Abstract
Acute respiratory failure (ARF) can necessitate mechanical ventilation and intensive care unit (ICU) admission in patients with COPD. We evaluated the reasons COPD patients are admitted to the ICU and assessed long-term outcomes in a retrospective cohort study in a respiratory level-III ICU of a teaching government hospital between November 2007 and April 2012. All COPD patients admitted to ICU for the first time were enrolled and followed for 12 months. Patient characteristics, body mass index (BMI), long-term oxygen therapy (LTOT), non-invasive ventilation (LT-NIV) at home, COPD co-morbidities, reasons for ICU admission, ICU data, length of stay, prescription of new LTOT and LT-NIV, and ICU mortality were recorded. Patient survival after ICU discharge was evaluated by Kaplan–Meier survival analysis. A total of 962 (710 male) patients were included. The mean age was 70 (SD 10). The major reasons for ICU admission were COPD exacerbation (66.7%) and pneumonia (19.7%). ICU and hospital mortality were 11.4%, 12.5% respectively, and 842 patients were followed-up. The new LT-NIV prescription rate was 15.8%. The 6-month 1, 2, 3, and 5-year mortality rates were 24.5%, 33.7%, 46.9%, 58.9% and 72.5%, respectively. Long-term survival was negatively affected by arrhythmia (p < 0.013) and pneumonia (p < 0.025). LT-NIV use (p < 0.016) with LTOT (p < 0.038) increase survival. Pulmonary infection can be a major reason for ICU admission and determining outcome after ICU discharge. Unlike arrhythmia and pneumonia, LT-NIV can improve long-term survival in eligible COPD patients.
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide, and its prevalence is expected to increase to be the third leading cause of death in 2020 (Citation1, 2). Patients with advanced stages of COPD can experience respiratory failure that requires oxygen and mechanical ventilation (non-invasive or invasive), both short term in the hospital/intensive care unit (ICU), and long term at home (Citation3–5).
Acute COPD exacerbation is a major cause of acute respiratory failure (ARF) that leads to ICU admission. Studies researching COPD patient outcome following ICU admission and a long-term ICU stay have largely excluded any other primary reasons, other than acute exacerbation of COPD (Citation6,7,Citation8). There are reports on ICU outcomes of COPD patients with pneumonia (Citation9, 10); however, there is limited data on these COPD patients regarding all causes for ICU demand and long-term stay, and mortality after ICU discharge. In the present study, we aimed to evaluate the reasons for ICU admission and long-term outcome after ICU discharge. Factors affecting mortality in the ICU were not assessed.
Methods
We designed a retrospective, observational, cohort study in a 22-bed, level III ICU of a tertiary teaching hospital for chest diseases, between November 2007 and April 2012. During the study period, a total of eight intensivist specialists worked in the ICU, which they staffed 24 hours a day. This study was approved by the local ethics committee of Sureyyapasa Chest Diseases and Thoracic Surgery Teaching and Research Hospital, Istanbul, Turkey. Ethical approval was in accordance with the Declaration of Helsinki. Due to the retrospective nature of the study design, informed consent was not obtained as half of the patients had died at the beginning of study.
Patients
All consecutive patients with previously diagnosed COPD, who were admitted to our ICU due to ARF and were followed-up for more than 12 months, were included. The COPD diagnosis was established by a physician who evaluated airflow obstruction on spirometry, i.e. forced expiratory volume in 1 second (FEV1) of 70% predicted or less, and an FEV1 and forced vital capacity ratio of 70% or less (Citation11). Spirometry test data were not recorded from the patients’ charts. Only those patients who were admitted to the ICU for the first time with ARF were evaluated in case of recurrent admissions to the ICU during the study period.
Data
Demographics and reasons for ICU admission associated with respiratory failure, such as sepsis/septic shock, pneumonia, pulmonary embolism, and pneumothorax, were recorded from the patients’ ICU files (Citation5, Citation12, Citation13). Co-morbidities including diabetes mellitus, arrhythmia (i.e. atrial fibrillation), hypertension, congestive heart failure, coronary artery disease (CAD), and depression were recorded (Citation14, 15). History of smoking, use of long-term oxygen therapy (LTOT), and long-term mechanical ventilation (non-invasive (NIV) or invasive (IMV)) via tracheostomy were also recorded. The acute physiology and chronic health evaluation II (APACHE II) score was calculated as part of the patients’ ICU severity index and on admission to the ICU (Citation16). Arterial blood gas (ABG) values at the time of ICU admission and discharge from ICU were recorded from the patients file. Serum C-reactive protein (CRP) levels, the application of NIV and/or IMV in the ICU, the length of ICU and hospital stay (days), and ICU mortality were recorded on admission to, and discharge from the ICU.
