Abstract
Co-morbid conditions are frequently found in patients with COPD. We evaluate the association of co-morbidities with mortality, in stable COPD. 224 patients, mean age 61.2 (±10.00), 48.2% female, mean FEV1 1.1 (±0.5) liters, median follow-up time 4.2 years, participated. Medical co-morbidities were scored according to the Charlson Co-morbidity Index (CCI). Depressive symptoms were assessed with the Hospital Anxiety and Depression Scale (HADS) and Symptom Checklist-90 (SCL-90). The Cox proportional hazard model was used for survival analyses. In our sample, 70% of all patients have a co-morbid medical condition or high depressive symptoms. During follow-up 51% of all patients died, and those with heart failure have the highest mortality rate (75%). Age, fat-free mass and exercise capacity were predictive factors, contrary to CCI-scores and high depressive symptoms. An unadjusted association between heart failure and survival was found. Although the presence of co-morbidities, using the CCI-score, is not related to survival, heart failure seems to have a detrimental effect on survival. Higher age and lower exercise capacity or fat-free mass predict mortality.
Keywords: :
Introduction
Medical and psychological co-morbid conditions are frequently found in patients with Chronic Obstructive Pulmonary Disease (COPD) (Citation1,2). Recently, Schnell et al. (Citation3) reported a prevalence rate of 96.4% and Anecchino et al. (Citation4) found 68.4% of their cohort received medication for cardiovascular diseases, diabetes or depression. An association between medical co-morbidities and mortality was demonstrated (Citation5), but not all investigators found an independent association (Citation6). However, many studies were performed in hospitalized patients (Citation5–10) and to the lesser extent in patients with stable COPD (Citation11–13). The aim of the present article is to investigate the association between co-morbidities and survival in stable COPD.
Methods
Participants
Patients were recruited from January 2004 through December 2007, before starting pulmonary rehabilitation at the Center for Rehabilitation of the University Medical Center Groningen (UMCG), the Netherlands. All patients were diagnosed with COPD according to GOLD guidelines and 242 consecutive patients participated. COPD had to be stable for at least 6 weeks, patients had to be able to fill out questionnaires, perform cycle ergometry and spirometry. Also, medical history had to be available. Eighteen patients were excluded: one patient refused to fill out questionnaires, 14 patients did not have fully available medical records, and 3 patients could not perform cycle ergometry due to their body weight (≥150 kg). Data of 224 patients were analyzed. All measurements took place as part of usual care, and each patient approved usage of his or her data. Therefore, no formal medical ethical approval was necessary due to local regulations.
Demographic variables
Age, sex, height, weight, marital status (‘living with a partner’ or ‘living without a partner’) and smoking status (‘never smoked or ex-smoker ≥1 year’ or ‘current smoker or ex-smoker <1 year’) were derived from medical records.
Physiological parameters
Spirometry (Masterlab, Viasys Healthcare) was performed to obtain FEV1 and Forced Vital Capacity (FVC). Total Lung Capacity (TLC) and Residual Volume (RV) were obtained using body plethysmography. Levels of arterial oxygen (PaO2), carbon dioxide tension (PaCO2) and lactate at rest were determined prior to cycle ergometry (OxyconPro, Viasys Healthcare). The Fat-Free Mass Index (FFMI) was determined by bioelectrical impedance analysis (Bodystat 1500).
Exercise capacity
The Incremental Shuttle Walk Test (Citation15) was used to assess walking capacity. A symptom limited cycle ergometry was performed using a 1-minute incremental schedule at 5 or 10 Watts (Citation16). Maximal workload sustained for at least 30 s (Wpeak), and maximal oxygen uptake (VO2peak) were measured.
Vital status
The primary endpoint was all-cause mortality. Although cause of death was mentioned in death certificates, it was considered not accurate enough. Survival status was obtained from municipal registrations on June 8, 2012.
