Abstract
The COPD assessment test (CAT) is a short questionnaire designed to assess the impairment in health status of COPD patients. We aimed to determine the change of the CAT in COPD patients after 1 year of treatment and test the association between the score and clinical and lung function variables. Methods A cohort of 111 newly diagnosed COPD patients in primary care was evaluated at baseline and one year after the implementation of the recommended treatment according to the Global Initiative for the management of COPD (GOLD). Results Most of the patients (82%) were diagnosed with mild to moderate airflow limitation (mean FEV1 72 ± 21.5% predicted) and the CAT score increased in proportion with the GOLD stage of severity. The CAT significantly correlated with the number of exacerbations, visits to general practitioners and days of hospitalization both at the beginning and at 1 year follow-up. A strong negative correlation between the CAT score and FEV1 predicted was also observed. The CAT was responsive to the application of treatment with a significant improvement in the mean score (95% confidence interval) following 12 months of treatment by –2.4 (–2.9, –1.9) despite the small decline in lung function indices. The number of exacerbations in the preceding year and FEV1 were independent predictors of the CAT score in the general linear model. Conclusion The CAT questionnaire may serve as a simple, measurable tool complementary to spirometry in the assessment of severity and of response to treatment in unselected COPD patients in primary care.
| Abbreviations | ||
| CAT | = | COPD assessment test |
| GOLD | = | Global Initiative for COPD |
| SGRQ | = | St George's Respiratory Questionnaire |
| GPs | = | general practitioners |
| ICS/LABA | = | inhaled corticosteroids/long-acting β2 agonists |
Introduction
The COPD assessment test (CAT) is a short, patient-completed questionnaire designed to provide a score that indicates the impact of the disease on health status of COPD patients (Citation1). CAT development has involved well-accepted methodologies used to develop psychometric tools (Citation1). Subsequent item reduction and validation studies resulted in the final 8-item CAT questionnaire with good sensitivity and reliability (Citation1,2). The eight questions forming the CAT cover the most burdensome symptoms of COPD such as breathlessness and limitations in daily activities. The design of the test aims to improve the communication between COPD patients and healthcare professionals, thus enabling a common understanding of the disease's severity and impact (Citation2). Interestingly, it has been demonstrated that it is possible to relate CAT scores to clinical scenarios descriptive of impaired health status in COPD, providing users of the CAT with a more rounded understanding of the effects of COPD associated with different CAT scores (Citation3). The questionnaire exhibits high reproducibility and is independent of various languages, as well (Citation4,5).
When compared with other more complex health status questionnaires, such as St. George's Respiratory Questionnaire (SGRQ) and clinical COPD Questionnaire, the CAT showed similar psychometric properties (Citation2,Citation6). Although SGRQ reflects the COPD health status very well, it is rather complicated, time consuming and scores can only be calculated by a computer-based scoring system (Citation6). On the other hand, the CAT is much shorter and easier to use, and can be completed within a few minutes at most (Citation1). The high correlation between SGRQ and CAT is remarkable, suggesting that the CAT provides a reliable measure of the impact of COPD on a patient's health status (Citation2,Citation6). Recent studies also indicate that the CAT is sensitive to changes in health status associated with recovery from exacerbations (Citation7), and is immediately responsive to pulmonary rehabilitation, not only in COPD, but also in unselected chronic respiratory disease patients (Citation8,9).
We hypothesized that CAT score, owing to its good discriminative properties, would be sensitive to detect treatment effects in a cohort of COPD patients. Accordingly, the aim of the present study is: (i) to establish the association between clinical variables and the CAT score in a cohort of first diagnosed COPD patients in primary care and (ii) to determine the change of CAT score and associate variables following 1 year of treatment with application of current GOLD guidelines in routine clinical practice.
Patients and methods
Details of the initial study design and entry requirements have been published previously and are summarized here (Citation10). Twenty-five general practitioners (GPs) in Northern Greece participated in the study. The first 50 patients aged >40 years, who visited each GP for any reason during the period for 1st March 2009 to 31 May 2009 and fulfilled the entry criteria, were included in the study. All participants completed the IPAG questionnaire (Citation11) and underwent spirometry with the handheld PiKo-6® flow meter, both of which were used as screening tools for the diagnosis of COPD.