Definitions
Hypoxic ARF was defined as the ratio of partial arterial oxygen pressure to inspired fractionated oxygen (PaO2/FiO2) < 300 and partial arterial carbon dioxide pressure (PaCO2) < 45 mmHg. Hypercapnic/hypoxemic ARF was defined as PaCO2 > 45 mmHg and PaO2/FiO2 < 300, and hypercapnic ARF was PaCO2 > 45 mmHg and PaO2/FiO2 > 300 (Citation5, Citation12). The definition of COPD exacerbation due to an infectious origin was defined by the presence of all three Anthonisen's criteria as follows: worsening of dyspnea, increased volume of pulmonary secretions (endotracheal, sputum), and increased purulence of respiratory secretions (Citation11,Citation17).
Mechanical ventilation
Initially, NIV was applied to all COPD patients with hypercapnic respiratory failure except when absolutely contraindicated (Citation5, Citation12, Citation18). Contraindications of NIV were defined as: 1) absolute, respiratory arrest and unable to fit mask, and 2) relative, medically unstable (hypotensive shock, uncontrolled cardiac ischemia or arrhythmia, uncontrolled copious upper gastrointestinal bleeding), agitation, uncooperativeness, inability to protect airway, impaired swallowing, excessive secretions not managed by secretion clearance techniques, multiple (two or more) organ failure, and recent upper airway or upper gastrointestinal surgery (Citation5, Citation12, Citation18).
NIV was provided in pressure assist-control mode with ICU mechanical ventilators via a double-tube circuit with a full-face mask. Pressure support (PS) was initially set at 8–10 cmH2O and gradually increased to a maximum of 30 cmH2O until the exhaled tidal volume was 5–7 mL/kg and guided by patient tolerance. Positive end-expiratory pressure (PEEP) was set at 5 cmH2O and raised or lowered to treat hypoxemia or enhance patient comfort, respectively. FiO2 was adjusted to maintain oxygen saturation (SaO2) at 90%. NIV was applied intermittently for periods of 1 to 4 hours, and initial ABG samples were obtained at the end of the first hour. The duration of each session was determined by ABG value improvement, consciousness level, and patient compliance. The definition of NIV failure in hypercapnic patients was no pH improvement, no change or a rise in breathing frequency after 1–2 hours, and lack of cooperation. For hypoxic COPD patients, failure was considered as no, or a minimal, rise in PaO2/FiO2 after 1–2 h (< 200) (Citation5).
IMV was applied in the presence of absolute or relative contraindications for NIV, as mentioned above. IMV was applied by assist control ventilation (A/C) in pressure control mode. Inspiratory pressure was set for a target tidal volume of 5–7 mL/kg ideal body weight (airway plateau pressure < 30 cmH2O) under a sedation protocol. The Richmond agitation sedation scale (RASS) was used for infusion and assessment of the daily need for sedation (Citation19). When patients met the previously described criteria for weaning, the pressure support ventilation (PSV) mode was used and gradually decreased (1–2 cm H2O every 1–2 hours) (Citation20). When the PS reached 8–10 cm H2O and PEEP was 0 or < 5 cm H2O the patient progressed to spontaneous breathing trials (SBT) using a T-piece with oxygen support; the T-piece trial duration was 30 minutes, after which time they were extubated. NIV was then applied in cases of moderate respiratory distress if there was no contraindication (Citation20).
Bronchodilator and anti-inflammatory treatment in the ICU (Citation11, Citation21, Citation22)
A short-acting ß2 agonist (salbutamol, 100 mcg per puff) and ipratropium bromide (100 mcg/20 mcg per puff) were given every 2–4 hours (4 to 10 puffs) via a metered dose inhaler chamber (Aerovent, Altech®, Altera Firm, Izmir-Turkey) when the patients were under NIV or IMV. A nebular form of salbutamol (2.5 mg/2.5 mL per nebule) was given every 15 minutes to 4 hours, or ipratropium bromide/salbutamol (0.5 mg/3.01 mg/2.5 mL per nebule) was given every 2 to 4 hours for patients breathing without mechanical ventilation support.
