Abstract
Malnutrition is prevalent in patients with chronic obstructive pulmonary disease (COPD) but is often neglected in clinical practice. This study examined the usefulness of the Mini Nutritional Assessment (MNA) for assessing the nutritional status of patients with COPD. We recruited 83 patients with COPD in stable condition from the pulmonary rehabilitation unit of a medical center in northern Taiwan. Each patient was interviewed with a structured questionnaire to elicit personal and health-related data, and measured for anthropometric and blood biochemical indicators. Nutritional status was rated with two Taiwanese-specific versions of the MNA, MNA-T1 and MNA-T2. Fat-free mass was measured with bioelectrical impedance analysis (BIA), and exercise capacity indicators with the 6-Minute Walk Test. The two MNA versions showed high agreement (kappa = 0.949) in predicting the nutritional risk, and both versions predicted the FFMI well (area under the curve of the Receiver Operating Characteristics = 0.804, p < 0.001 for MNA-T1; and 0.813, p < 0.001 for MNA-T2). MNA scores decreased with increasing disease severity and were highly correlated with FFMI, BMI, mid-arm circumference, calf circumference, and oxygen saturation at rest and during exercise (all p < 0.01). The MNA score was positively correlated with FEV1, FVC and 6-minute walking distance, and negatively correlated with GOLD stages (all p < 0.05). However, the MNA score was not significantly correlated with blood biochemical indicators, perhaps due to inflammatory status associated with COPD. The MNA appears appropriate for rating the nutritional risk of patients with COPD. Routine use of the MNA may help reduce the risk of malnutrition in patients with COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by airflow limitation that is not fully reversible. Body weight loss and muscle wasting is often a common and serious problem with poor prognosis and malnutrition is a systemic manifestation in these patients. COPD decreases muscle strength especially the diaphragm, reduces exercise capacity, and weakens immunity of the respiratory system, resulting in increased risk of mortality.
Although malnutrition is common in patients with COPD, nutritional assessment is not carried out routinely and often ignored in clinical settings. Ideal nutritional marker or gold standard for diagnosis of nutritional risk for these patients is not yet available. Because weight loss, especially lean body mass is a common problem in patients with COPD (Citation1), clinicians often use anthropometric or biochemical indicators to get a feeling of their nutritional status. Percent of ideal body weight, mid-arm circumference (MAC) and triceps skinfolds thickness were often the indicators used to gauge nutritional changes in these patients (Citation2) and a multi-indicator method including albumin, prealbumin, total lymphocyte count, percent of ideal body weight, triceps skinfolds thickness, mid-arm circumference and BMI has been used to compute a nutritional index to confirm the diagnosis (Citation3–6). Although these methods may yield satisfactory results and successfully identify the nutritional status of patients with COPD, the process is time consuming, expensive and impractical for routine clinical practice.
Bioelectrical impedance analysis (BIA) is a method that determines lean and fat body compartments. It has been used to assess body composition of patients with COPD (Citation7–9). Using BIA, body fat-free mass index (FFMI), ≤ 15 kg/m2 for women and ≤ 16 kg/m2 for men, has been derived to suggest muscle depletion in patients with COPD (Citation10–12). However, few families are equipped with BIA.
In recent decades, several measurement scales have been developed for rating the nutritional status of elderly persons. The Mini-Nutritional Assessment (MNA) is one of the most widely used ones. It is a simple, low-cost, fast and noninvasive tool that has been shown to perform well in elderly living in various settings or with various health conditions (Citation13). The MNA consists of 18 items and evaluates 4 areas of nutritional status—anthropometric, dietary, global and self-rated status. A normalized version (MNA-T1) has been developed for Taiwanese elderly by adopting population-specific anthropometric cutoff points. An alternative version that replaced calf circumference (CC) for BMI (MNA-T2) was also developed (Citation14). These Taiwanese-specific versions have been shown to be suitable for assessing the nutritional status of elderly Taiwanese living in various settings (Citation15, 16) or with various health conditions (Citation17).