Exclusion criteria were previous medically confirmed diagnosis of respiratory diseases, previous use of inhaler medication and uncontrolled cardiac disease. Guided by a respiratory physician who visited the GPs, all subjects performed spirometry (Vitalograph Ltd, Buckingham, UK) according to American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines (Citation12). Post-bronchodilator forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC) and FEV1/FVC were recorded. Patients with significant (>12%) acute bronchodilator reversibility were excluded from the present study. COPD diagnosis was based on physicians’ clinical evaluation and was confirmed by post-bronchodilator spirometry on stable condition according to Global Initiative for Obstructive Lung Disease (GOLD) guidelines (Citation13). Then, 111 newly diagnosed COPD patients were finally enrolled in the present study. The Medical Ethics Committee of the G. Papanikolaou Hospital, Thessaloniki, Greece approved the study protocol.
The patients were categorized in GOLD stages according to the severity of airflow limitation (post-bronchodilator FEV1% predicted >80% for stage I, 50–80% for stage II, 30–50% for stage III and <30% for stage IV). Pharmacological treatment was assigned by the GPs according to the GOLD stage. In particular, short acting bronchodilators (salbutamol 200 mcg prn) were recommended as “rescue” relief therapy for GOLD stage I patients, long-acting anticholinergic or long-acting β2 agonists (tiotropium 18 mcg od or formoterol 12 mcg bid) for GOLD stage II patients and finally a combination of inhaled corticosteroids with long-acting β2 agonists (ICS/LABA) or/and long-acting anticholinergic (budesonide/formoterol 320/9 mcg bid or/and tiotropium 18 mcg od) for GOLD severity stage III and IV patients. Compliance to treatment was monitored by the GPs during the next 12 months at regular follow-up visits every 3 months. All subjects performed a second spirometry, conducted by the respiratory physicians, in a follow-up visit after 12 months of treatment.
All participants completed an 8-item CAT questionnaire both at the time of diagnosis (baseline-CAT1) as well as after one year of treatment (end of study-CAT2). The history of exacerbations, hospitalization and visit to GPs related to COPD during the previous 12 months was recorded at baseline and 1 year after the implementation of the recommended treatment. An exacerbation was defined as a worsening of symptoms that required oral corticosteroids and/or antibiotics and/or hospitalization. The presence of cardiovascular disease in general, as well as the presence of systemic hypertension, coronary artery disease, congestive heart failure, arrhythmias or stroke, was specifically recorded.
CAT questionnaire
The patients in this study completed the CAT questionnaire that was validated by Jones (Citation1). The CAT consists of eight items, each formatted as a semantic 6-point differential scale, making the tool easy for patients to complete.
The items are related to cough and phlegm, chest tightness, breathlessness going up hills/stairs, activity limitations at home, confidence leaving home, sleep and energy. Each item is scored from 0 to 5, giving a total score range from 0 to 40 and providing a reliable measure of the impact of COPD on a patient's health status. CAT scores are categorized into severity bands, as described in the CAT users guide (http://www.catestonline.org): Low Impact (CAT score 1 to 10), Medium Impact (11 to 20), High Impact (21 to 30), and Very High Impact (31 to 40) (Citation14). The questionnaire was accessed via www.CATestonline.org and was translated into the Greek language.
Statistical analysis
The paired samples t-test was used to test pre- versus post-treatment differences between continuous measurements, while the chi-square test was used for the comparison of proportions. One-way analysis of variance (ANOVA) was employed in order to check differences of continuous measurements among GOLD stages, and the Tukey-Kramer HSD test was used as post-hoc. The Pearson correlation coefficient was used for the assessment of linear relationships between continuous variables.
The General Linear Model was used for the assessment of the dependence between the CAT score and FEV1, adjusting for the presence of exacerbations, while binary logistic regression was used to model the probability of exacerbation based on CAT scores.
Any p-values less than 0.05 were considered statistically significant. The statistical package SPSS version 17.0 (SPSS Inc., Chicago, IL) and JMP 8.0 (SAS Inst., Cary, NC) were used for data analysis.