Co-morbidity
Patients’ co-morbidities were obtained from medical histories, using prior diagnoses and current medication. In addition, hospital records and data from the primary care physician were obtained. With respect to medications: if patients used cardiovascular medications such as b-blockers or ACE-I, it was investigated for which indication it had been prescribed, e.g., CHF or systemic hypertension. Heart disease was defined to be present in case of documented STEMI or non-STEMI myocardial infarction, cardiological interventions and evidence for heart failure by echocardiography or scintigraphy. The presence of renal failure was looked for in medical records and by calculation of the eGFR, using serum creatinine, which was present in 178/224 patients and, classified as normal (eGFR> 90 ml/min, 1.73m2) renal function or mild (eGFR 60–89 ml/min, 1.73 m2) or moderate (eGFR 30–59 ml/min, 1.73 m2) renal function failure. The Charlson Co-morbidity Index (CCI) (Citation17) was used to classify co-morbidities and assign a weighted score. No score was attributed to COPD.
Depressive symptoms were assessed with Dutch translations of the Hospital Anxiety and Depression Scale, depression subscale (HADS-D) (Citation18,19) and the 16-item depression subscale of the Symptom Checklist-90 (SCL-90) (Citation20,21). We used the conventional HADS-Depression cut-off (≥8) to indicate high depressive symptoms. For the SCL-90 no cut-off is available (Citation26).
Statistical analyses
For all analyses SPSS 18.0.3 for Windows (SPSS Inc., Chicago, Illinois) was used. Patient characteristics were calculated in terms of means, standard deviations, medians or percentages. Comparison of non-survivors to survivors was carried out using independent samples’ t-tests. Presence of medical co-morbidities (CCI-score of 0 versus ≥1) and high depressive symptoms (HADS-D ≥8) were included in regression analyses. All variables significant at p < .05 on a bivariate level were included in a multivariate analysis, adjusting for confounders (Cox proportional hazard model). Results were expressed in hazard ratios (HR) and 95% Confidence Intervals (CI).
Results
Patient characteristics
Characteristics of all patients (108 women, 116 men) are presented in . Of the 224 participants, 114 (51%) had died at the end of the observation period. The mortality rate was highest in patients with co-morbid CHF. The overall median follow-up time is 4.2 years, ranging from 14 days to 7.6 years. The cumulative mortality rate is as follows: one year, 8%; two years, 16%; three years, 25%; four years, 33%; five years, 39%; six years, 46%; and seven years, 49%. Survivors are significantly younger than non-survivors, have higher FEV1 and FEV1%predicted, less hyperinflation, and a better exercise capacity (Wpeak, VO2peak and ISWT%predicted).
Table 1. Characteristics of the total sample of patients with stable COPD
Medical co-morbidities are present in 56% of all patients. The most prevalent co-morbidity is moderate or severe renal disease, followed by diabetes mellitus without organ damage (DM), congestive heart failure (CHF) and myocardial infarction (AMI, ). Co-morbidity scores are significantly higher in non-survivors. High depressive symptoms are present in 28% of all patients.
Table 2. Frequencies of comorbidities in the sample of patients with stable COPD, classified according to the Charlson Comorbidity Index (CCI)
Predictors of mortality in all patients with stable COPD
On a bivariate level, CCI-scores or high depressive symptoms are not related with mortality (). Lower FEV1, lower PaO2, higher RV%TLC, higher FFMI, lower Wpeak, lower VO2peak and a lower ISWT%predicted are significantly related to mortality.
Table 3. Predictors of mortality in all patients with stable COPD
Multivariate Cox regression analyses further demonstrate ISWT%predicted (hazard ratio = 0.997, 95% C.I. 0.995–0.999) is an independent predictor of increased mortality, adjusting for age, FEV1 and FFMI. Analyses with Wpeak or VO2peak, substituting ISWT%predicted, show Wpeak is associated with mortality (hazard ratio = 0.98, 95% C.I. 0.97–0.99) but VO2peak (hazard ratio = 0.999, 95% C.I. 0.998–1.000) is not.
Adjusted for age, FEV1 and ISWT%predicted, FFMI (hazard ratio = 0.93, 95% C.I. 0.88–0.98) is independently predictive of increased mortality, whereas BMI is not. An additional bivariate analysis with three subsamples (underweight; BMI < 21, normal weight; BMI ≥21 and < 30, overweight; BMI >30), reveals no significant result either.