Long-acting ß2 agonists were not used in COPD patients with ARF in the ICU. Intravenous methylprednisolone (40–60 mg) was given one to two times daily as an anti-inflammatory for those patients unable to take oral medications or with impaired gastrointestinal absorption. The steroid dose was tapered gradually and discontinued over 7 to 10 days. Methylxanthines (theophylline and aminophylline) were not given. All patients received oxygen for COPD, as well as medication to treat the underlying cause of ICU admission or ARF and co-morbidities, such as antibiotics and anti-arrhythmia or anticoagulant therapies (Citation11).
Follow-up
Long-term NIV was prescribed according to the national guidelines (based on international guidelines) for COPD patients after ICU discharge with day time hypercapnia (e.g. PaCO2 greater than 50 mmHg) and oxygen desaturation during sleep (e.g. SpO2 ≤ 88% for ≥5 minutes of ≥ 2 hours of nocturnal sleep oximetry), despite the use of supplemental oxygen at ≥2 L/min. It was also prescribed to those with an acute exacerbation that necessitated the use of continuous NIV in hospital more than two times per year (Citation23). A spontaneous bi-level positive airway pressure NIV device was prescribed if the need for inspiratory pressure was greater than 20 cmH2O, or in the presence of ineffective respiratory effort/apnea in the ICU. LTOT was prescribed when PaO2 was less than 55 mmHg or SaO2 was 88% or less in room air at the time of discharge from ICU, and when hypoxemia had occurred for 1 to 2 months prior, in a clinically stable period (Citation11). The prescription of LTOT and NIV devices after ICU discharge, and long-term survival were recorded from the patient's files. The time of death was determined from outpatient files and online in May 2013.
Statistical analysis
A descriptive analysis was used to investigate patient demographics and ICU data. Groups were compared with Mann Whitney U-tests for non-parametric continuous variables or Student's t-tests for parametric continuous variables. Chi-square tests were employed for dichotomous variables. The median with interquartile range (IQR) was employed for non-parametric continuous variables, and mean ± standard deviation (SD) was used for parametric continuous variables. Count and percentage were used when applicable.
To predict the long-term mortality of COPD patients after ICU discharge, Kaplan-Meier survival analyses were performed. Patients’ co-morbidities, reasons for ICU admission, CRP levels, APACHE II score on admission, PaCO2 and PaO2/FiO2 at ICU discharge, HCO3, prescription of LT-NIV at home, and LTOT after ICU discharge were included as variables. Long-term mortality risk factors were analyzed with the Cox regression model. We included the variables that were of statistical significance following univariate analyses of survival and non-survival in patients following ICU discharge. A p value < 0.05 was accepted as statistically significant.
Results
Patients
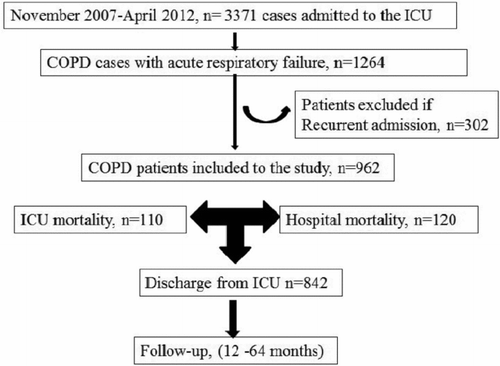
During the study period, 3371 patients were admitted to the ICU, and 1264 admissions (including re-admission) were due to ARF in COPD patients. Only those who were admitted to the ICU were evaluated and, as such; 962 patients were ultimately included in the study. Inclusion criteria are summarized in .
Patient demographics (age, gender, body mass index (BMI), history of cigarette smoking), co-morbidities (diabetes mellitus, hypertension, arrhythmias, coronary artery disease (CAD), depression) and baseline data of utilization of LTOT and LT-NIV or IMV before ICU admission are shown in . The majority of COPD patients were male, elderly (age > 65 years), and overweight.
Table 1. Characteristics of chronic obstructive pulmonary diseases patients with acute respiratory failure in the intensive care unit
ICU data
ICU data including reasons for ICU demand (acute COPD exacerbation, pneumonia, embolus, pneumothorax), APACHE II score on ICU admission, CRP and ABG values on ICU admission and discharge, length of ICU stay, and ICU mortality are shown in .