A few recent reports have used the MNA to evaluate the nutritional risk of patients with COPD (Citation18–21). However, the appropriateness of using the MNA to screen for nutritional risk in patients with COPD has not been robustly examined. Thus, we conducted this study to determine the appropriateness of using the MNA for assessing the nutritional risk of patients with COPD.
Methods
Design and subjects
We recruited 83 (79 males and 4 females) patients with COPD from the pulmonary rehabilitation unit of a medical center in northern Taiwan to serve as study subjects during November 2009 to April 2011. The diagnosis of COPD and the grading of the severity of the disease were based on the 2007 version of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (Citation22). Only non-hospitalized patients who were able to communicate verbally and without acute infection or exacerbation during the study period were allowed to participate. The study protocol was approved by the Ethics Committee of the Chang-Gung Memorial Hospital (No. 98-3601B) and conducted according to the guidelines laid down in the Declaration of Helsinki. All participants signed a consent form in the presence of a nursing staff and family members.
Outcome measurements
Each participant underwent a person-to-person interview with a structured questionnaire for eliciting personal, demographic, lifestyle and disease-related data and answers to items in the MNA. Each subject was also measured for anthropometric indicators and body composition, and taken a fasting blood specimen for measuring blood/serum biochemical indicators. Weight, height, MAC and CC were measured according to standard methods (Citation23). Bioelectrical impedance analysis (TANITA's, BF-800 Body Fat Monitor, Tokyo, Japan) was performed to determine percentage of body fat. Fat-free mass was calculated from body weight minus fat mass. FFMI was calculated according to fat-free mass (kg)/height (m)2. Hemoglobin, total lymphocyte count, and serum albumin and C-reactive protein (CRP) concentrations were measured in the clinical laboratories of the hospital.
The nutritional status of each patient was rated with both versions of the MNA. The MNA has a maximum score of 30. A score ≤ 16.5 suggests malnourishment; 17–23.5 suggests at risk of malnutrition, and ≥ 24 suggests normal (Citation14). Lung function indicators including FVC and FEV1 were measured with a spirometer (SpiroAnalyzer ST-250, Fukuda Sangyo, Tokyo, Japan). Each subject was also evaluated for exercise capacity with the 6-Minute Walk Test (6-MWT) according to American Thoracic Society guidelines (Citation24). Patients were instructed to walk as far as possible but were allowed to stop and rest when necessary. During the test, pulse oxygen saturation (SaO2) was continuously monitored with a finger pulse oximeter (BCI, 3301, Wisconsin, USA).
Statistical analysis
Data were analyzed with PASW Statistics 18.0 (SPSS Inc., Chicago, IL). Simple descriptive statistics such as mean and standard deviation or percentage were calculated for continuous or categorical data. Kappa statistics were performed to evaluate the agreement of the two MNA versions in discriminating the nutritional status. Receiver operating characteristic (ROC) curves were generated for both MNA versions using the FFMI as a reference. The area under the curves (AUC) was used to indicate the discriminating ability by each scale. Pearson's correlation analysis was performed to determine the strength of relationship of age, anthropometric, biochemical, lung function (FEV1 and FVC), physical functional and health-related parameters with the MNA scores whereas Spearman's correlation analysis was performed to determine the relationship between the severity of COPD (GOLD stages) with the MNA scores. The level of significance for all statistical tests was set at α = 0.05.
Results
shows the characteristics and the status of health indicators of subjects. Most subjects (75/83) aged over 65 years; 55 (66%) were past smokers and 15 (18%) were current smokers; Average FEV1% predicted was 60.7 ± 20.4%, and FVC% predicted was 75.6 ± 18.2%. Fourteen subjects (16.9%) had mild, 43 (51.8%) had moderate, 22 (26.5%) had severe and 4 (4.8%) had very severe airway obstruction according to GOLD guidelines. The MNA scores were generally lower with increasing COPD severity. The average BMI was 23.7 ± 3.9 kg/m2, and FFMI was 17.8 ± 2.5 kg/m2. Only 12 patients (14.4%) were <90% of ideal weight, and 15 (19.2%) were < 4 g/dL (averaged 4.3 ± 0.3 g/dL) in serum albumin. Average CRP was 9.9 ± 17.3 mg/L.