FEV1 does not predict survival independently (after adjusting for age, FFMI and ISWT%predicted). Additionally, RV%TLC (hazard ratio = 0.99, 95% C.I. 0.97–1.01) and PaO2 (hazard ratio = 0.92, 95% C.I. 0.80–1.06) are not associated with mortality when tested in separate multivariate analyses, substituting FEV1.
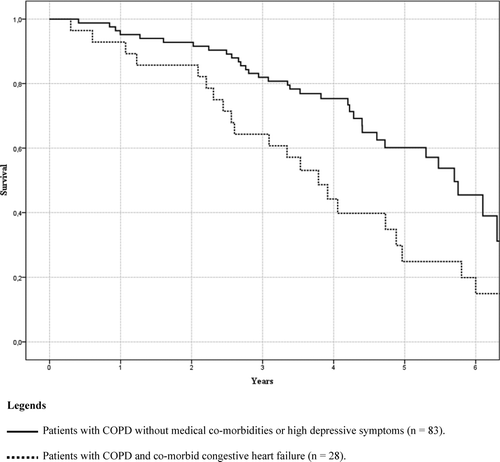
Because of the high mortality rate in patients with co-morbid CHF, an additional survival analysis was performed with co-morbid heart failure as a dichotomy (present or absent) in our total sample shows an association with increased mortality (p = .005, hazard ratio = 1.97, 95% C.I. 1.23–3.18). But, this association was no longer significant after adjusting for age, ISWT and FFMI (p = .208, hazard ratio = 1.39, 95% C.I. 0.83–2.32). A survival curve using Kaplan–Meier estimates was plotted for this subsample and a sample of patients without co-morbid conditions, to graphically illustrate the high mortality rate ().
Discussion
In our sample of patients with stable COPD, 56% is diagnosed with at least one co-morbid medical condition, 28% has high depressive symptoms and 70% has either at least one medical co-morbid condition or high depressive symptoms. Having a co-morbid condition in itself (Charlson Co-morbidity Index-score) is not related to long-term mortality. However, heart failure is, unadjusted, associated with survival this particular sample has the most unfavorable survival prospects (75% died). Acknowledged survival predictors age, exercise capacity test ISWT, cycle ergometry derived Wpeak and fat-free mass do independently predict survival. For each year a patient gains, the estimated survival chance decreases with 4%. Further, a low score increases the risk of dying per year with 3% for the ISWT (%predicted), with 2% for Wpeak (per 5 W) and with 7% for fat-free mass (per 1 kg/m2).
Our finding that the Charlson Co-morbidity Index-scores did not predict mortality contradicts with the work of some (Citation11,12), but is in line with the results of others (Citation13). One explanation for the absence of an association with mortality might lie in the possibly exponential relationship between CCI-scores and survival (Citation23). But, our finding might as well indicate more pervasive problems with the CCI exist. Therefore the CCI should be handled carefully, especially in clinical samples. Alternative indices need to be developed (Citation2,Citation24).
The observed mortality (51%) is more or less similar to the mortality in two other cohorts we earlier investigated: n = 121, age 61 years, follow-up 8.5 years, mortality 63% (7.5 %/year) (14) and n = 122, age 61 years, follow-up 7 years, mortality 39% (5.6 %/years) (Citation25). The mortality in our cohorts is somewhat higher than in other ‘landmark’ cohorts: Moberg et al. (Citation26): n = 674, age 69 years, mean follow-up 5.5 years, mortality 48.2%, (8.8 %/year), the medical group from the NETT (Citation27): n = 610, age 67 years, follow-up 6 years, mortality 50% (8.3 %/year) and Marin et al. (Citation28): n = 210, age 57 years, follow-up 9 years, mortality 24% (2.7 %/year).
Differences between the study groups with respect to age and phenotype (lung function, exacerbations) largely account for the differences in mortality between our groups and others. Our cohorts consisted of subjects specifically referred for rehabilitation, being severely ill, whilst other groups consisted of more or less stable patients. We found that 56% of all patients have at least one co-morbid medical condition. This is higher than prevalence rates found in other survival studies in stable COPD-patients which used the CCI. One study found a percentage of 38% (Citation11) and another report 43.8% of their sample has at least one co-morbid condition, but they excluded patients with certain co-morbidities (e.g. heart failure) (Citation13).