Table 2. Intensive care unit data of chronic obstructive pulmonary diseases with acute respiratory failure
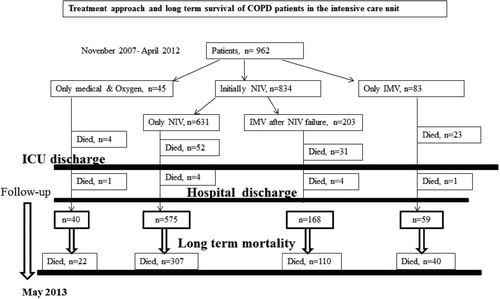
Nearly all patients were hypercapnic and hypoxemic, and one-fourth of the patients were severely acidotic (pH < 7.25). The majority of COPD patients with ARF were admitted to the ICU due to an infection, including pneumonia (86.4%). The pathogens responsible for the infections were not recorded. Airway infection was observed in 66.7% of patients without lung parenchymal infiltration. Patients with pneumonia (n = 189, 19.7%) required initial IMV (n = 17) and NIV (n = 167). Among pneumonia patients who initially received NIV, the intubation rate i.e., NIV failure, was 32.3% (n = 54). The overall mortality rate of patients with pneumonia was 21.7% (n = 41) in the ICU; but the mortality rate of pneumonia patients with IMV, NIV failure and those who only used NIV were 7/17, 18/54 and 13/113, respectively. Three patients with pneumonia who did not consent to intubation were only treated medicinally and died in the ICU. One patient with pneumonia died in the ward after ICU discharge.
shows the treatment approach and mortality of COPD patients with ARF. Although 86% of COPD patients were treated initially with NIV, initial IMV was applied in only 8.6% of cases. Overall, intubation was performed in 24.3% of patients, including those with NIV failure. The mortality rate of patients with NIV followed by IMV (NIV failure), those with NIV success, and those with initial IMV use, were 15%, 8%, and 28%, respectively, and were significantly different from each other (p < 0.001). ICU and hospital mortality rate were 11.4% and 12.5%, respectively.
Long-term Survival
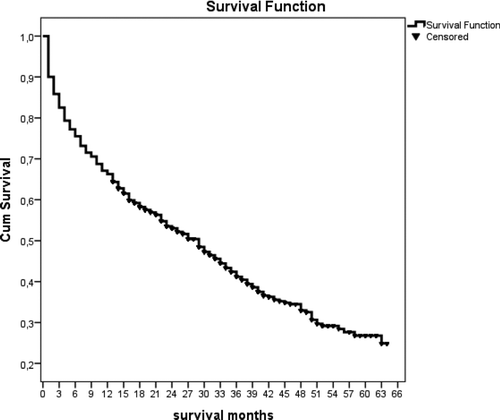
After ICU discharge, 842 patients were followed-up for a minimum of 12 months and a maximum of 64 months. The median and IQR of survival was 15 months (3 to 29 months). During follow-up, the 3- and 6-month mortality rates were 17.1% and 24.5%, respectively (). The numbers of and mortality rates of patients followed for 1, 2, 3, 4, and 5 years were n = 842 (33.7%), n = 612 (46.9%), n = 393 (58.9%), n = 214 (64.4%), and n = 80 (72.5%), respectively ().
Figure 3. COPD patient survival after ICU discharge shown in months as determined by Kaplan–Meier analysis.
demonstrates the utilization of long-term oxygen and noninvasive mechanical ventilation therapy in COPD patients with acute respiratory failure after ICU discharged.
Table 3. The utilization of long-term oxygen and noninvasive mechanical ventilation therapy in COPD patients with acute respiratory failure after ICU discharge
shows a a univariate analyzes of those patients who survived (n = 363) and those who did not survive (n = 479) in the follow-up period. Those patients who did not survive in the long-term were older, had a lower BMI, higher APACHE II score on admission to the ICU, and a higher rate of IMV use after NIV failure than survivors (). Long-term survivors had a statistically significant higher rate of NIV and shorter length of ICU and hospital stay than non-survivors.