Table 1. Basic characteristics and functional and nutritional status of 83 patients with chronic obstructive pulmonary disease (COPD)
shows the MNA item score patterns. Forty-three patients (51.8%) had moderate loss of appetite over the past 3 months; 10 (12.0%) had weight loss ≥1 kg; 23 (27.7%) had psychological stress or acute disease; 66 (79.5%) were on ≥ prescription drugs; about a quarter of patients consumed inadequate protein-rich foods or fruits/vegetable; nearly half (48.2%) did not consume enough water; 43.4% thought self as malnourished or were unsure about their nutritional state; and 42.2% thought self had poorer health relative to peers or were unsure about own health status. MNA-T1 rated 4 (4.8%) as malnourished, 28 (33.7%) as at risk of malnutrition, and 51 (61.4%) as normal; whereas MNA-T2 rated 4 (4.8%) as malnourished, 26 (31.3%) as at risk of malnutrition, and 53 (63.9%) as normal. κ = 0.949 for the two versions (p < 0.001) ().
Table 2. MNA item-score patterns of 83 patients with chronic obstructive pulmonary disease
Table 3. Cross-tabulation test of the nutritional scores between MNA-T1 and MNA-T2 of 83 patients with chronic obstructive pulmonary disease
shows the correlations the MNA scores with age and various COPD-related indicators. The scores of both MNA versions were significantly correlated with BMI, MAC, CC, FFMI, FEV1, FVC, and O2 saturation at rest or during exercise (all p < 0.05), but not with age and blood biochemical indicators (all p > 0.05) on the basis of Pearson's correlation. The scores of both MNA versions were also correlated with GOLD stages on the basis of Spearman's correlation. 6MWD was correlated with the score of MNA-T2 but not MNA-T1 on the basis of Pearson's correlation (p < 0.05).
Table 4. Correlation coefficients (r) of MNA scores with anthropometric, biochemical and pulmonary function status of 83 patients with chronic obstructive pulmonary disease
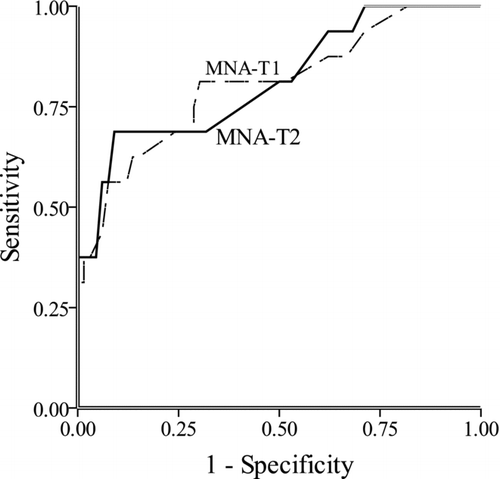
shows the ROC generated for MNA-T1 and -T2 in predicting FFMI of patients with COPD. The AUC of ROC was 0.804, (95% CI = 0.673–0.935, p < 0.001) for MNA-T1 and 0.813, 0.686–0.940, p < 0.001 for MNA-T2 (FFMI was sex-adjusted).
Discussion
Results show the MNA predicts the FFMI (a widely used nutritional indicator for patients with COPD) well and the MNA score is significantly correlated with most other anthropometric indicators or exercise capacity/pulmonary functional indicators. Thus, the MNA appears to be an appropriate tool for rating the nutritional status of patients with COPD. However, the MNA scores were not significantly (p > 0.05) correlated with blood biochemical indicators.