The percentages of the specific co-morbidities in our study correspond to percentages in earlier studies (Citation2). We relied on a thorough retrospective analysis of medical records in our study. This is considered an accepted way to calculate CCI scores. Prevalence rates for studies that relied on analysis of medical records range from 7–23% for congestive heart failure (Citation29–32). In our study we found 12.5% of our sample of stable COPD patients to have a co-morbid diagnosis of congestive heart failure. In COPD literature, we found two studies who relied on a prospective analysis of congestive heart failure (echocardiography) (Citation33,34). Macchia et al. found 17% of their sample of stable COPD suffered from (diastolic or systolic) ventricular dysfunction. Rutten et al. showed 20.5% of their sample of primary care patients with stable COPD suffered from co-morbid heart failure.
As far as we know, the study by Macchia et al. is the only study that examined the mortality prognosis for COPD patients with or without CHF. It showed a nearly significant increase in mortality when left ventricular dysfunction was present (HR 2.3, p = 0.053). Our study investigated whether co-morbidity influences prognosis. We are of course aware that an analysis of medical records has limitations, compared to echocardiography, but as we found quite similar prevalences with previous studies, we are confident that the results of our study yields useful and valid information. The negative effect of heart failure on mortality in COPD was found in several studies using prior diagnosis of heart failure (Citation5,Citation35) or high levels of NT-prBNP (Citation36,37). These studies were in hospitalized patients, and follow-up was up to one year. These and our findings point at awareness of adequate diagnosis and treatment of heart failure in COPD, not only in an acute situation, but also in a stable condition.
In this study, the ISWT is as strongly associated to survival as cycle ergometry derived Wpeak and performs even better than VO2peak. Our finding that the ISWT independently predicts survival is in accordance with recent findings by others (Citation38,39). The ISWT is easy to perform, relatively cheap and might in certain cases be considered as an alternative to ergometry tests (Citation40,41).
Fat-free mass predicts mortality in our study, whereas BMI does not. The predictive value of fat-free mass is in accordance with other studies in stable COPD outpatients (Citation42,43), or in patients following a rehabilitation program (Citation44). In addition, a low fat-free mass was predictive of postoperative complications following Lung Volume Reduction Surgery (Citation45). BMI does not predict survival in our study, as in the study of Schols et al., perhaps partly due to overlap between low fat-free mass and low BMI. On the other hand, BMI and FFM are supposed to be of additional value to each other (Citation43) and our and other results may suggest that in patients with worse COPD, e.g., those attending a rehabilitation program, the FFMI is a better predictor of mortality than the BMI.
Contrary to earlier findings of our research group (Citation14,Citation25) depressive symptoms are not associated to survival. One of the issues in explaining this finding is homogeneity of measures over studies. Earlier, the Beck Depression Index and the Brief Assessment Schedule Depression Cards did not independently associate to survival (Citation42,Citation46). The HADS was studied twice before and both studies generated an independent association with mortality (Citation25,Citation47). We are the first to study SCL-90 depression subscale scores as a predictor for mortality, which were not predictive.
Conclusions
Exercise capacity parameters, fat-free mass and age independently predict survival. The Charlson Co-morbidity Index (CCI) is not a significant predictor in this group with stable COPD and we question the value of the CCI in relatively small samples. Patients with co-morbid heart failure have the worst outcomes in terms of survival.
Acknowledgments
We thank Eric van Sonderen, methodologist, for his assistance in the data analysis.
Declaration of Interest Statement
All authors have no conflicts of interest to disclose. The work was funded by the Dutch Asthma Foundation. The sponsor had no role in the study design, data collection, data analysis, writing and reviewing of the manuscript.
References
- Chatila WM, Thomashow BM, Minai OA, Criner GJ, Make BJ. Co-morbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008; 5(4):549–555.