Results
Of the 1,250 subjects examined (50 subjects × 25 GPs), 172 refused to participate in the second phase of the study (spirometry) or did not meet the ATS/ERS criteria for spirometry. Thus, data on 1,078 subjects (57.1% males, mean age 65.3 ± 11.4 years) were collected and analyzed. The percentage of smokers was 48.4% (38 ± 29 pack-years). We diagnosed 111 (10.3%) patients with COPD who were enrolled in the current extension study. Five patients were withdrawn due to lack of compliance to treatment (3/5) and due to serious adverse event unrelated to treatment (death 1-stroke 1). In the subgroup of smokers the prevalence of COPD was 17.2% (90/522). The clinical characteristics of COPD patients are summarized in .
Table 1. Demographic and clinical characteristics of patients with COPD (N = 111)
The majority of COPD patients were males with a mean age of 71 years, mean smoking history of 44 pack-years and mean FEV1 of 1.89 L (72 ± 21.5% predicted). Most of them (92/111, 82%) were classified in GOLD stages I and II. In one year follow-up, mean FEV1 decreased by 0.06 (95% confidence interval: −0.08, −0.03) and FVC by 0.06 (95% CI: −0.09, −0.02). Following 12 months of treatment, a substantial decrease in the number of exacerbations was also observed from the patients’ medical records (paired t-test, t105 = 4.83, p < 0.001). A proportional decrease was found in the number of visits to GPs (t105 = 7.52, p < 0.001). No difference in the number of hospitalizations (t105 = −0.38, p = 0.71) and the days of hospitalizations (t105 = 0.96, p = 0.34) was noted ().
Table 2. Change of the CAT score, the clinical and lung function parameters after 12 months of treatment
CAT scores and COPD severity
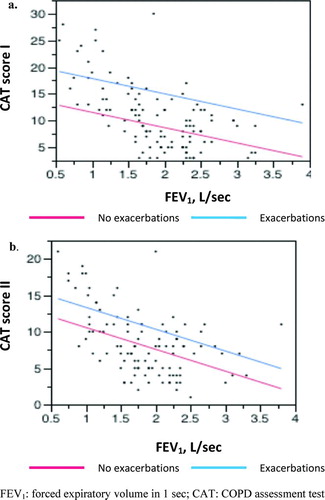
There was a significant improvement in the mean CAT score following 12 months of treatment by −2.4 (95% CI: −2.9, −1.9); p < 0.001. CAT score increased along with severity according to the GOLD stage, demonstrating impairment in health status of the patients across all stages. This result was found both at baseline (one-way ANOVA, F3,107 = 59.17, p < 0.001) and at the end of the study (F3,102 = 30.98, p < 0.001) (). A significant correlation between FEV1 and total CAT score was detected at baseline (Pearson correlation, r = −0.583, p < 0.001) and at the end of study as well (r = −0.523, p < 0.001).
Table 3. Differences in COPD assessment test (CAT) scores between GOLD stages before (CAT score 1) and after (CAT score 2) treatment
Association between CAT scores and exacerbations
Based on binary logistic regression modeling, we sought to assess if exacerbations lead to poorer health outcomes as measured by the CAT and whether the CAT score could further predict future exacerbations. The estimated probability of an exacerbation in the preceding year increased accordingly to the value of the CAT score at the beginning of the study (OR = 1.473, p < 0.001). Moreover, a significant relationship was found between the baseline CAT score and the probability of exacerbations at 1-year follow-up after adjusting for possible confounders, such as sex, age, smoking status, diabetes and cardiovascular co-morbidities at baseline (OR = 1.167, p = 0.002).
The CAT1 score was positively correlated with the number of exacerbations (r = 0.646, p < 0.001), the number of hospitalizations (r = 0.243, p = 0.010) and the days of hospitalization (r = 0.289, p = 0.003). Simple linear regression modeling suggests that the number of exacerbations increased per 0.12 with each point rise of CAT1. There was no statistically significant association between the CAT1 score and the probability of hospitalization.
Similar results were found at the end of the study based on binary logistic regression modeling, with the CAT2 score increasing proportionally with the estimated probability of exacerbation in the preceding year (OR = 1.256, p < 0.001). There was a statistically significant association between CAT2 score and the number of exacerbations (r = 0.400, p < 0.001). The CAT2 score was positively correlated with the number of hospitalizations (r = 0.352, p < 0.001) and also with the days of hospitalization (r = 0.351, p < 0.001).