Table 4. The univariate analyzes of survival and non-survival COPD patients’ characteristics and ICU data after hospital discharge
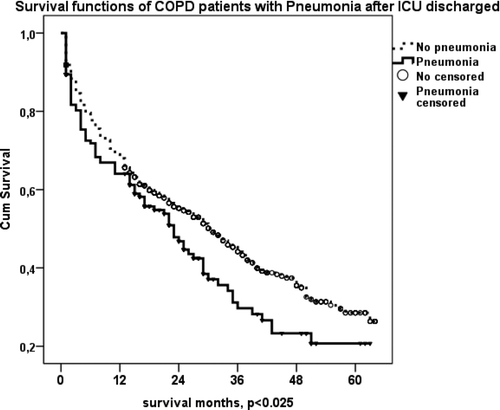
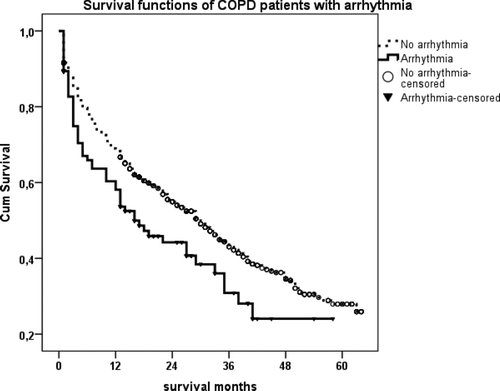
Gender, co-morbidities, reasons for ICU admission (pneumonia, pulmonary embolus, pneumothorax, COPD exacerbation), and utilization of LTOT and LT-NIV after ICU discharge, were analyzed with Kaplan–Meier analyses to identify factors affecting long-term survival. We observed statistically significant shorter survival in COPD patients with arrhythmia or pneumonia after ICU discharge (p < 0.013 and p < 0.025, respectively) (, ).
Figure 4a. (a) Kaplan–Meier survival curves of COPD patients with arrhythmia after ICU discharge. Patients with arrhythmia died earlier than patients without arrhythmia after ICU discharge (p < 0.013). (b) Kaplan–Meier survival curves of COPD patients with pneumonia as a reason for ARF after ICU discharge. Patients with pneumonia died earlier than those without (p < 0.025).
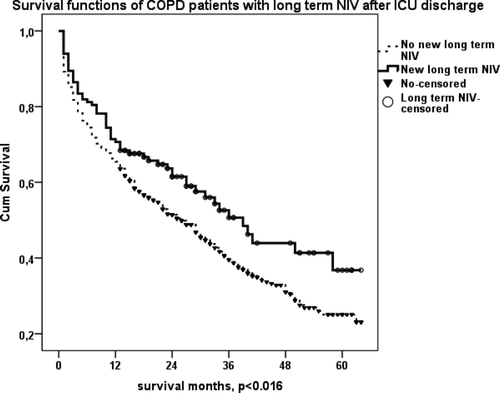
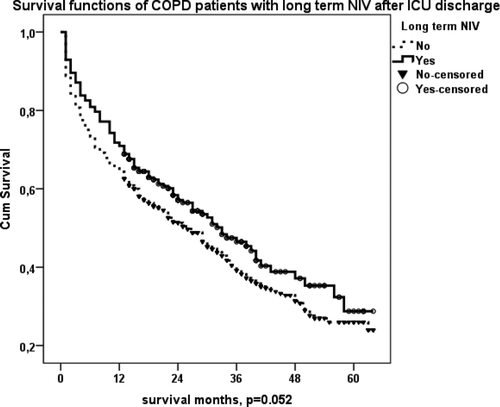
LT-NIV and LTOT prescription after ICU discharge were associated with significantly increased survival of COPD patients (p < 0.016 and p < 0.038, respectively) (, ). shows the survival of COPD patients with LT-NIV at home (previously, plus newly prescribed) after ICU discharge. Patients with LT- NIV after ICU discharge appeared to live longer than others, but this result did not quite reach significance (p = 0.052).
Figure 5. (a) Kaplan–Meier survival curve of COPD patients with new LTOT use after ICU discharge. Those with LTOT lived longer than patients without (p < 0.038). (b) Kaplan–Meier survival curve of COPD patients with new LT-NIV use at home after ICU discharge. Those who used LT-NIV after ICU discharge lived longer than patients who did not (p < 0.016).
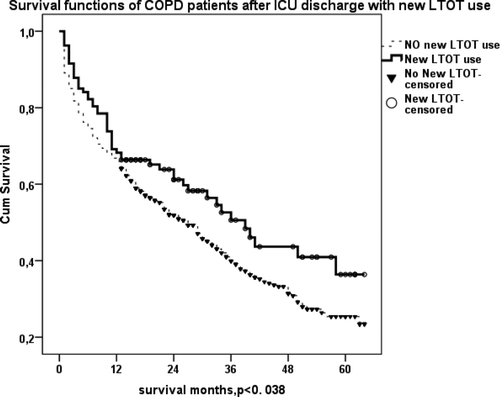
Figure 5. (c) Kaplan–Meier survival curve of COPD patients with LT-NIV at home (previously plus newly prescribed) after ICU discharge. Patients with LT- NIV after ICU discharge lived longer than others who did not use LT-NIV (p = 0.052).