Validating the MNA for rating patients with COPD
Without a universally accepted gold standard, we used a divergent list of anthropometric, lung function, exercise capacity, and biochemical markers, especially FFMI as reference standards for the validation. We found that MNA scores were significantly correlated with all anthropometric indices examined including FFMI, BMI, MAC and CC. Patients with COPD generally have lower fat-free mass compared to healthy elderly (Citation25). Depletion of fat-free mass is associated with impaired peripheral muscle strength (Citation12) and FFMI is an independent predictor of mortality in patients with COPD (Citation26) and also a stronger predictor of mortality than BMI (Citation27). In patients with COPD, muscle wasting is common, thus fat-free mass is an important nutritional indicator (Citation7, Citation28).
We used FFMI as the major reference standard for rating the functional ability of the MNA. BMI is probably the most frequently used indicator of general nutritional status, especially for the elderly. Higher BMI has been shown to be a better predictor of long-term survival in patients with acute exacerbation in patients with COPD (Citation29). Furthermore, patients with COPD with lower BMI took longer time to improve symptoms and had longer hospital stay (Citation1). MAC reflexes body muscle mass and subcutaneous fat and it complements BMI in the diagnosis of protein-energy malnutrition and mid-arm muscle area and it is a better predictor of mortality than BMI in patients with stable COPD (Citation30). CC is a major biomarker of malnutrition in hospitalized elderly patients (Citation31).
It can reflect nutritional status, lean body mass, functional activity and general health conditions in elderly population better than BMI or MAC (Citation32). Results suggest that the MNA predicts FFMI well and the MNA scores are highly correlated with anthropometric indicators examined in these patients.
Rating the nutritional status with the MNA
Both MNA versions rated 4.8% (4/83) of patients with COPD as malnourished and roughly one-third were as at risk of malnutrition. Cross-tabulation test (combining malnourished and at risk vs. normal) showed that the two versions have high agreement (κ = 0.949). The MNA scores were also significantly correlated (p < 0.05) with all anthropometric and most exercise capacity markers.
To date, only a few studies have used the MNA to rate the nutritional status of patients with COPD. Odencrants et al. (Citation19) rated 50 patients with COPD admitted to an acute care hospital with the MNA and found all but two were malnourished or at risk of malnutrition. Scichilone et al. (Citation20) observed that the MNA score was associated with dyspnoea in 32 ≥ 60-year-old patients with COPD. Benedik et al. (Citation18) found that 14% of patients with COPD were malnourished and another 55% were at risk of malnutrition, and there was a positive correlation between MNA score and lean body mass. Battaglia et al. (Citation33) suggested that the MNA was valuable for gaining an insight into the nutritional problems in patients with COPD, and might provide useful clues for treatment strategies. Yuceege et al. (Citation21) rated 60 male patients with COPD from outpatient clinics with SGA and MNA. They found that the MNA identified more patients as malnutrition than SGA and suggested that SGA and MNA could be used for the evaluation of the nutritional state in patients with COPD. The present study has further shown that the MNA predicts FFMI of patients with COPD well and their MNA scores are significantly (p < 0.05) correlated with most other anthropometric, lung function and exercise capacity indicators.
Rating lung function with the MNA
Our results suggest that the MNA is capable of predicting lung function capacity in patients with COPD. The MNA score is significantly correlated with FEV1, FVC and GOLD stages of COPD patients. This finding is generally in line with the observations of earlier studies. Steuten et al. (Citation9) observed that COPD stage 4 patients had lower BMI compared to less severe patients. Gupta et al. (Citation1) found the correlation between body weight and FEV1/FVC% was good and the correlation between BMI and FEV1was statistically significant among patients in hospital with COPD acute exacerbation. Yuceege et al. (Citation21) found FEV1 and FVC values were less in the malnourished group evaluated with the SGA, but not with the MNA in male patients with COPD. Exercise capacity is important in patients with COPD and it also reflects their nutritional status. We observed that the score of both versions of the MNA were significantly (p < 0.05) correlated with O2 saturation at rest and during exercise and with 6MWD, an indicator of exercise capacity. MNA-T2 shows slightly stronger correlations with all three indicators than MNA-T1. Clinical observations suggest that exercise limitation in patients with COPD might be related to their weakening in lung function and worsening of nutritional conditions (Citation8, Citation34).