Concerning the visit to the GPs related to COPD deterioration, a statistically significant association was found between CAT scores of those who visited the GP vs. those who did not, both at the beginning (independent samples t-test, t109 = 9.7, p < 0.001) and at the end of the study (t104 = 8.93, p < 0.001). Similarly the number of visits increased accordingly with the CAT score at baseline (r = 0.687, p < 0.001) and at the end of study (r = 0.684, p < 0.001).
Use of the general linear model in the whole population revealed that the number of exacerbations in the preceding year and FEV1 were independent predictors of the CAT score, resulting in the following linear regression models: CAT1 = 14.818649 − 2.964921 (FEV1) + 2.5106965 (exacerbation/year), R2 = 0.49 at the time of diagnosis and CAT2 = 13.402661 − 2.932829 (FEV1) +2.4211109 (exacerbation/year), after 12 months of treatment, R2 = 0.3 ().
Association between CAT scores and co-morbidities
There was no difference in CAT scores between patients with and without cardiovascular co-morbidities both at the beginning and at the end of the study.
Association between CAT score and pharmacological treatment
A statistically significant difference was found among the means of CAT score along with the escalation of recommended therapies both at baseline (one-way ANOVA, F3,107 = 70.49, p < 0.001) and at the end of treatment (F3,102 = 39.94, p < 0.001). The values of CAT score were higher at more severe GOLD stages, so that the patient was treated more often with ICS/LABA or/and tiotropium combination.
Discussion
In the present study we prospectively used the CAT questionnaire to assess health status in a cohort of newly diagnosed COPD patients in primary care at baseline and after 12 months of treatment. Our results indicate health status impairment across all COPD severity stages evaluated with the CAT score. Furthermore, the CAT score was strongly associated with clinical variables, such as the number of exacerbations, visits to GPs and days of hospitalization both at the beginning and at 1 year follow-up. In contrast to previous studies, a strong negative correlation was observed between the CAT score and lung function indices, such as FEV1 predicted. Importantly, our results indicate that the CAT score presented with a significant improvement after application of the recommended treatment strategy in the cohort of COPD patients, despite the small decline of the FEV1 observed at 1 year follow-up.
Baseline CAT values were found elevated even in COPD patients with mild airway obstruction. Patients in GOLD stages I and II present with a low impact CAT score (1 to 10). However, as it was previously shown, even this low severity band of the CAT score corresponds to a clinically significant impairment on daily activities of COPD patients (Citation14).
It is noteworthy that the CAT scores were clearly different between patients categorized to be mild or moderate according to the GOLD guidelines at the time of diagnosis. Small but not significant difference in health status impairment between these two GOLD stages was still detected after one year of treatment. In accordance to previous studies, these results suggest that the CAT questionnaire provides GPs with an objective tool complementary to spirometry in the assessment of severity especially in COPD patients with mild to moderate airflow limitation (Citation2,Citation15). Moreover, owning to its good discriminative properties even between the low stages of disease severity, the CAT can serve as a potentially useful instrument for guiding the management decisions at the time of COPD diagnosis in primary care.
In this study we have shown that both baseline and follow-up CAT scores are associated with the exacerbation frequency, the visits to GPs and the number and days of hospitalization. Previous studies have shown that patients with a history of frequent exacerbations present with a more rapid decline in lung function, increased risk of hospitalization, and a greater mortality (Citation16,17). Our results indicate that CAT may serve as a simple measurable tool to discriminate high-risk COPD patients at first evaluation in primary care.
Importantly, CAT score could further identify COPD patients at risk for future exacerbations; in the middle range of the baseline CAT values (approximately around 20), a rise of the CAT score per 1 point is estimated to increase the average odds of a future exacerbation per 16.7%. Similarly, in previous studies, exacerbation rate was identified as an important contributor to impaired health status expressed by the CAT. In the study of Kelly et al., CAT score rose by approximately 1 point per reported exacerbation per year and differed by 8 points between the low and high exacerbation rate groups (Citation15). Moreover, in previous studies the CAT appeared to be sensitive to changes in health status in patients experiencing COPD exacerbations (Citation18) and provided a reliable score of exacerbation severity (Citation19,20). CAT scores increased at exacerbation and reflected exacerbation severity as determined by lung function and exacerbation length (Citation20).