The Cox regression model was used to analyze risk factors for mortality. The variables included were chosen from those described in that were statistically significant, as well as some variables that were not significant but that were suspected to be potentially significant. Thus, the variables analyzed were age, body mass index, gender, smoke history, length of ICU and hospital stay, co-morbidities, pneumonia, type of mechanical ventilation, history of previous and/or new long-term oxygen and NIV at home. The Cox-regression model showed that age above 65 years, ICU admission due to pneumonia, and each additional day in the ICU increased the risk ratio for death.On the other hand, NIV use during the ICU stay and the prescription of LTOT after ICU discharge decreased the risk of mortality during the long-term follow-up ().
Table 5. Cox regression analysis of long term survival of COPD patients after ICU discharge
Discussion
This study analyzed the ICU data and long-term outcomes in a large number of COPD patients with ARF. The main reason for ICU admission in these COPD patients was infectious in origin. After ICU discharge, COPD patients with newly prescribed LT-NIV and LTOT lived significantly longer than others. The primary risk factors for long-term mortality after ICU discharge in this group were a longer hospital stay, older age, and a lower BMI. LT-NIV after ICU discharge was associated with decreased long-term mortality.
ICU data
Previous studies investigating respiratory failure in COPD ICU patients mainly looked at acute COPD exacerbation (Citation7), (Citation24). In the present study, two-thirds of patients were admitted to the ICU due to acute COPD exacerbation. Beside COPD exacerbation, pneumonia is an important reason for ICU admission and IMV (Citation25). Rello and coworkers demonstrated the ICU mortality was 39% for COPD patients initially intubated, and 50% for those with failed NIV (Citation25). In the present study, the pneumonia rate was 19.7%, however, the mortality rate was nearly doubled when these patients were intubated initially for IMV, or after NIV failure (37.6%).
Among those cases who received IMV, a 41.2% mortality rate was seen in those who were initially intubated, and 33.3% mortality was observed in those who were intubated after NIV failure. The mortality rate following NIV failure observed here was lower than was reported in Rello's study. Pulmonary embolism is known to be a reason for acute COPD exacerbation. Our data showed that nearly one-fourth of the COPD patients with ARF were severely hypoxemic and had a higher rate of clinical suspicion of pulmonary embolism; and nearly all were intubated for IMV.
NIV is the gold standard for treating hypercapnic ARF in COPD patients (Citation5, Citation18). The success rate of NIV in this group is between 54% and 85% (Citation7, Citation26, Citation27). Confalonieri and colleagues generated a prediction chart that estimates a 70% NIV failure rate if: the pH on admission to ICU is < 7.25, a Glasgow Coma Scale <11, and an APACHE II score >29 (Citation28). In the present study, 86.7% of the COPD patients with ARF had NIV initially applied, and 24.3% of these were intubated. Overall, the NIV failure rate, including those who were intubated and those who were deceased patients during NIV, was 30.8%, which is similar to previously published results. Three fourths of NIV failure patients in our study had blood pH values <7.28, PaCO2 >76 mmHg, and APACHE II scores >23. These values are in accordance with the chart predicting NIV failure in Confalonieri's study (Citation28).
The mean length of ICU stay was longer for COPD patients with ARF requiring IMV (Citation29). Although we observed similar lengths of ICU stay in both the NIV and IMV groups, a two-fold longer ICU stay was noted for patients who were intubated for IMV after NIV failure. The overall ICU mortality rate was 12.2%, and this was similar to other studies with the exception of Alaitian and coworkers, who recently reported only 6% ICU mortality (Citation7), (Citation29–33).
Long-term follow-up
LTOT is known to be the only treatment proven to increase COPD patient survival (Citation31). However, studies have shown that 60% of COPD patients with LTOT die within 5 years (Citation26, 27), similar to our results. In the present study, LTOT was recommended after ICU discharge in 13.6% of cases, according to international guidelines and during follow-up these patients appeared to live longer. LT-NIV has been demonstrated in several studies to improve COPD patient survival. Other randomized studies have reported positive long-term effects (months) of ventilation on CO2 retention and better quality of life in stable hypercapnic (PaCO2 >50 mmHg) patients on LTOT and using LT-NIV (Citation34, 35).