- Patel AR, Hurst JR. Extrapulmonary co-morbidities in chronic obstructive pulmonary disease: state of the art. Expert Rev Respir Med 2011; 5(5):647–662.
- Schnell K, Weiss CO, Lee T, Krishnan JA, Leff B, Wolff JL, The prevalence of clinically-relevant co-morbid conditions in patients with physician-diagnosed COPD: a cross-sectional study using data from NHANES 1999–2008. BMC Pulm Med 2012; 12(26):567–574.
- Anecchino C, Rossi E, Fanizza C, De Rosa M, Tognoni G, Romero M, Prevalence of chronic obstructive pulmonary disease and pattern of co-morbidities in a general population. Int J Chron Obstruct Pulmon Dis 2007; 2(4):567–574.
- Almagro P, Cabrera FJ, Diez J, Boixeda R, Ortiz B, Murio C, Co-morbidities and short-term prognosis in patients hospitalized for acute exacerbation of COPD. The ESMI study. Chest 2012; 142(5):1126–1133.
- Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest 2003; 124(2):459–467.
- Almagro P, Calbo E, Ochoa de Echaguen A, Barreiro B, Quintana S, Heredia JL, Mortality after hospitalization for COPD. Chest 2002; 121(5):1441–1448.
- Holguin F, Folch E, Redd SC, Mannino DM. Co-morbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest 2005; 128(4):2005–2011.
- Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med 2003; 163(10):1180–1186.
- Antonelli Incalzi R, Fuso L, De Rosa M, Forastiere F, Rapiti E, Nardecchia B, Co-morbidity contributes to predict mortality of patients with chronic obstructive pulmonary disease. Eur Respir J 1997; 10(12):2794–2800.
- Marti S, Munoz X, Rios J, Morell F, Ferrer J. Body weight and co-morbidity predict mortality in COPD patients treated with oxygen therapy. Eur Respir J 2006; 27(4):689–696.
- Casanova C, Cote C, de Torres JP, Aguirre-Jaime A, Marin JM, Pinto-Plata V, Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171(6):591–597.
- Soler-Cataluna JJ, Sanchez-Sanchez L, Martinez-Garcia MA, Sanchez PR, Salcedo E, Navarro M. Mid-arm muscle area is a better predictor of mortality than body mass index in COPD. Chest 2005; 128(4):2108–2115.
- de Voogd JN, Wempe JB, Koeter GH, Postema K, van Sonderen E, Ranchor AV, Depressive symptoms as predictors of mortality in patients with COPD. Chest 2009; 135(3):619–625.
- Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 1992; 47(12):1019–1024.
- Varray A, Prefaut C. Exercise capacity in patients with respiratory disease: procedure and results. Eur Resp Rev 1995; 5:51–58.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic co-morbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40(5):373–383.
- Van Hemert B, Ormel J. Nederlandse versie van de Hospital Anxiety and Depression Scale (HADS); vragenlijst en regels voor scoring –Dutch version of the Hospital Anxiety and Depression Scale (HADS); questionnaire and scoring instructions. Leiden/Groningen: Vakgroep psychiatrie/Vakgroep gezondheidswetenschappen; 1993.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67(6):361–370.
- Derogatis LR. SCL-90: administration, scoring and procedures manual-I for the R(evised) version. Baltimore: Johns Hopkins University School of Medicine, Clinical Research Unit; 1977.
- Arrindell WA, Ettema JHM. SCL-90: Handleiding voor een multidimensionele psychopathologie-indicator-SCL-90: Manual for a multifaceted measure of psychopathology. Lisse: Swets & Zeitlinger; 2003.
- Wagena EJ, Arrindell WA, Wouters EF, van Schayck CP. Are patients with COPD psychologically distressed? Eur Respir J 2005; 26(2):242–248.
- Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: Role of co-morbidities. Eur Respir J 2006; 28(6):1245–1257.
- Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto-Plata V, Co-morbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186(2):155–161.
- de Voogd JN, Wempe JB, Postema K, van Sonderen E, Ranchor AV, Coyne JC, More evidence that depressive symptoms predict mortality in COPD patients: is type D personality an alternative explanation? Ann Behav Med 2009; 38(2):86–93.