Interestingly, we observed a significant inverse correlation between FEV1 and total CAT score both at baseline and after one year of treatment. Both the number of exacerbations in the preceding year and FEV1 were independent predictors of the CAT score in linear regression analysis. Similar results were not seen in a large European health status study evaluating the properties of the questionnaire where CAT score had only a weak negative correlation with FEV1% predicted (Citation2). In the study of Kelly et al. as well, an absolute reduction of 10% in predicted FEV1% produced less than a 1-point change in the CAT score (Citation15). The same applies for other more complex health status questionnaires such as SGRQ. As shown by a meta-analysis aiming to identify markers of COPD severity, SGRQ is only able to differentiate between health condition and presence of COPD, but it is not a real indicator of the severity of the disease according to the degree of airway obstruction (Citation21).
The most notable finding of the present study is that the CAT score was responsive to the application of treatment. After one year of treatment, the mean CAT score improved by 2 units, which is considered a clinically meaningful difference or change in health status (Citation14). To our knowledge this is the first study in the literature to evaluate the use of the CAT score as a marker of the effectiveness of treatment in a general population of COPD patients. In accordance to the results of previous large placebo-control trials (Citation22,Citation23), a substantial decrease in the number of exacerbations and visits to GPs following treatment was observed in our study.
Small number of hospitalizations in the year before diagnosis may account for the absence of improvement in the number and the days of hospitalizations after treatment. Despite the small decline of the FEV1 in 1 year follow-up, the CAT responded to the application of treatment, largely reflecting the amelioration of symptoms related to COPD exacerbations. Previous studies have evaluated the CAT in the context of clinical pulmonary rehabilitation programs (Citation8,9). The CAT score was immediately responsive to rehabilitation and remained improved at 6 months (Citation9), suggesting that the CAT can be used as an outcome measure in patients with COPD taking part in rehabilitation programs.
The majority of patients with COPD are managed in primary care. Most GPs are now familiar with spirometry which remains the standard method for confirming a clinical diagnosis of COPD and monitoring the efficacy of pharmacological treatment. However, spirometric measures are poor descriptors of the impact of disease and it is now recognized that FEV1 measurements alone do not represent the complexity of COPD (Citation24). A number of clinical and physiological outcomes, such as dyspnea, exercise capacity and health status, are recognized as being important for the characterization of response to treatment (Citation25).
In a recent study, using cluster analysis, Burgel et al. proved that variables such as age, symptoms, health status, exacerbations and co-morbidities were markedly different among COPD subjects with the same airflow limitation (Citation26). These results highlight the value of the novel multidimensional approach adopted by the current guidelines that combine the symptomatic assessment with the patient spirometric classification and the risk of exacerbation (Citation24).
The strength of this study is that it included a relatively large sample of unselected patients in primary care with data collected prospectively across multiple sites. As such, it is likely to be generalisable to routine clinical practice. One limitation of the present study is that we have not included a well-established measure, such as the SGRQ, in parallel with the CAT, in order to test the ability of the CAT to exhibit responsiveness to treatment. Another weakness of the study is that it was not designed to detect whether the CAT affected any management decisions made by the GPs. This was a necessary limitation since there were no management guidelines based on the CAT at the time that the study was designed. The situation has changed, and the CAT is now incorporated in 2014 updated GOLD together with lung function testing and exacerbation history.
For the first time in the literature, we have shown that the CAT score provides an objective tool to evaluate the effectiveness of treatment in COPD patients, at a primary care level. Since the questionnaire is quick to complete and score, it could therefore be used as a measurable marker to track changes in health status following interventions in routine clinical practice or even as outcome measure in future clinical trials to investigate the effectiveness of novel treatments in prevention of COPD exacerbations.
Acknowledgments
We thank Dr. Hellie Lithoxopoulou for helping us prepare the manuscript.
Declaration of Interest Statement
The authors declare that they do not have a conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jones PW. COPD assessment test- rationale, development, validation and performance. COPD 2013; 10(2):269–271.
- Jones PW, Brusselle G, Dal Negro RW, Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J 2011; 38:29–35.
- Jones PW, Tabberer M, Chen WH. Creating scenarios of the impact of COPD and their relationship to COPD Assessment Test (CAT™) scores. BMC Pulm Med 2011; 11:42.
