(4):385–392.
- Benrahmoune M, Therond P, Abedinzadeh Z. The reaction of superoxide radical with N-acetylcysteine. Free Rad Biol Med 2000; 29(8):775–782.
- Sutherland ER, Crapo JD, Bowler RP. N-acetylcysteine and exacerbations of chronic obstructive pulmonary disease. COPD 2006; 3(4):195–202.
- Stey C, Steurer J, Bachmann S, Medici TC, Tramer MR. The effect of oral N-acetylcysteine in chronic bronchitis: a quantitative systematic review. Euro Respir J Off J Euro Soc Clin Respir Physiol 2000; 16(2):253–262.
- Sadowska AM, Manuel YKB, De Backer WA. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: discordant in vitro and in vivo dose-effects: a review. Pulmon Pharmacol Ther 2007; 20(1):9–22.
- Boman G, Backer U, Larsson S. Oral acetylcysteine reduces exacerbation rate in chronic bronchitis: Report of a trial organized by the Swedish Society for Pulmonary Diseases. Euro J Respir Dis 1983; 64(6):405–415.
- Multicenter Study Group. Long-term oral acetylcysteine in chronic bronchitis.a double-blind controlled study. Euro J Respir Dis Suppl. 1980; 111:93–108.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, Assessing the quality of reports of randomized clinical trials: is blinding necessary? Contr Clin Trials 1996; 17(1):1–12.
- Bachh AA, Shah NN, Bhargava R, Ahmed Z, Pandey DK, Dar KA, Effect of oral N-acetylcysteine in COPD—A randomised controlled trial. JK Practitioner 2007; 14(1):12–16.
- Rasmussen JB, Glennow C. Reduction in days of illness after long-term treatment with N-acetylcysteine controlled-release tablets in patients with chronic bronchitis. Euro Respir J 1988; 1(4):351–355.
- McGavin CR, Prescott RJ, Nariman S, Macfarlane JT. Oral N-acetylcysteine and exacerbation rates in patients with chronic bronchitis and severe airways obstruction. Thorax 1985; 40(11):832–835.
- Grassi C, Morandini GC. A controlled trial of intermittent oral acetylcysteine in the long-term treatment of chronic bronchitis. Euro J Clin Pharmacol 1976; 9(5–6):393–396.
- Grandjean EM, Berthet P, Ruffmann R, Leuenberger P. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: A meta-analysis of published double-blind, placebo-controlled clinical trials. Clin Therap 2000; 22(2):209–221.
- Patil AB, Kale AB, Singhal SS, Khan TA. Study of malondialdehyde as an indicator of oxidative stress and its modulation by N-acetylcysteine in chronic obstructive pulmonary disease. J Clin Diag Res 2011; 5(1):48–51.
- Stav D, Raz M. Effect of N-acetylcysteine on air trapping in COPD: a randomized placebo-controlled study. Chest. 2009; 136(2):381–6. Epub 2009/05/19. doi: 10.1378/chest.09-0421. PubMed PMID: 19447919.
- Van Overveld FJ, Demkow UA, Gorecka D, Bialas-Chromiec B, Sadowska AM, Goljan A, The effects of N-acetylcysteine and inhaled steroid on inflammatory and oxidative stress markers in chronic obstructive pulmonary disease (COPD). Central-European Journal of Immunology. 2008; 33(3):135–41.
- Sadowska AM, van Overveld FJ, Gorecka D, Zdral A, Filewska M, Demkow UA, The interrelationship between markers of inflammation and oxidative stress in chronic obstructive pulmonary disease: modulation by inhaled steroids and antioxidant. Respiratory medicine. 2005; 99(2):241–9. Epub 2005/02/18. PubMed PMID: 15715193.
- Dorow P, Weiss T, Felix R, Kohler M. Influence of tasuldine and acetylcysteine on mucociliary clearance in patients with chronic airways obstruction. Arzneimittel-Forschung. 1986; 36(1):131–3. Epub 1986/01/01. PubMed PMID: 3954815.
- Patil AB, Kale AB, Singhal SS, Khan TA. Study of malondialdehyde as an indicator of oxidative stress and its modulation by N-acetylcysteine in chronic obstructive pulmonary disease. Journal of Clinical and Diagnostic Research. 2011; 5(1):48–51.
- Zuin R, Palamidese A, Negrin R, Catozzo L, Scarda A, Balbinot M. High-dose N-acetylcysteine in patients with exacerbations of chronic obstructive pulmonary disease. Clinical drug investigation. 2005; 25(6):401–8. Epub 2007/05/30. PubMed PMID: 17532680.
- Barbaro MPF, Serviddio G, Resta O, Rollo T, Tamborra R, Carpagnano GE, Oxygen therapy at low flow causes oxidative stress in chronic obstructive pulmonary disease: Prevention by N-acetyl cysteine. Free Radical Research. 2005; 39(10):1111–8.
- De Benedetto F, Aceto A, Dragani B, Spacone A, Formisano S, Pela R, Long-term oral n-acetylcysteine reduces exhaled hydrogen peroxide in stable COPD. Pulmonary Pharmacology and Therapeutics. 2005; 18(1):41–7.
- Zheng JP, Wen FQ, Bai CX, Wan HY, Kang J, Chen P, High-Dose N-Acetylcysteine in the Prevention of COPD Exacerbations: Rationale and Design of the PANTHEON Study. Copd. 2013; 10(2):164–71. Epub 2012/10/16. doi: 10.3109/15412555.2012.732628. PubMed PMID: 23061828.
- Black PN, Morgan-Day A, McMillan TE, Poole PJ, Young RP. Randomised, controlled trial of N-acetylcysteine for treatment of acute exacerbations of chronic obstructive pulmonary disease [ISRCTN21676344]. BMC pulmonary medicine. 2004; 4:13. Epub 2004/12/08. doi: 10.1186/1471-2466-4-13. PubMed PMID: 15581425; PubMed Central PMCID: PMC539269.
- van Overveld FJ, Demkow U, Gorecka D, de Backer WA, Zielinski J. New developments in the treatment of COPD: comparing the effects of inhaled corticosteroids and N-acetylcysteine. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2005; 56 Suppl 4:135–42. Epub 2005/10/06. PubMed PMID: 16204787.