The present study observed no significant correlation between the MNA score and serum albumin or other biochemical indicators (all p > 0.05). Similar findings have also been observed by others. Braun et al. (Citation2) found that albumin was only slightly reduced and prealbumin was within the normal range in patients with COPD. Giron et al. (Citation35) observed that serum albumin values remained normal in most (82%) of hospitalized patients with COPD. Gupta et al. (Citation36) found that nutritional status of patients with COPD newly admitted for hospitalization rated with the Subjective Global Assessment (SGA) was not correlated with albumin or any other blood nutritional indicators. Even in the severely malnourished patients, serum albumin was within the normal range (averaged 4.08 g/dL). Gupta et al. (Citation1) further showed that serum total protein, albumin, urea and creatinine were all within the normal ranges in patients with COPD admitted in hospital with acute exacerbation.
Yazdanpanah et al. (Citation37) found that serum albumin was associated with the severity of the disease (GOLD stages), but was only minimally reduced, from 4.43 mg/dL for stage 2 patients to 4.06 and 3.6 mg/dL for stages 3 and 4, respectively. Thus, Batres et al. (Citation38) concluded that biochemical parameters including serum albumin, prealbumin and transferrin were generally not useful for assessing nutritional status in patients with COPD and suggested that the reason might be because these indicators are influenced by non-nutritional factors, such as infections and renal or hepatic disease. Inflammation, hydration status and medication especially corticosteroids may also play a role (Citation39).
Limitations of the study
This study has some limitations. (a) Subjects were recruited from one hospital and therefore might not represent the entire spectrum of patients with COPD in Taiwan. (b) The prevalence of COPD in female was relatively low. We were able to recruit only four female patients. This small sample size may not accurately reflect the status of female subjects. Further confirmation with larger samples is needed. (c) Most subjects were elderly adults and some might have difficulty in correctly recalling certain information in the MNA questionnaire. This might have a slight impact on the accuracy of the MNA score. (d) The full MNA involves more items/questions compared to the SGA or single item indicators such as FFMI or FVC1. Thus, it may have greater chance of revealing nutritional inadequacy at an earlier stage than other tools/indicators. However, more items mean more time consuming which is an important factor in today's clinical settings.
Conclusion
Our results suggest that both MNA-T1 and MNA-T2 appear appropriate for assessing the nutritional status of patients with COPD. Nutritional scores rated with both versions correlated well with most nutrition-related anthropometric and pulmonary function indicators. The MNA appears appropriate not only for rating the nutritional status but also for suggesting exercise capacity of patients with COPD. Since patients with COPD may have greater risk of malnutrition than their non-COPD counterparts, routine screening with the MNA may enhance timely intervention to prevent the development of malnutrition in these patients.
Funding
The study was supported by a grant from the Chang-Gung Memorial Hospital (CMRPG391921).
Declaration of Interest Statement
All authors declare no conflict of interest involved in this study.
Authorship contributions: MFH and SCH conceived the idea, designed and carried out the study, performed statistical analyses, and drafted the manuscript. HPK directed and supervised the study. JYW provided statistical advice; ACT directed the nutritional assessment; and both interpreted the results and edited the manuscript. All authors read and approved the final version of the manuscript.
Acknowledgments
Disclosures: Min-Fang Hsu and Shu-Chuan Ho contributed equally to this paper. The authors wish to thank the Pulmonary Rehabilitation unit of the Chang Gung Memorial Hospital at Linkou and the participants for their collaboration during the course of this study.
References
- Gupta B, Kant S, Mishra R, Verma S. Nutritional status of chronic obstructive pulmonary disease patients admitted in hospital with acute exacerbation. J Clin Med Res 2010; 2(2):68–74.