- Kwon N, Amin M, Hui DS, Validity of the COPD assessment test translated into local languages for Asian patients. Chest 2013; 143:703–710.
- Jones PW, Shahrour N, Nejjari C, Psychometric evaluation of the COPD assessment test: data from the BREATHE study in the Middle East and North Africa region. Respir Med 2012; 106 Suppl 2:S86–99.
- Tsiligianni IG, van der Molen T, Moraitaki D, Assessing health status in COPD. A head-to-head comparison between the COPD assessment test (CAT) and the clinical COPD questionnaire (CCQ). BMC Pulm Med 2012; 12:20.
- Jones PW, Harding G, Wiklund I, Berry P, Tabberer M, Yu R, Leidy NK. Tests of the responsiveness of the COPD assessment test following acute exacerbation and pulmonary rehabilitation. Chest 2012; 142:134–140.
- Kon SS, Clark AL, Dilaver D, Canavan JL, Patel MS, Polkey MI, Man WD. Response of the COPD assessment test to pulmonary rehabilitation in unselected chronic respiratory disease. Respirology 2013; 18:974–977.
- Dodd JW, Marns PL, Clark AL, The COPD Assessment Test (CAT): short- and medium-term response to pulmonary rehabilitation. COPD 2012; 9(4):390–394.
- Sichletidis L, Spyratos D, Papaioannou M, Chloros D, Tsiotsios A, Tsagaraki V, Haidich AB. A combination of the IPAG questionnaire and PiKo-6® flow meter is a valuable screening tool for COPD in the primary care setting. Prim Care Respir J 2011; 20:184–189.
- Grouse L, DeWeerdt S, editors. COPD diagnosis track. In: International Primary Care Airways Group (IPAG) Diagnosis and Management Handbook, Jan 2005. MCR Vision, Inc., p11–12.
- Miller MR, Hankinson J, Brusasco V, et al; ATS/ERS Task Force: Standardisation of spirometry. Eur Respir J 2005; 26:319–338.
- Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2007. Available from: http://www.goldcopd.org/.Guidelines/guidelines-global-strategy-for-diagnosis-management-2007-3.html. Last Accessed: April 16, 2014.
- COPD Assessment Test: Healthcare Professional User Guide. Issue 3: February 2012. On behalf of the CAT Development Steering Group. www.catestonline.org /CATHCPUser guideEn-pdf. Last Accessed: April 16, 2014.
- Kelly JL, Bamsey O, Smith C, Health status assessment in routine clinical practice: the chronic obstructive pulmonary disease assessment test score in outpatients. Respiration 2012; 84:193–199.
- Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60:925–931.
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57:847–852.
- Agustí A, Soler JJ, Molina J, Is the CAT questionnaire sensitive to changes in health status in patients with severe COPD exacerbations? COPD 2012; 9:492–498.
- Chetta A, Olivieri D. The COPD Assessment Test in the evaluation of chronic obstructive pulmonary disease exacerbations. Expert Rev Respir Med 2012; 6:373–375.
- Mackay AJ, Donaldson GC, Patel AR, Jones PW, Hurst JR, Wedzicha JA. Usefulness of the Chronic Obstructive Pulmonary Disease Assessment Test to evaluate severity of COPD exacerbations. Am J Resp Crit Care Med 2012; 185:1218–1224.
- Franciosi LG, Page CP, Celli BR, Markers of disease severity in chronic obstructive pulmonary disease. Pulm Pharmacol Ther 2006; 19:189–199.
- Barr RG, Bourbeau J, Camargo CA, Ram FS. Inhaled tiotropium for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005; (2):CD002876.
- Tashkin DP, Fabbri LM. Long-acting beta-agonists in the management of chronic obstructive pulmonary disease: current and future agents. Respir Res 2010; 11:149.
- Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) Updated 2014. Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Last Accessed: April 16, 2014.
- Cazzola M, MacNee W, Martinez FJ, on behalf of the American Thoracic Society/European Respiratory Society Task Force on outcomes of COPD. Outcomes of COPD pharmacological trials: From lung function to biomarkers. Eur Respir J 2008; 31:416–469.
- Burgel PR, Paillasseur JL, Caillaud D, Initiatives BPCO Scientific Committee. Clinical COPD phenotypes: A novel approach using principal component and cluster analyses. Eur Respir J 2010; 36:531–539.