- Moberg M, Vestbo J, Martinez G, Williams JE, Ladelund S, Lange P, Validation of the i-BODE Index as a predictor of hospitalization and mortality in patients with COPD participating in pulmonary rehabilitation. COPD 2013 epub ahead of print.
- National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume–reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003 05/22; 2014/01;348(21):2059–2073.
- Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med 2010; 182(3):325–331.
- Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL. Patterns of co-morbidities in newly diagnosed COPD and asthma in primary care. Chest 2005; 128(4):2099–2107.
- Noteboom B, Jenkins S, Maiorana A, Cecins N, Ng C, Hill K. Co-morbidities and medication burden in patients with chronic obstructive pulmonary disease attending pulmonary rehabilitation. J Cardiopulm Rehabil Prev 2014; 34(1):75–79.
- Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003; 107(11):1514–1519.
- Sidney S, Sorel M, Quesenberry CP Jr, DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest 2005; 128(4):2068–2075.
- Rutten FH, Moons KG, Cramer MM, Grobbee DE, Zuithoff N, Hoes AW. Recognising heart failure in elderly patients with stable chronic obstructive pulmonary disease in primary care: cross sectional diagnostic study. BMJ 2005; 331(7529):1379.
- Macchia A, Rodriguez Moncalvo JJ, Kleinert M, Comignani PD, Gimeno G, Arakaki D, Unrecognised ventricular dysfunction in COPD. Eur Respir J 2012; 39(1):51–58.
- Slenter RH, Sprooten RT, Kotz D, Wesseling G, Wouters EF, Rohde GG. Predictors of 1-year mortality at hospital admission for acute exacerbations of Chronic Obstructive Pulmonary Disease. Respiration 2012 Oct 2; 85(1):15–26.
- Hoiseth AD, Omland T, Hagve TA, Brekke PH, Soyseth V. NT-proBNP independently predicts long term mortality after acute exacerbation of COPD—a prospective cohort study. Respir Res 2012 Oct 29;13:97–99.
- Medina AM, Marteles MS, Saiz EB, Martinez SS, Laiglesia FR, Rodriguez JA, Prognostic utility of NT-proBNP in acute exacerbations of chronic pulmonary diseases. Eur J Intern Med 2011; 22(2):167–171.
- Ringbaek T, Martinez G, Brondum E, Thogersen J, Morgan M, Lange P. Shuttle walking test as predictor of survival in chronic obstructive pulmonary disease patients enrolled in a rehabilitation program. J Cardiopulm Rehabil Prev 2010; 30(6):409–414.
- Williams JE, Green RH, Warrington V, Steiner MC, Morgan MD, Singh SJ. Development of the i-BODE: validation of the incremental shuttle walking test within the BODE index. Respir Med 2012; 106(3):390–396.
- Arnardottir RH, Emtner M, Hedenstrom H, Larsson K, Boman G. Peak exercise capacity estimated from incremental shuttle walking test in patients with COPD: a methodological study. Respir Res 2006; 7:127.
- Luxton N, Alison JA, Wu J, Mackey MG. Relationship between field walking tests and incremental cycle ergometry in COPD. Respirology 2008; 13(6):856–862.
- Waschki B, Kirsten A, Holz O, Muller KC, Meyer T, Watz H, Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest 2011; 140(2):331–342.
- Vestbo J, Prescott E, Almdal T, Dahl M, Nordestgaard BG, Andersen T, Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med 2006; 173(1):79–83.
- Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr 2005; 82(1):53–59.
- Nezu K, Kawaguchi T, Kimura M, Yasukawa M, Kushibe K, Taniguchi S, Lung volume reduction surgery and nutritional status in patients with severe emphysema. Jpn J Thorac Cardiovasc Surg 2001; 49(9):552–556.
- Yohannes AM, Baldwin RC, Connolly MJ. Predictors of 1-year mortality in patients discharged from hospital following acute exacerbation of chronic obstructive pulmonary disease. Age Ageing 2005; 34(5):491–496.
- Ng TP, Niti M, Tan WC, Cao Z, Ong KC, Eng P. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med 2007; 167(1):60–67.