- Braun SR, Keim NL, Dixon RM, Clagnaz P, Anderegg A, Shrago ES, The prevalence and determinants of nutritional changes in chronic obstructive pulmonary disease. Chest 1984; 86(4):558–563.
- Soler JJ, Sanchez L, Roman P, Martinez MA, Perpina M. Prevalence of malnutrition in outpatients with stable chronic obstructive pulmonary disease. Arch Bronconeumol 2004; 40(6):250–258.
- Schols A, Mostert R, Soeters P, Greve LH, Wouters EF. Inventory of nutritional status in patients with COPD. Chest 1989; 96(2):247–249.
- Thorsdottir I, Gunnarsdottir I, Eriksen B. Screening method evaluated by nutritional status measurements can be used to detect malnourishment in chronic obstructive pulmonary disease. J Am Diet Assoc 2001; 101(6):648–654.
- Laaban JP, Kouchakji B, Dore MF, Orvoen-Frija E, David P, Rochemaure J, Nutritional status of patients with chronic obstructive pulmonary disease and acute respiratory failure. Chest 1993; 103(5):1362–1368.
- King DA, Cordova F, Scharf SM. Nutritional aspects of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008; 5(4):519–523.
- Ischaki E, Papatheodorou G, Gaki E, Papa I, Koulouris N, Loukides S, Body mass and fat-free mass indices in COPD: relation with variables expressing disease severity. Chest 2007; 132(1):164–169.
- Steuten LM, Creutzberg EC, Vrijhoef HJ, Wouters EF. COPD as a multicomponent disease: inventory of dyspnoea, underweight, obesity and fat free mass depletion in primary care. Prim Care Respir J 2006; 15(2):84–91.
- Kuznar-Kaminska B, Batura-Gabryel H, Brajer B, Kaminski J. Analysis of nutritional status disorders in patients with chronic obstructive pulmonary disease. Pneumonol Alergol Pol 2008; 76(5):327–333.
- Pirabbasi E, Najafiyan M, Cheraghi M, Shahar S, Abdul Manaf Z, Rajab N, Predictors’ factors of nutritional status of male chronic obstructive pulmonary disease patients. ISRN Nurs 2012; 2012:782626.
- Vermeeren MAP, Creutzberg EC, Schols AMWJ, Postma DS, Pieters WR, Roldaan AC, Prevalence of nutritional depletion in a large out-patient population of patients with COPD. Respir Med 2006; 100:1349–1355.
- Cereda E. Mini nutritional assessment. Curr Opin Clin Nutr Metab Care 2012; 15(1):29–41.
- Tsai AC, Ho CS, Chang MC. Population-specific anthropometric cut-points improve the functionality of the Mini Nutritional Assessment (MNA) in elderly Taiwanese. Asia Pac J Clin Nutr 2007; 16(4):656–662.
- Tsai AC, Chang TL, Chen JT, Yang TW. Population-specific modifications of the short-form Mini Nutritional Assessment and Malnutrition Universal Screening Tool for elderly Taiwanese. Int J Nurs Stud 2009; 46(11):1431–1438.
- Tsai AC, Chang TL, Yang TW, Chang-Lee SN, Tsay SF. A modified mini nutritional assessment without BMI predicts nutritional status of community-living elderly in Taiwan. J Nutr Health Aging 2010; 14(3):183–189.
- Tsai AC, Chou YT, Chang TL, Chang-Lee SN, Tsay SF. A modified Mini Nutritional Assessment without BMI can effectively assess the nutritional status of neuropsychiatric patients. J Clin Nurs 2009; 18(13):1916–1922.
- Benedik B, Farkas J, Kosnik M, Kadivec S, Lainscak M. Mini nutritional assessment, body composition, and hospitalisations in patients with chronic obstructive pulmonary disease. Respir Med 2011; 105:S38–43.
- Odencrants S, Ehnfors M, Ehrenberg A. Nutritional status and patient characteristics for hospitalised older patients with chronic obstructive pulmonary disease. J Clin Nurs 2008; 17(13):1771–1778.