These therapies also effectively reduce hospital admissions and minimize costs (Citation36). Casanova and coworkers reported a decreased number of hospital re-admissions, but no positive effect on survival (Citation37). More recently, a randomized control study described a promising effect of prolonged NIV on COPD patient survival of at least 12 months during a 5-year follow-up (Citation38). In the present study, it is important to note that COPD patients with newly prescribed LT-NIV after ICU discharge lived longer than other COPD patients, regardless of whether or not they had previously used NIV.
COPD is a systemic inflammatory disease, and the presence of co-morbidities affects quality of life and long-term survival. Previous reports that evaluated long-term mortality risk factors in COPD patients after ICU discharge failed to identify any risk factors (Citation8, Citation30). In these studies, the patient numbers were 57 and 74, which are insufficient sample sizes to identify risk factors by logistic regression analyses. In the present study, according to Cox regression model analysis, the long-term mortality risk factors of 842 COPD patients were evaluated over a period of at least 12 months, and 393 patients were included in a 3-year follow-up.
Older age, especially above 80 years, and a longer hospital stay, were identified as risk factors for increased long-term mortality. On the other hand, the prescription of LTOT after ICU discharge and NIV application during the ICU stay were associated with a decreased mortality risk in our study. Kaplan–Meier survival curves revealed that, unlike LT-NIV prescription after ICU discharge, patients with pneumonia or arrhythmia had a shorter survival period after ICU discharge.
There are some limitations to our study. First, it was retrospective and conducted in a single-center. Nonetheless, this large, specific patient group was followed by experienced ICU pulmonologist-intensivists and could provide important results to guide future studies. Secondly, spirometry test scores were not recorded from the patients’ files. Third, no cultures were taken to identify specific infectious pathogens. Finally, our results are not generalizable to all patients. The strengths of this study are the large sample size of a particular group of patients, and the length of the follow-up time.
In conclusion, the primary reasons COPD patients with ARF were admitted to the ICU were acute COPD exacerbation and pneumonia. Initial respiratory support mainly consisted of NIV, but IMV was applied after NIV failure, and a small number of patients were initially ventilated with IMV. Half of the patients survived 2 years after ICU discharge. COPD patients with pneumonia or an arrhythmia had shorter survival times after ICU discharge and, as such, these patients should be closely monitored. The prescription of LT-NIV and LTOT at home prolonged the survival in COPD patients with ARF Thus we suggest prescribing LTOT and LT-NIV to COPD patients with ARF (both hypoxemic and hypercapnic) when discharged from ICU. These findings may be helpful for designing future studies and for a COPD treatment approach.
Declaration of Interest Statement
None of the authors have any conflict of interest, and all contributed to the preparation of the manuscript. The authors alone are responsible for the content and writing of the paper.
References
- Lopez AD, Shibuya K, Rao C, Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 2006; 27(2):397–412.
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006; 3(11):e442.
- Report of the Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet 1981; 1(8222):681–686.
- Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation–a consensus conference report. Review. Chest 1999; 116(2):521–534.
- Nava S, Hill N. Non-invasive ventilation in acute respiratory failure. Lancet 2009; 374(9685):250–259. Review.
- Khilnani GC, Banga A, Sharma SK. Predictors of mortality of patients with acute respiratory failure secondary to chronic obstructive pulmonary disease admitted to an intensive care unit: a one year study. BMC Pulm Med 2004; 4:12.
- Alaithan AM, Memon JI, Rehmani RS, Qureshi AA, Salam A. Chronic obstructive pulmonary disease: hospital and intensive care unit outcomes in the Kingdom of Saudi Arabia. Int J Chron Obstruct Pulmon Dis 2012; 7:819–823.
- Breen D, Churches T, Hawker F, Torzillo PJ. Acute respiratory failure secondary to chronic obstructive pulmonary disease treated in the intensive care unit: a long term follow up study. Thorax 2002; 57(1):29–33.
- Carrillo A, Gonzalez-Diaz G, Ferrer M, Non-invasive ventilation in community-acquired pneumonia and severe acute respiratory failure. Intens Care Med 2012; 38(3):458–466.
- Confalonieri M, Potena A, Carbone G, Porta RD, Tolley EA, Umberto Meduri G. Acute respiratory failure in patients with severe community-acquired pneumonia. A prospective randomized evaluation of noninvasive ventilation. Am J Respir Crit Care Med 1999; 160(5 Pt 1):1585–1591.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for diagnosis, management, and prevention of COPD (updated 2008) (http://www.goldcopd.org).