- Scichilone N, Paglino G, Battaglia S, Martino L, Interrante A, Bellia V, The mini nutritional assessment is associated with the perception of dyspnoea in older subjects with advanced COPD. Age Ageing 2008; 37(2):214–217.
- Yuceege MB, Salman SO, Duru S, Saygıdeğer Y, Sonmez Z, Ardıç S The evaluation of nutrition in male COPD patients using subjective global assesment and mini nutritional assesment. Inter J Intern Med 2013; 2(1):1–5.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Amer J Respir Crit Care Med 2007; 176(6):532–555.
- Lee RD, Nieman DC. Assessment of the hospitalized patient in Nutritional Assessment. 3rd ed. New York: McGraw-Hill; 2003: 216–250.
- American Thoracic Society. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166(1):111–117.
- Sergi G, Coin A, Marin S, Vianello A, Manzan A, Peruzza S, Body composition and resting energy expenditure in elderly male patients with chronic obstructive pulmonary disease. Respir Med 2006; 100(11):1918–1924.
- Slinde F, Gronberg A, Engstrom CP, Rossander-Hulthen L, Larsson S. Body composition by bioelectrical impedance predicts mortality in chronic obstructive pulmonary disease patients. Respir Med 2005; 99(8):1004–1009.
- Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr 2005; 82(1):53–59.
- Lerario MC, Sachs A, Lazaretti-Castro M, Saraiva LG, Jardim JR. Body composition in patients with chronic obstructive pulmonary disease: which method to use in clinical practice? Br J Nutr 2006; 96(1):86–92.
- Lainscak M, von Haehling S, Doehner W, Sarc I, Jeric T, Ziherl K, Body mass index and prognosis in patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease. J Cachexia Sarcopen Musc 2011; 2(2):81–86.
- Soler-Cataluna JJ, Sanchez-Sanchez L, Martinez-Garcia MA, Sanchez PR, Salcedo E, Navarro M. Mid-arm muscle area is a better predictor of mortality than body mass index in COPD. Chest 2005; 128(4):2108–2115.
- Bonnefoy M, Jauffret M, Kostka T, Jusot JF. Usefulness of calf circumference measurement in assessing the nutritional state of hospitalized elderly people. Gerontology 2002; 48(3):162–169.
- Tsai AC, Lai MC, Chang TL. Mid-arm and calf circumferences (MAC and CC) are better than body mass index (BMI) in predicting health status and mortality risk in institutionalized elderly Taiwanese. Arch Gerontol Geriatr 2012; 54(3):443–447.
- Battaglia S, Spatafora M, Paglino G, Pedone C, Corsonello A, Scichilone N, Ageing and COPD affect different domains of nutritional status: the ECCE study. Eur Respir J 2011; 37(6):1340–1345.
- Sabino PG, Silva BM, Brunetto AF. Nutritional status is related to fat-free mass, exercise capacity and inspiratory strength in severe chronic obstructive pulmonary disease patients. Clinics (Sao Paulo) 2010; 65(6):599–605.
- Giron R, Matesanz C, Garcia-Rio F, de Santiago E, Mancha A, Rodriguez-Salvanes F, Nutritional state during COPD exacerbation: clinical and prognostic implications. Ann Nutr Metab 2009; 54(1):52–58.
- Gupta B, Kant S, Mishra R. Subjective global assessment of nutritional status of chronic obstructive pulmonary disease patients on admission. Int J Tuberc Lung Dis 2010; 14(4):500–505.
- Yazdanpanah L, Shidfar F, Moosavi J, Heidarnazhad H, Haghani H. Assessment of nutritional status in chronic obstructive pulmonary disease patients. Iran J Publ Health 2009; 38(3):39–45.
- Batres SA, Leó JV, Álvarez-Sala R. Nutritional Status In COPD. Arch Bronconeumol 2007; 43(5):283–238.
- Fuhrman MP. The albumin-nutrition connection: separating myth from fact. Nutrition 2002; 18(2):199–200.