- Ambrosino N, Vagheggini G. Non-invasive ventilation in exacerbations of COPD. Review. Int J Chron Obstruct Pulmon Dis 2007; 2(4):471–476.
- Levy MM, Fink MP, Marshall JC, International Sepsis Definitions Conference. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Review. Intensive Care Med 2003; 29(4):530–538.
- Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest 2005; 128(4):2099–2107.
- Fabbri LM, Luppi F, Beghé B, Rabe KF. Complex chronic comorbidities of COPD. Eur Respir J 2008; 31(1):204–212.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13(10):818–829.
- Anthonisen NR, Man Freda J, Warren CP, Hersfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Inter Med 1987; 106(6):196–204.
- Ambrosino N, Vagheggini G. Noninvasive positive pressure ventilation in the acute care setting: where are we? Eur Respir J 2008; 31(4):874–886.
- Sessler CN, Gosnell MS, Grap MJ, The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002; 166(10):1338–1344.
- Boles JM, Bion J, Connors A, Weaning from mechanical ventilation. Eur Respir J 2007; 29(5):1033–1056.
- Snow V, Lascher S, Mottur-Pilson C; Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001; 134(7):595–599.
- Nava S, Karakurt S, Rampulla C, Braschi A, Fanfulla F. Salbutamol delivery during non-invasive mechanical ventilation in patients with chronic obstructive pulmonary disease: a randomized, controlled study. Inten Care Med 2001; 27(10):1627–1635.
- Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation–a consensus conference report. Chest 1999; 116:521.
- Wildman M, Harrison DA, Brady AR, Rowen K. Case mix and outcomes for admissions to UK adult, general critical care units with chronic obstructive pulmonary disease: a secondary analysis of the ICNARC Case Mix Programme Database. Crit Care 2005; 9:S38–S48.
- Rello J, Rodriguez A, Torres A, Implications of COPD in patients admitted to the intensive care unit by community-acquired pneumonia. Eur Respir J 2006; 27(6):1210–1216.
- Afessa B, Morales IJ, Scanlon PD, Peters SG. Prognostic factors, clinical course, and hospital outcome of patients with chronic obstructive pulmonary disease admitted to an intensive care unit for acute respiratory failure. Crit Care Med 2002; 30(7):1610–1615.
- Scarpazza P, Incorvaia C, Melacini C, Shrinking the room for invasive ventilation in hypercapnic respiratory failure. Int J Chron Obstruct Pulmon Dis 2013; 8:135–137.
- Confalonieri M, Garuti G, Cattaruzza MS, Italian noninvasive positive pressure ventilation (NPPV) study group. A chart of failure risk for noninvasive ventilation in patients with COPD exacerbation. Eur Respir J 2005; 25(2):348–355.
- Nevins ML, Epstein SK. Predictors of outcome for patients with COPD requiring invasive mechanical ventilation. Chest 2001; 119(6):1840–1849.
- Ai-Ping C, Lee KH, Lim TK. In-hospital and 5-year mortality of patients treated in the ICU for acute exacerbation of COPD: a retrospective study. Chest 2005; 128(2):518–524.
- Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 1980; 93:391–398.
- Miyamoto K, Aida A, Nishimura M, Aiba M, Kira S, Kawakami Y. Gender effect on prognosis of patients receiving long-term home oxygen therapy. The Respiratory Failure Research Group in Japan. Am J Respir Crit Care Med 1995; 152(3):972–976.
- Marti S, Muñoz X, Rios J, Morell F, Ferrer J. Body weight and comorbidity predict mortality in COPD patients treated with oxygen therapy. Eur Respir J 2006; 27(4):689–696.
- Clini E, Sturani C, Rossi A, Rehabilitation and Chronic Care Study Group, Italian Association of Hospital Pulmonologists (AIPO). The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J 2002; 20(3):529–538.
- Meecham Jones DJ, Paul EA, Jones PW, Wedzicha JA. Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD. Am J Respir Crit Care Med 1995; 152(2):538–544.
- Tuggey JM, Plant PK, Elliott MW. Domiciliary non-invasive ventilation for recurrent acidotic exacerbations of COPD: an economic analysis. Thorax 2003; 58(10):867–871.
- Casanova C, Celli BR, Tost L, Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest 2000; 118(6):1582–1590.
- McEvoy RD, Pierce RJ, Hillman D, Australian trial of non-invasive Ventilation in Chronic Airflow Limitation (AVCAL) Study Group. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax 2009; 64(7):561–566.